Assessment of tuberculosis drug efficacy using preclinical animal models and in vitro predictive techniques

Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (M.tb), is a communicable bacterial infection spread through airborne transmission and predominantly infects the lungs. TB has been a major public health concern for many years, with an estimated 10.6 million people worldwide falling ill with the disease in 2022 alone1,2. The COVID-19 pandemic has further compounded the global TB burden, with recent reports indicating significant increases in TB-related deaths and an overall decline in TB diagnosis and treatment1,3,4. This highlights the urgent need for continued efforts to address the global TB epidemic.
One of the main challenges in the treatment of TB is the development of resistant mutants to almost every antibiotic in use, including multi-drug resistant (MDR) TB. MDR-TB is defined as resistance to the two most potent first-line drugs used to treat TB, isoniazid (INH, H) and rifampicin (RIF, R), which complicates treatment and increases the risk of death1. Globally, there were an estimated 450,000 incident cases of MDR and rifampicin resistant (RR)-TB in 2021, with 191,000 deaths occurring due to MDR/RR-TB1. This represents a 3.1% increase from the 437,000 cases reported in 2020. The increase in TB incidence from 2020 to 2021, primarily driving this rise, is believed to stem from the COVID-19 pandemic’s disruption of TB detection efforts. Furthermore, first-line therapies for TB are increasingly failing, with 600,000 new cases of tuberculosis resistant to the most effective first-line antibiotic, rifampin1. The emergence of DR-TB and XDR-TB strains pose a significant challenge to TB control initiatives, especially in low- and middle-income countries, where TB incidence is most prevalent. Managing MDR-TB cases necessitates the use of more costly and toxic second-line antibiotics, prolonged intake periods, elevated treatment expenses, extended hospital stays, and increased risk of adverse drug reactions5,6. To overcome these challenges, a comprehensive approach that includes innovative strategies for TB prevention, diagnosis, and treatment is essential. A central tenet of TB treatment is the use of combination therapy to reduce the likelihood of bacterial resistance developing7,8.
In the pursuit of more efficacious drug regimens for the treatment of M.tb, the aerosol infection mouse model has proved to be a valuable tool enabling novel drug, regimen, and shortened treatment time evaluation compared to the first line rifampicin-isoniazid-pyrazinamide-ethambutol (RHZE) regimen9,10,11,12,13,14,15,16,17,18,19,20,21,22. These investigations have included regimens that substitute rifapentine for rifampin and/or moxifloxacin for isoniazid or ethambutol, as well as the more novel PaMZ (Pretomanid, Moxifloxacin, and Pyrazinamide), BPaMZ (Bedaquiline, Pretomanid, Moxifloxacin, and Pyrazinamide), and BPaL (Bedaquiline, Pretomanid, and linezolid) regimens20.
The diarylquinoline Bedaquiline (BDQ; Sirturo®, TMC207) stands as a milestone in TB treatment, securing approval from the Food and Drug Administration in 2012 after a span of more than four decades, since the last TB drug approval23,24,25. BDQ’s remarkable potency extends to both actively replicating and nonreplicating bacterial subpopulations, as demonstrated in various studies26,27,28. Its efficacy is further supported by results from preclinical animal models, which provide valuable insights into its potential clinical application29,30 and in real-world cases of multidrug-resistant TB infection31,32. In the murine model of TB, the combination of BDQ, Pretomanid (PMD), and Pyrazinamide (PZA, Z) demonstrated superior efficacy compared to the RIF, INH, and PZA regimen, indicating a higher potential to reduce treatment time by 2 to 3 months33. In a 14-day Early Bactericidal Activity (EBA) study34,35, the BDQ-PMD-PZA combination displayed activity either similar to or exceeding that of the RIF-INH-PZA-EMB regimen. To ensure the longevity of BDQ’s effectiveness and to counteract the emergence of drug resistance, careful attention must be dedicated to designing and implementing combination regimens.
The current study aimed to examine the efficacy of BDQ when combined with Capreomycin (CAP), a cyclic peptide antibiotic with a unique mechanism of action that inhibits bacterial protein synthesis, was chosen for its potent anti-tubercular activity, particularly against MDR strains of M.tb. Other antibiotics were also evaluated, based on their distinct yet complementary mechanisms of action, including Linezolid (LIN) and Sutezolid (SUT), both of which are oxazolidinones that interfere with protein synthesis. Specifically, we hypothesize that the addition of CAP to the BDQ base regimen will enhance its effectiveness, compared with the performance of the standard RHZ regimen, which served as our control group. Utilizing the SynCidy checkerboard assay36, we aimed to discover potential synergies between BDQ and various antibiotics and to evaluate how they may differ across distinct M.tb lineages. The results indicated that the BDQ/CAP combination is effective against infection with the Lineage 2 strain (M.tb HN878). In preclinical animal models, including both mice and guinea pigs, treatment with both BDQ and the BDQ/CAP combination significantly decreases bacterial burden after 1 month of drug treatment. This work is intended to provide ways in which in vitro models can be used in concert with in vivo preclinical animal models to predict meaningful synergistic drug regimens against M.tb.
Methods
Bacterial strains and culture conditions
Very low passage mycobacterial strains were grown in Middlebrook 7H9 medium containing 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC) supplement (Becton Dickinson) and 0.05% (wt/vol) Tween 80 (7H9-Tw-OADC) under aerobic conditions. Specifically, the strains were limited to a maximum of five passages after revival from the frozen stock to maintain clinical relevance37. This approach helps to limit genetic duplications and ensures the phenotypic consistency of the bacterial strains.
