Prevalence, clinicopathologic features and long-term overall survival of early breast cancer patients eligible for adjuvant abemaciclib and/or ribociclib

Introduction
Hormone-receptor-positive (HR+) breast cancers without HER2 amplification (HER2−) represent the vast majority of breast cancers, accounting for 70–80% of cases1. These forms are most often diagnosed at the localized stage, with the possibility of curative treatment, which will most often involve local treatment combining surgery to remove the tumor, followed by radiotherapy. Depending on the risk of metastatic relapse, different systemic treatments may be indicated. Historically, the main systemic treatment for these HR+/HER2- tumors has been endocrine therapy, prescribed for a variable duration (5–10 years, depending on the risk of relapse).
Overall, the long-term prognosis of localized HR+/HER2− breast cancer is quite favorable, and the majority of patients will not experience metastatic relapse with these standard treatments. However, around 20-30% of patients experience local or distant relapse2, some of which may be late, up to 20 years after treatment of the primary tumor3,4,5. The main risk factors for relapse are related to the initial tumor burden at diagnosis (tumor size and extent of axillary lymph node invasion (with 20-year distant recurrence risk ranging from 22% for N0 initial stage, to 52% for patients with more than 3N+))3,6, and to a lesser extent, to certain characteristics of the tumor biology, such as Ki67 expression, or tumor grade2. For patients at risk of relapse despite local treatment and adjuvant endocrine therapy, several additional systemic treatment strategies have been developed, such as adjuvant cytotoxic chemotherapy, or the extension of endocrine therapy beyond 5 years7. Despite these additional treatments, a significant risk of metastatic relapse persists for patients at highest risk, with a substantial unmet medical need in this setting.
Over the past decade, CDK4/6 inhibitors (such as palbociclib, ribociclib and abemaciclib) used in combination with standard endocrine therapy have considerably improved progression-free survival (and for some, overall survival) in patients with HR+/HER2− metastatic breast cancer8,9,10,11,12,13,14,15,16,17,18. These results obtained in the advanced phase of the disease led to the testing of these molecules in combination with adjuvant endocrine therapy for 2–3 years for high-risk localized forms of HR+/HER2− cancers, with results differing between the various molecules. Indeed, unlike the PALLAS19 and PENELOPE-B20 trials with palbociclib (which showed no benefit in terms of reduction of the risk of recurrence), the monarchE trial with abemaciclib for 2 years21, and more recently, the NATALEE trial with ribociclib for 3 years22, showed a statistically and clinically significant benefit of this strategy in patients with forms at high risk of relapse, making these treatments a new standard of care in this indication. The advent of these new treatments in the management of early HR+/HER2− breast cancer does, however, raise a number of practical questions, particularly regarding the eligible populations, depending on the molecule, since the definition of “high risk” used in the monarchE and NATALEE trials was very different, with a much broader population in the NATALEE trial that included, in particular, patients without axillary lymph node invasion22.
Using unique data from a French population-based registry over a 10 years diagnostic period, and with a median follow-up of 12 years, we aimed to assess the proportion of patients with HR+/HER2− localized breast cancer who are now potentially eligible for adjuvant treatment incorporating abemaciclib or ribociclib (using the inclusion criteria defining high-risk from each of the pivotal trials), and what the long-term prognosis is for these different populations who are potentially set to receive these new adjuvant treatments.
Results
Prevalence of “high risk” patients according to monarchE or NATALEE inclusion criteria
Overall, among 15,936 breast cancer cases collected in the registry, 4675 patients were treated between 2005 and 2015, of whom 3,103 were treated for HR+/HER2− localized breast cancer (Supplementary Fig. 1). The median follow-up of the whole cohort of eBC patients was 144.7 months (95%CI: [142.9 – 146.9]).