Initial enhanced effect screenings used the laboratory M.tb isolate H37Rv. We also incorporated clinical isolates representing diverse M.tb lineages to ensure a broad evaluation of therapeutic efficacy. These included the lineage 2 Beijing clinical strain M.tb HN878, the lineage 4 M.tb Erdman strain and the lineage 1 M.tb T46 strain. Additionally, we extended our assessment to include a lineage 1 strain, M.tb N0072, and another lineage 4 representative, M.tb N1216, both of which were sourced from the Belgian Coordinated Collections of Microorganisms (BCCM)38, ensuring a cross-lineage analysis, and highlighting the diversity within M.tb strains.
Enhanced effect testing
Microtiter plates containing a two-dimensional array of drug were prepared in a 96-well format (Supplementary Fig. 1). The D300e digital dispenser was used to distribute the drugs into the wells. Gradient concentrations of Bedaquiline fumarate (BDQ) (ThermoFisher Scientific, Pittsburgh, PA) and Capreomycin sulphate (CAP) (AdooQ®, Bioscience LLC, Irvine CA), Linezolid (LIN) (ThermoFisher Scientific, Pittsburgh, PA), and Sutezolid (Sigma-Aldrich, St. Louis, MO) were added to wells per checkerboard SynCidy design (Table 1, Supplementary Fig. 2). Subsequently, 5×105 CFU of the specified M.tb strain were added to each well and incubated for 7 days at 37 °C. After 1-week incubation, cultures were diluted at a 1:10 ratio using a plate stamper, INTEGRA VIAFLO (INTEGRA Biosciences AG, Zizers, Switzerland). A 5 μl sample from each well was then stamped onto 7H10 agar plate supplemented with 10% v/v oleic acid, albumin, dextrose, catalase (OADC) for determination of bacterial killing. Each experiment was performed with three biological replicates to ensure reproducibility and reliability of the results.
Microbial cell viability was assayed using the BacTiter-Glo microbial viability assay (Promega, Madison, WI) according to the manufacturer’s instructions and as previously described39. Briefly, 25 μl of M.tb culture subjected to designated drug concentration were mixed with an equal volume of freshly prepared BacTiter-Glo reagent in white 96-well flatbottom plates and incubated in the dark for 30 min. Luminescence was measured using a Tecan Safire 2 plate reader and is reported as relative luminescence units.
The minimal inhibitory concentration (MIC) values for the drugs used in our study were as follows: capreomycin (3.1 μM), sutezolid (0.8 μM), bedaquiline (0.1 μM), and linezolid (1.6 μM). These concentrations guided the setup of our assays to include relevant concentrations for evaluating potential synergistic effects. This was determined based on liquid growth readout in 7H9 media, and the CFU formation was determined by plating on 7H10 agar.
Preclinical animal models
Mice
In this study, C57BL/6 female mice aged between 5 to 6 weeks, were procured from the Jackson Laboratory (Bar Harbor, ME). These mice were housed in the biosafety level 3 (BSL-3) animal facility at Seattle Children’s Research Institute (SCRI). The handling and experimental procedures involving these mice adhered to the protocols approved by the SCRI Institutional Animal Care and Use Committee (IACUC). All experimental methods were conducted in compliance with the relevant animal welfare guidelines and regulations. The mice were infected with a low dose aerosol (LDA) challenge with M.tb HN878, calibrated to deliver 50-100 colony forming units (CFU), using the Glas-Col inhalation whole body exposure system, as described previously40. To confirm initial infection, a subset of 3 mice were euthanized 24 hours post-infection. This exposure resulted in an average lung deposition of 75 bacteria enumerated the day after challenge.
Post-infection, animals were segregated into various treatment groups, with a set of negative control mice left untreated for comparison.
Guinea pigs
Four-week-old female Dunkin–Hartley guinea pigs sourced from Elm Hill Laboratories were housed in a BSL-3 facility. This facility is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). The care and experimental use of these guinea pigs were in strict compliance with the National Research Council’s guidelines for the Care and Use of Laboratory Animals with all procedures approved under protocol 1536 by the Colorado State University Animal Care and Usage Committee. Guinea pigs were exposed to a LDA of M.tb HN878, calibrated to deliver 20-50 bacilli per animal, using a Glas-Col Airborne whole-body exposure apparatus. Twenty guinea pigs were exposed per run over two days. Each run included a single guinea pig designated for euthanasia and necropsy 24 hours after exposure to confirm M.tb low-dose delivery to the lungs. For confirmation by bacterial enumeration at 24 hours, all lung lobes were collected, homogenized in 15 ml of PBS, and the total liquid homogenate was plated across 15 7H11 agar plates, employing approaches described in the methods section for bacterial enumeration.
Drug treatment regimen
Mice
Treatment began 28 days after aerosol infection with M.tb and continued for 4 weeks. Bedaquiline fumarate (BDQ) (ThermoFisher Scientific, Bothell, WA) was administered at 25 mg/kg once daily, by gavage, 5 days per week, consistent with the dosing used by Irwin S.M. et al. and Robertson G.T. et al.41,42. Capreomycin sulphate (CAP) (AdooQ, Bioscience LLC, Irvine CA) was administered at 150 mg/kg intraperitoneally (i.p.) 5 days per week, consistent with the dosing used by Zhao W. et al43 and Klemens S.P. et al.43,44,45. Rifampin (RIF, R), isoniazid (INH, H), and pyrazinamide (PZA, Z) (collectively abbreviated as RHZ) were obtained and formulated for oral administration as described previously30,46. Briefly, a combination of R (10 mg/kg), H (25 mg/kg) and Z (150 mg/kg) was administered in the drinking water.
Guinea pig
Guinea pigs were infected with a LDA of the virulent HN878 M.tb strain and allowed to progress to day 21 of infection. At day 21, treatment was initiated in each of four groups (n = 6 per group) including mock treated, BDQ alone, CAP alone, or both in combination. Guinea pigs were treated for four weeks, followed by a 72-hour washout period, then euthanized for tissue collection at day 49 of infection. BDQ was prepared as a suspension in water containing 30% w/v Captisol at concentrations of at least 15 mg/ml and administered orally at a dose of 15 mg/kg, 5 days per week47. CAP was dissolved in normal saline at 20 mg/ml for administration to guinea pigs by intramuscular injection at a dose of 20 mg/kg, 5 days per week.
Monitoring of treatment efficacy
Mice
To monitor treatment efficacy and detect recurrence, two primary assessment criteria were employed: the measurement of bacterial burden in both the lung and spleen at specific time points after antimicrobial treatment, serving as a measure of bactericidal activity. Secondly, the proportion of mice with a culture-positive bacterial counts post-treatment completion was evaluated, zero bacterial counts would be indicative of sterilizing activity. For this purpose, mice were euthanized with CO2 aligning to the approved IACUC protocols. All experimental methods were conducted in compliance with the relevant animal welfare guidelines and regulations.
Lung and spleen tissues were harvested and homogenized in RPMI + FBS (for lung) or PBS + 10% Tween-80 (for spleen). The homogenates were then serially diluted and plated on Middlebrook 7H10 agar plates supplemented with OADC. Plates were incubated at 37 °C for up to 42 days to determine the final CFU counts, providing a measure of the bacterial load. Lung CFU counts, indicative of M.tb burden, were systematically obtained from five mice per treatment group at each time point.
Guinea pigs
At the end of treatment (seven weeks of infection), guinea pigs were euthanized by intraperitoneal overdose of pentobarbital.
The right cranial lung lobe and ½ of the spleen were collected per animal and weighed. The tissue was homogenized with a volume of PBS containing 1% BSA to result in a 10% homogenate volume by weight. The tissue homogenate was serial diluted in PBS and plated on 150 mm quadrant 7H11 plates with and without charcoal. Plates were incubated at 37 °C for 6-8 weeks and Log10 CFU per gram of tissue was calculated.
Histopathology
Tissue was fixed in 4% PFA for 48 hours and then embedded in paraffin. Slides were cut and stained following H&E standard procedure. Slides were digitally scanned at 20X using an Olympus VS120 or Akoya Vectra Polaris scanning microscopes. Digital slide images were analyzed using Visiopharm analysis software. For each tissue section, a region of interest (ROI) was generated at a low magnification with a custom tissue detecting algorithm using decision forest training and classification to differentiate tissue versus background based on color and area. Lesions were identified within tissue ROI’s at a high magnification with an additional custom-made algorithm using decision forest training and classification based on staining intensity, color normalization and deconvolution, area, and morphological features. Percent lesion calculations were integrated into the same algorithm and calculated from tissue area and lesion area as designated by the ROI and lesions detected. Lesion identification and quantification were then reviewed and edited by a pathologist as needed.
Statistical analysis
CFU were Log transformed, and group means were compared by one-way analysis of variance with Dunnett’s post-test to control for multiple comparisons. All analyses were performed using GraphPad Prism 9.4.1 (GraphPad Software, San Diego CA, United States). Significant differences are labeled accordingly in the figures where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Results
Antimicrobial combination screening for enhanced effect
In order to observe potential combinatory effects of antimicrobial agents against M.tb, we leveraged the checkerboard assay (SynCidy assay)48,49,50,51. Enhanced effect was inferred from a reduction in CFU abundance at the intersection points of the two drugs at specific concentrations, compared to the effects of each drug alone or with DMSO. This interaction often manifests as an arching effect across the plate, where the zones of inhibited bacterial growth delineate the synergistic interaction (Supplementary Fig. 2). The key findings of our screening are summarized in Table 1. CAP demonstrated enhanced effect against M.tb H37Rv with Linezolid (LIN) and Sutezolid (SUT), both belonging to the Oxazolidinone class. CAP and BDQ also demonstrated anti-tubercular enhanced effect in this assay, depicted in Fig. 1, suggesting an elevated potential for combination therapy efficacy. Although LIN exhibited some enhanced effect with CAP, no synergistic effect was observed with BDQ. SUT, similar to LIN in its class, displayed enhanced effect with CAP in our study. Like LIN, SUT did not synergize with BDQ. However, CAP demonstrated enhanced effect with both Oxazolidinones (LIN and SUT) and BDQ, indicating its versatile potential in combination treatments. Based on these promising results, we decided to expand our investigation to include other representative M.tb isolates from different lineages to determine if the observed sensitive phenotype is retained across strains or is a strain-specific phenomenon. This extension aims to further validate the potential of these drug combinations for broader therapeutic applications against tuberculosis.
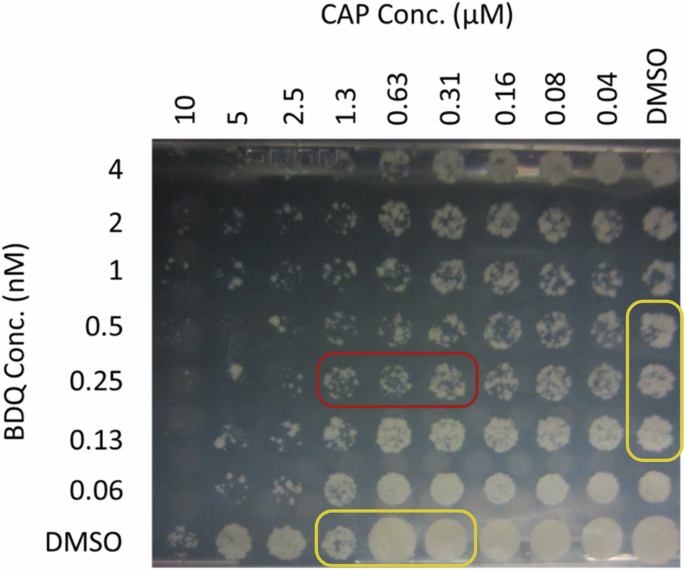
The M.tb H37Rv cultures were treated with escalating concentrations of BDQ and CAP in 96-well plates for 7 days. Cultures were stamped onto 7H10 agar plate supplemented with OADC agar for determination of bacterial killing.