Among these 3103 patients, N = 440 (14.2%) would have been eligible for adjuvant abemaciclib, because they would have been considered at “high-risk” according to the monarchE inclusion criteria, as follows: N = 279 (9%) had ≥4 positive lymph nodes (≥pN2 stages), and N = 161 (5.2%) had 1-3 positive lymph nodes and at least 1 of the following criteria: primary tumor size ≥50 mm (N = 45) or tumor grade III (N = 108), or both (N = 8). Thus, the population of patients eligible for adjuvant abemaciclib with ≥4 N+ represents 63.4% (279/440) of eligible patients (Fig. 1A).

A Eligibility for adjuvant abemaciclib according to the monarchE risk-based inclusion criteria. B Eligibility for adjuvant ribociclib according to the NATALEE risk-based inclusion criteria.
Concerning the NATALEE inclusion criteria, among the 3103 patients, N = 1068 (34.4%) would have been eligible for adjuvant ribociclib, as follows: N = 279 (9%) because of tumor stage III, N = 317 (10.2%) because of tumor stage IIB, N = 384 (12.4%) because of tumor stage IIA with at least one lymph node involved, and finally N = 88 (2.8%) with tumor stage IIA without nodal involvement but with grade 3 tumor (N = 55), or grade 2 tumor associated with a Ki67 proliferation index of at least 20% (N = 33) (Fig. 1B).
Table 1 gives a detailed distribution, by AJCC clinical stage, of patients eligible for adjuvant abemaciclib and/or ribociclib according to the inclusion criteria of the monarchE and NATALEE trials respectively.
Clinicopathological features of early breast cancer patients eligible for adjuvant abemaciclib and/or ribociclib
We next examined the classical clinicopathological characteristics of our cohort of patients with early HR+/HER2− breast cancer, according to their eligibility for adjuvant abemaciclib or ribociclib.
Compared to non-eligible patients, patients eligible for adjuvant abemaciclib according to the monarchE inclusion criteria were more likely to have axillary lymph node involvement (50.8% vs 6.8% p < 0.0001), higher AJCC clinical and pathological stages (p < 0.0001), and higher tumor grade (44.8% vs 6.6% grade III; p < 0.0001). In line with these findings, adjuvant abemaciclib-eligible patients were more likely to have undergone mastectomy (68.4% vs 24.9%; p < 0.0001), and axillary lymph node dissection (80.9% vs 29.2%; p < 0.0001). Among this early-stage HR+/HER2− eBC population treated between 2005 and 2015, N = 347 patients (11.2%) underwent BRCA1/2 germline mutation testing, of whom N = 37 were found to have a pathogenic BRCA mutation (N = 26 (1%) in the non-eligible population, and N = 11 (2.5%) in the abemaciclib-eligible population; p = 0.008). Age, menopausal status at diagnosis, as well as tumor expression of progesterone receptors were not significantly different between the adjuvant abemaciclib eligible and non-eligible populations (Supplementary Table 1).
The conclusions were similar for the comparison between patients eligible and non-eligible for adjuvant ribociclib according to the NATALEE inclusion criteria (Supplementary Table 2), except for menopausal status (with more premenopausal patients and progesterone-negative tumors in the eligible population). During the 10-year period, 89.1% of the population eligible for adjuvant ribociclib was node-positive. Patients with node-negative status who would have been eligible for adjuvant ribociclib represent only 10.9% of the eligible population (and only 5.7% of the total population of node-negative HR+/HER2− eBC).
The comparison of clinicopathological features between adjuvant abemaciclib eligible patients, adjuvant ribociclib eligible patients, and non-eligible patients for adjuvant CDK4/6 is presented in Table 2.
Adjuvant treatments received by early breast cancer patients eligible or not for adjuvant abemaciclib and/or ribociclib
After noting that patients eligible for adjuvant abemaciclib and/or ribociclib were significantly more likely to have undergone mastectomy and axillary lymph node dissection than patients not eligible for these treatments, we examined adjuvant treatments in these different populations.