Assessing in vitro enhanced effect of a BDQ/CAP treatment across various M.tb lineages
To assess the efficacy of the BDQ/CAP combination across different M.tb lineages, we conducted a detailed enhanced effect analysis using representative strains from lineages 1, 2, and 4 to address the genetic diversity and relevance to global TB epidemiology. The aim was to determine whether the observed synergistic effects in our initial screenings were consistent across various lineages or if there were lineage-specific responses to the drug combination.
In Fig. 2, for M.tb T46 (lineage 1) higher concentrations of BDQ (0.5 nM) in combination with CAP beginning at 1.3 μM, moderate suppression of bacterial growth was observed. This suppression decreases when BDQ concentrations fall below 0.5 nM. For the M.tb N0072 strain, there is reduced susceptibility to the BDQ/CAP combination at lower concentrations. Notable bacterial killing is only evident at the highest tested CAP concentration of 0.63 μM, especially effective when used in conjunction with 0.25 nM of BDQ.
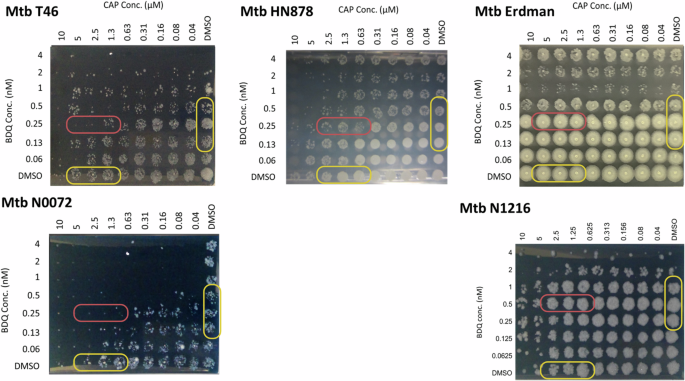
The cultures from M.tb strains representing Lineages 1 (M.tb T46, M.tb N0072), 2 (M.tb HN878), and 4 (M.tb Erdman and M.tb N1216) were treated with escalating concentrations of BDQ and CAP in 96-well plates for 7 days. Cultures were stamped onto 7H10 agar plates supplemented with OADC agar for determination of bacterial killing.
In contrast, M.tb HN878 (lineage 2) displays significant bacterial killing across a spectrum of BDQ concentrations, particularly when combined with CAP concentrations exceeding 0.63 μM. At lower CAP concentrations, the combination still proves effective, with BDQ showing considerable bacterial growth suppression, even at a concentration as low as 0.06 nM as shown in Fig. 2.
For M.tb Erdman (lineage 4), there appears to be no synergistic effect of the BDQ/CAP combination. Almost complete bacterial growth is observable across various concentrations of CAP (0.04 to 10 μM) even when combined with BDQ concentrations above 0.5 nM. A similar trend is observed in the M.tb N1216 strain, also from lineage 4, where a wide range of BDQ concentrations (0.06 to 4 nM) used alongside CAP results in substantial bacterial growth. This may suggest an innate refractory property within lineage 4 to these two compounds.
The selected concentrations of BDQ and CAP were verified using the BacTiter-Glo assay for detection of microbial viability after adding the concentrations from BDQ and CAP designated for M.tb HN878 (CAP 1.25 μM/BDQ 0.5 nM), M.tb Erdman (CAP 5μM/BDQ 1 nM), and T46 (CAP 1.25 μM/BDQ 0.5 nM) as representative isolates from both lineage 1 and 2 as shown in Supplementary Fig. 2.
Overall, these observations highlight the variable sensitivity of different M.tb lineages to the BDQ/CAP combination in an enhanced effect assay. This suggests the need for tailored treatment strategies depending on the lineage. While some lineages, like lineage 2, are highly sensitive to varying concentrations of the drug combination, others, such as lineage 1, require higher concentrations for effective bacterial killing. These results underscore the BDQ/CAP combination’s potential as a versatile therapeutic approach against multiple M.tb lineages, albeit with a need for careful optimization of drug concentrations to target specific strains effectively.
In vivo validation of antimicrobial efficacy
Mouse experiments
In order to validate the antimicrobial efficacy of BDQ and CAP in vivo, we investigated the responses of single and combination drug treatments over a period of 1 month. Two separate animal infection experiments were conducted in mice, detailed in Fig. 3 and Supplementary Fig. 4, to evaluate whether the BDQ and CAP combination could enhance treatment efficacy against TB.
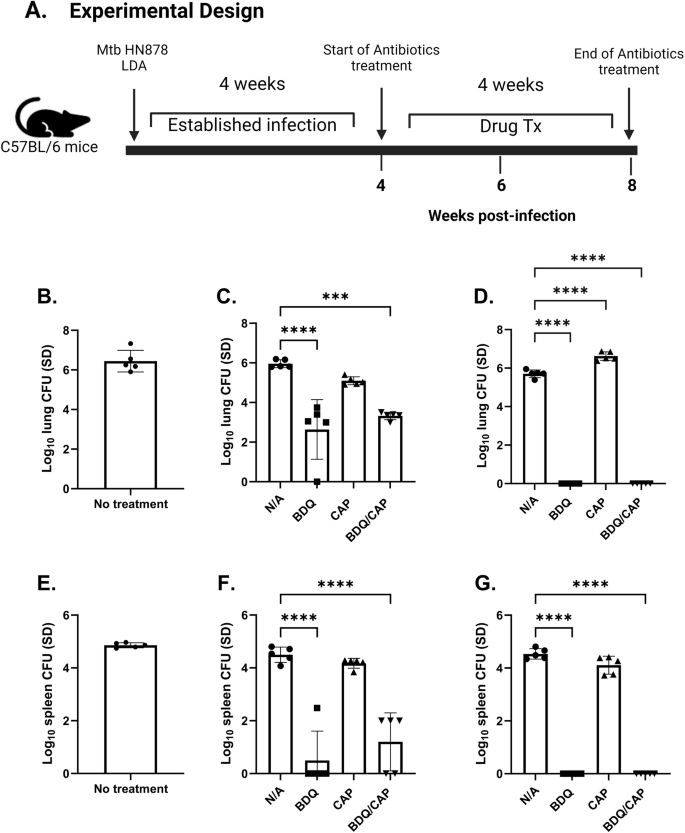
C57BL/6 mice were infected with a LDA of M.tb HN878. Timeline of the in vivo experimental design (A). Bacterial burden was assessed by colony forming unit (CFU) in the lung (B), and the spleen (E) 4 weeks post-infection (prior to treatment). Bacterial burden in the lung (C), and the spleen (F) 6 weeks post- infection (2 weeks post-treatment); and 8 weeks post-infection (4 weeks post-treatment) in the lung (D), and spleen (G). CFU means were compared between each group using one-way ANOVA with Dunnett’s multiple comparisons test. Black line and error bars show mean ± SEM, dots represent individual mice, n = 6–7/group. Asterisks indicate statistical significance, where ***p < 0.001 and ****p < 0.0001.
Initially, 4-weeks post-infection (wpi), we established a baseline bacterial count in the lungs and spleens before initiating drug treatment (Fig. 3B, E, respectively). Subsequent bacterial burden assessments at 6- and 8-wpi, reflecting 2- and 4-week timepoints following drug treatment, revealed no marked decrease in bacterial counts for both untreated and CAP-treated groups, as depicted in the lung (Fig. 3C) and in the spleen (Fig. 3F). Conversely, groups treated with BDQ alone, and the combination of BDQ/CAP, showed significant decreases in lung bacterial load at 6 wpi, 2 weeks after the start of drug treatment. This reduction was amplified at 8 wpi, with a further decrease in bacterial counts (Fig. 3D) following 4 weeks of drug treatment. Spleen tissues exhibited a similar pattern of response, underscoring the systemic efficacy of the treatments (Fig. 3G). Both at 6 weeks post-infection (wpi) and 8 wpi, samples were replated on charcoal plates to avoid any possible inhibitory effects of the antibiotics. The observed CFU in these replated samples were similar to those in the initial experiment (plated without charcoal), confirming the reliability of our bacterial count assessments (data not shown).
A repeat experiment confirmed these findings. Supplementary Figs. 4A, 3B, shows that, compared to the RHZ drug treatment control, given for the same 4-week time period, mice treated with BDQ, or the BDQ/CAP combination presented a significant decrease in both lung and spleen bacterial loads after one month of treatment. The CAP alone group did not exhibit a statistically significant change. Notably, the combination therapy’s impact on bacterial reduction was validated, with a marked decrease in bacterial loads akin to the initial experiment’s outcomes.
In both experimental iterations (Fig. 3, Supplementary Fig. 4), the BDQ/CAP treatment demonstrated similar efficacy to the BDQ single drug regimen. While there was no advantage of the combined treatment over BDQ alone, both BDQ and BDQ/CAP regimens were superior when compared to the RHZ control group (Supplementary Fig. 4), suggesting a potential for the application of either BDQ or the BDQ/CAP combination in preclinical tuberculosis treatment models, where 1 month of treatment could be combined with host-directed therapy and subsequently assessed for relapse. Similarly, as 1 month of treatment with RHZ resulted in significant reduction in bacterial load in both the lung and spleen, this suboptimal regimen could be combined with host-directed therapy to study synergistic reductions in CFU.
Guinea pig experiment
In a parallel study, the antimicrobial efficacy of BDQ and CAP was further assessed using a guinea pig model of M.tb HN878 infection, as depicted in Fig. 4A. This model was chosen for its pathological similarities in the lungs of M.tb infected animals compared to human TB, providing a relevant physiological context for evaluating therapeutic interventions52.
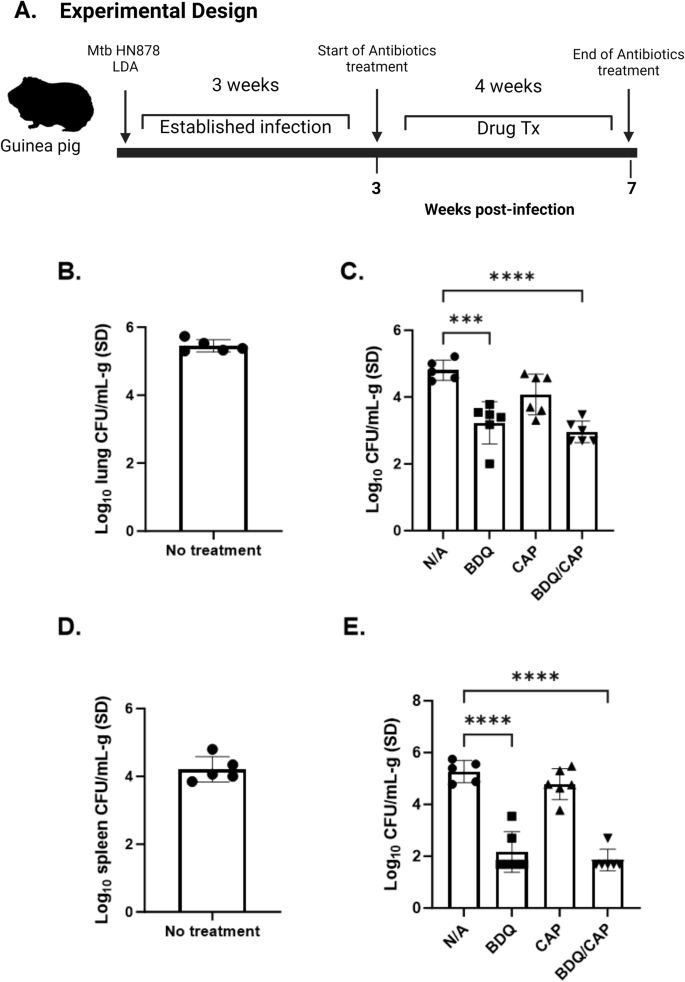
Guinea pigs were infected with M.tb HN878 by aerosol route. Timeline of antimicrobial therapy and M.tb HN878 aerosol challenge (A). Bacterial burden was assessed by colony forming unit (CFU) in lung (B) and spleen (D) before start of antimicrobial therapy, 3 weeks post challenge lung (C), Spleen (E), organ homogenates, 7 weeks post challenge. CFU means were compared between each group using one-way ANOVA with Dunnett’s multiple comparisons test. Black line and error bars show mean ± SEM, dots represent individual guinea pigs, n = 6–7/group. Asterisks indicate statistical significance, where ***p < 0.001 and ****p < 0.0001.
Performance of the pharmacodynamic study
The primary goal of our initial pharmacodynamic study was to discriminate therapeutic efficacy between compounds, BDQ, CAP or both in combination, when delivered by conventional oral (BDQ) and IM (CAP) routes of administration and to determine whether there was enhanced effect in the BDQ/CAP combination against M.tb HN878. As previously described, the liquid formulation of BDQ demonstrates improved serum pharmacokinetic profiles compared to a dry powder formulation when delivered by intrapulmonary route47, suggesting that the dry powder delivery requires further optimization, therefore we used a standardized oral delivery of BDQ for this experiment. In this study, guinea pigs exposed to a low-dose aerosol of M.tb were allowed to progress untreated for 21 days prior to initiation of BDQ and CAP monotherapy or combination therapy for a 28-day treatment course. Antimicrobial efficacy was evaluated by reduction in tissue bacterial burden and improvement in pulmonary and extrapulmonary pathology.
BDQ containing regimens offer superior therapeutic efficacy in the guinea pig
Three weeks post-exposure, allowing for infection to become established, guinea pigs were subjected to four weeks of drug treatment similar to the murine infection experiments. As shown in Fig. 4B, D, we established a baseline lung and spleen bacterial load before initiating drug treatment, where mean lung and spleen CFU counts were at Log10 of 5.5 and Log10 of 4.5, respectively.
Bacterial burden in the lung reached a maximum of 5.25 Log10 CFU/mL-g of tissue at day 21 of infection and reduced slightly by day 49 to a mean of 4.87 Log10 CFU/g (Fig. 4C), consistent with previously reported kinetics of M.tb Erdman growth in the guinea pig model53. All treatments containing BDQ significantly reduced mean lung bacterial burden by a range of 1.5 to 1.85 Log10 CFU/g. In contrast, similar to the mouse studies, CAP alone did not reduce CFU burden in lung or spleen. No significant differences were observed between regimens containing BDQ alone and those containing both BDQ and CAP at the end of treatment at day 49 of infection (1 month after treatment) (Figs. 4C, 4E).
In addition to assessing bacterial burden, our investigation extended to evaluating the lung and spleen lesion burden following a one-month BDQ/CAP drug treatment in guinea pigs infected with M.tb HN878. As represented in Fig. 5A, B, 4 weeks post-antimicrobial treatment, the regimen containing BDQ alone reduced lung lesion area following oral administration (14.9% +/− 4.83). Reduced lesion area was also observed in guinea pigs treated with a combination of BDQ and CAP. In contrast, CAP alone offered a limited, if any, impact on lung lesion burden (Fig. 5B). Histological evidence of resolution among pulmonary pathology was achieved only in formulations containing BDQ, while active and larger inflammatory lesions persisted in guinea pigs treated with CAP alone (Fig. 5B). Notably, the large variances observed are attributed to the genetic diversity within the guinea pig population, which can result in variable immune responses to the infection and treatments.
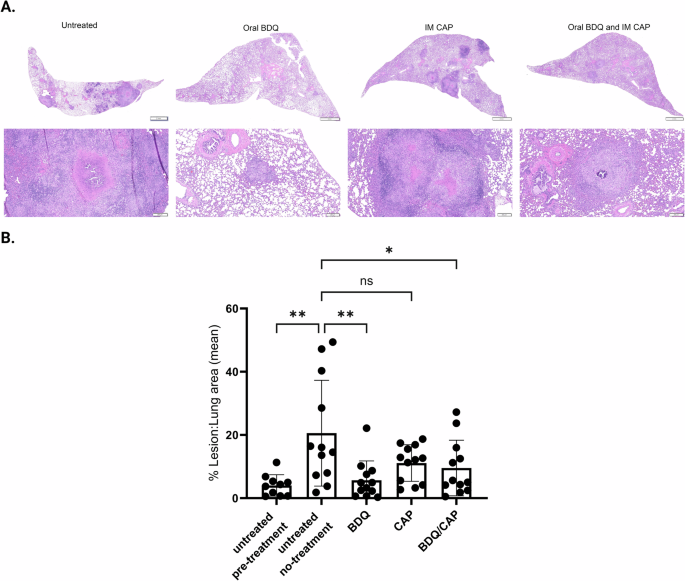
Bars show mean ± SEM percent lesion area, dots represent individual animal, n = 12/group. Asterisks indicate statistical significance, where *p < 0.05, **p < 0.01 using one-way ANOVA with Dunnett’s multiple comparisons test.
These findings from the guinea pig model resonate with those obtained from the mouse experiments, consistently showing that the BDQ and the BDQ/CAP combination therapy not only attenuates the bacterial load (mice and guinea pigs) but also protects against pulmonary lesion (guinea pigs) in the context of preclinical animal models. Importantly, the in vivo efficacy of BDQ and the BDQ/CAP combination regimens stands in contrast to the limited effectiveness of CAP alone, highlighting a discrepancy between in vitro and in vivo enhanced effect testing that warrants further investigation. Furthermore, the decrease in lung lesions correlates with the bacterial load reductions, providing multiple protective efficacy endpoints for future studies. These observations underscore the need for careful consideration of drug performance across different experimental models and warrants testing across different M.tb lineages.
Discussion
The objective of our study was to explore the potential enhanced effect between BDQ and other antibiotic candidates, primarily focusing on the enhanced effect between BDQ and CAP. Whereas we observed in vitro enhanced effect with BDQ/CAP, our findings did not provide compelling evidence for the synergistic effect of BDQ and CAP in vivo, in the treatment of TB. Our approach is in alignment with the National Institute of Health (NIH)‘s TB Strategic Priority 4, which emphasizes the need for innovative research methods to advance the understanding and treatment of tuberculosis and shorten treatment period of TB. We showed similar efficacy with BDQ and BDQ/CAP in the mouse and guinea pig models after 1 month of drug treatment. BDQ alone not only reduced bacterial load in both the lungs and spleens in the guinea pig model, but also provided protection from disease, as demonstrated by a reduction in the percentage of lung lesion involvement. Others have also shown reductions in CFU in guinea pigs with BDQ against M.tb H37Rv54. Shang et al. showed similar decreases in pathology in the guinea pig model using TMC207 (now BDQ) combined with Rifampin and Pyrazinamide against M.tb Erdman52. In humans, BDQ is used for treatment of MDR-TB and extensively drug resistant TB (XDR-TB) and WHO recommendations on the treatment of DR-TB are published55.
In our initial screening for synergistic antimicrobial agents, we utilized the checkerboard SynCidy assay to examine a wide range of drug combinations. The assay’s results were particularly striking when BDQ was paired with CAP, showing a level of enhanced effect that suggests a potent amplification of antimicrobial activity. Notably, the BDQ/CAP combination showed enhanced bactericidal activity compared to individual drugs. This finding aligns with previous reports that highlight the importance of combination therapy in TB to prevent the emergence of drug resistance and improve treatment outcomes42,43,56,57,58,59,60,61,62,63,64,65,66,67,68,69.
It is important to point out that the checkerboard assay used in this study was a killing assay, rather than a growth inhibition assay. In a killing assay, bacteria are plated at Day 0, and a reduction in the number of colonies over time indicates bacterial killing, as opposed to mere inhibition of growth. If there was only growth inhibition, full circles of bacterial growth would be observed at all timepoints. A reduction in the number of colonies, as observed in our assay, indicates bacterial killing. Consequently, FIC scores, which are typically calculated from liquid growth assays using optical density (OD) readouts for actual growth inhibition, are not applicable to our assay.
Checkerboard assays are valuable for determining whether molecules exhibit enhanced effect at concentrations below the MIC, but by their nature they cannot determine interactions or improved activity when concentrations are above the MIC of either agent. The advantage of running a kill assay is that concentrations above the MIC can be used and enhanced effect for bacterial kill noted. While MIC’s are valuable for estimating the relative potency of molecules, we believe that measuring the kill rate is more relevant for the clinical situation.
Although the Bliss independence method can be used with kill studies, it relies on the independence of the mode of action of two agents (drug A and drug B). The calculation is that SAB = SA * SB, where S represents the surviving proportion. For instance, if 1% of bacteria survive each drug individually, the surviving proportion for both drugs would be 0.01%. Since our experiment does not measure the surviving proportion, this calculation cannot be applied. Additionally, Bliss assumes that the surviving population after drug A treatment is equivalent to the initial population. This method does not account for antibiotic tolerance, where a subpopulation inherently survives drug treatment. As this tolerance mechanism is general, it cannot be assumed that drug B would eliminate the same proportion of the surviving population as the input population. Thus, we conclude that the two drugs do not act independently.
A simpler method for defining enhanced effect is discussed in the work by Ni W. et al70. This method, applicable in time-kill assays, defines enhanced effect as a > 2 log10 CFU/mL decrease in comparison with the most active single agent and antagonism as a > 2 log10 CFU/mL increase. As we did not conduct kill kinetic studies with CFU counts, this method could not be applied in our study.
Notably, the observed differences in enhanced effect between BDQ and CAP among M.tb H37Rv, Erdman, and M.tb N1216 strains, despite all belonging to lineage 4, can be attributed to the fact that H37Rv is a laboratory strain that has been maintained in controlled environments and passaged countless times in various laboratories71,72. Despite retaining its virulence in mice, it has adapted to laboratory conditions. This extensive passaging and adaptation can lead to differences in drug susceptibility and interaction73. This lack of environmental exposure may result in differences in drug susceptibility. H37Rv might respond to antibiotic treatments in a way that does not accurately reflect the responses seen in more environmentally exposed strains74.
In vivo studies were initiated based on the initial enhanced effect results observed between CAP and BDQ in M.tb H37Rv. Enhanced effect was also observed in the checkerboard assay with M.tb HN878 before the commencement of in vivo studies. Following these initial observations, additional M.tb lineages were included in checkerboard assays to evaluate the broader applicability of the drug combination. Moreover, we aimed to develop a spray-dried powder form of CAP based on the study by Pitner et al.75, as CAP is not bioavailable orally. This approach aligns with our previous success in formulating a spray-dried powder form of BDQ, providing a potential pathway for combined inhalation therapy47.
Whereas we did not observe enhanced effect with BDQ/CAP compared to BDQ alone in either the mouse or guinea pig models, other studies have reported enhanced effect with this drug combination. Almeida et al. found that the combination of BDQ and TBAJ-876, diarylquinoline analogues, is effective against multidrug-resistant tuberculosis (MDR/XDR-TB), demonstrating that these drugs can amplify each other’s antibacterial effects and may prevent the emergence of drug resistance when used in combination therapy76. Ismail et al. also reported that the BDQ and CAP combination improved early detection of resistance, although cross-resistance was limited to isolates with a specific mutation77. However, not all studies found synergistic effects for BDQ combinations. For example, Pang et al. did not observe synergistic effects against XDR-TB when BDQ was combined with other drugs like moxifloxacin (MFX), gatifloxacin (GAT), linezolid (LZD), or clofazimine (CLO)78. Our findings set the stage for the subsequent in vivo experiments that would provide a more comprehensive understanding of the therapeutic potential of the combination using two different therapeutic animal models.
Additionally, the checkerboard SynCidy assay was unable to predict the enhanced effect of the BDQ/CAP drug combination in our in vivo studies. This discrepancy underscores the importance of implementing additional systems for assessing drug combinations beyond the checkerboard assay, specifically evaluating multiple lineages and genetic backgrounds of M.tb. In vitro, while some M.tb lineages were more susceptible to the BDQ/CAP combination, others required higher concentrations to achieve a similar level of bactericidal activity. Ordaz-Vázquez et al. characterized M.tb genetic diversity and measured transmission rates of primary and acquired resistance, emphasizing the need for careful monitoring79. Singh et al. discussed the genotypic and phenotypic diversity of multidrug-resistant M.tb strains in India, noting the prevalence of lineage-2 strains resistant to fluoroquinolones, showing the need for personalized treatment strategies80. Importantly, Van et. Al. focused on developing predictive models and assays designed to bridge the gap between in vitro and in vivo outcomes in tuberculosis treatment. They utilized advanced computational models and in vitro assays that aim to more accurately predict how anti-TB drugs will behave in clinical settings based on in vitro data81. This highlights the potential for more targeted approaches to TB treatment, which could enhance efficacy and reduce the emergence of drug resistance. However, it is essential to consider the practical challenges of applying such personalized strategies in resource-limited settings where TB is most prevalent. Efforts to address the genetic diversity of M.tb strains and their varying responses to antibiotics must be balanced with the need for feasible and scalable treatment solutions in these regions.
While our in vitro experiments did not validate our in vivo experiments, we were able to show that CAP did not interfere or antagonize the significant decreases in bacterial load in both lung and spleen tissues observed with both BDQ alone and in combination with CAP. More work will also be needed to assess long-term effects with BDQ versus the BDQ/CAP regimen This is particularly relevant in the context of multidrug-resistant TB, where the need for new therapeutic strategies is urgent.
The utilization of both murine and guinea pig models in our investigation was instrumental in establishing the broad-spectrum efficacy of drug treatment in the mouse and guinea pig models. These models are being developed to assess combination treatment with host-directed therapies. In addition to these models, the in vitro model requires further evolution in order to more adequately predict in vivo responses. Taken together, the murine model was able to provide initial insights into the combination’s effectiveness and allowed for controlled exploration of treatment parameters. The guinea pig model, was able to validate the responses observed in the mouse model; and provided additional insight in terms of disease pathology, which can also offer additional validation of the treatment’s impact on complex disease manifestations such as granuloma formation and lung consolidation82,83,84. The congruence of findings across these two distinct animal models reinforces the potential translatability of our results to human TB and underscores the comprehensive approach of our study in evaluating new TB therapies.
In our investigation, one month of BDQ and the BDQ/CAP combination therapy demonstrated similar efficacy when compared to each other, and enhanced efficacy compared to the standard RHZ regimen13,16,20,85,86. Both BDQ alone and the BDQ/CAP combination therapy not only surpassed the standard RHZ regimen in reducing bacterial burden in lung and spleen tissues, but also demonstrated its potential to shorten the treatment duration significantly, which is beneficial for use in preclinical model applications where a reduction in per diems is cost effective.
Limitations of our study include the need for extended follow-up to assess the long-term efficacy and prevention of relapse, which are key goals in TB treatment. Moreover, translating findings from animal models to human patients requires careful consideration due to interspecies differences in drug metabolism and immune responses.
In conclusion, a mouse and guinea pig model were successfully used to validate different endpoints of BDQ efficacy against the M.tb HN878 clinical isolate after only one month of drug treatment. These models will enable further studies aimed as assessing host-directed immunotherapy and drug enhanced effect. Further efforts to optimize the in vitro assay so that it is more predictive for in vivo applications are needed to more rapidly translate new and effective drug combinations into clinical practice to address the persistent challenge of tuberculosis.


Responses