In line with their higher risk of relapse, patients eligible for adjuvant abemaciclib were more prone to receive adjuvant or neoadjuvant chemotherapy (78.3% vs 25.3%; p < 0.0001). In this real-world high-risk population, 21.7% of patients now eligible for adjuvant abemaciclib did not receive (neo)adjuvant chemotherapy. Most of this node-positive high-risk population also received adjuvant endocrine therapy (with only 3.9% of patients not receiving it, compared to 21% in non-eligible population; p < 0.0001). In accordance with higher tumor burden and axillary node involvement, patients eligible for adjuvant abemaciclib also received adjuvant radiotherapy in almost all cases (94.7%), compared to 85.3% of non-eligible patients (p < 0.0001) (Supplementary Table 3).
The results were similar for patients eligible for adjuvant ribociclib, who received significantly more frequent (neo)adjuvant chemotherapy (67.9% vs 14.4%; p < 0.0001), endocrine therapy (96.2% vs 73.7%; p < 0.0001) and radiotherapy (91.4% vs 84.2%; p < 0.0001) compared to non-eligible patients (Supplementary Table 4). In this cohort, 32.1% of patients now eligible for adjuvant ribociclib did not receive adjuvant chemotherapy.
The comparison of adjuvant treatments received by adjuvant abemaciclib eligible patients, adjuvant ribociclib eligible patients, and patients non-eligible for adjuvant CDK4/6 is presented in Table 3.
Long term outcome of early breast cancer patients eligible or not for adjuvant abemaciclib and/or ribociclib
We then assessed the long-term prognosis in terms of overall survival (OS) at 10 years, among different groups of patients who would now be eligible for adjuvant abemaciclib and/or ribociclib, compared with patients without any indication for adjuvant CDK4/6 inhibitors.
As relapse data and type of relapse were not collected in our registry, we assessed long-term prognosis using only OS, based on the knowledge of vital status (dead/alive) for each patient as of December 31, 2022. Vital status was obtained from the French Nationale Institute of Statistics and Economic Studies (Institut National de la Statistique et des Etudes Economiques, INSEE). Since the median follow-up for this entire cohort of HR+/HER2− eBC was around 12 years, we assessed not only observed OS, but we also estimated net survival, which is the survival that would be observed if breast cancer was the only cause of death. In the absence of relapse-free survival data (particularly metastatic relapse-free survival), this approach enables us to estimate the proportion of patients who died directly or indirectly from their cancer23.
Concerning patients eligible for adjuvant abemaciclib, our analyses show that this “high risk” group of patients had worse prognosis, with significantly poorer 10-year OS (60.6% (95%CI[55.7–65.1])), compared to abemaciclib-non-eligible patients (84.9% (95%CI[83.4–86.3])) (p < 0.0001) (Fig. 2A). Analysis of the estimated net survival for these 2 groups also showed a significant difference, showing excellent 5- and 10-year survival (100% and 98.9% (95%CI[96.7–100]) respectively) of adjuvant abemaciclib non eligible patients, compared with eligible patients (88.6% (95%CI[84.3–93.2]) and 71.2% (95%CI[64.7–78.3]) respectively) (Supplementary Fig. 2A). Among these 2 different populations, we analysed OS according to the burden of involved lymph nodes (N+), thus separating patients eligible for adjuvant abemaciclib with 4 or more N+, vs only 1-3 N+, and in patients not eligible for adjuvant abemaciclib by separating patients with or without N+ status (Fig. 2B). Kaplan-Meier curves for these 4 groups differed significantly (p < 0.0001), highlighting the prognostic importance of axillary lymph node involvement, with 10-year OS ranging from 53.9% (95%CI[47.6–59.7]) to 86.5% (95%CI[84.9–88]) according to the risk category (Fig. 2B). Estimated net survivals were also significantly different (p < 0.0001), ranging from 60% (95%CI[52.4–68.7]) to 100% at 10 years (Supplementary Fig. 2B).
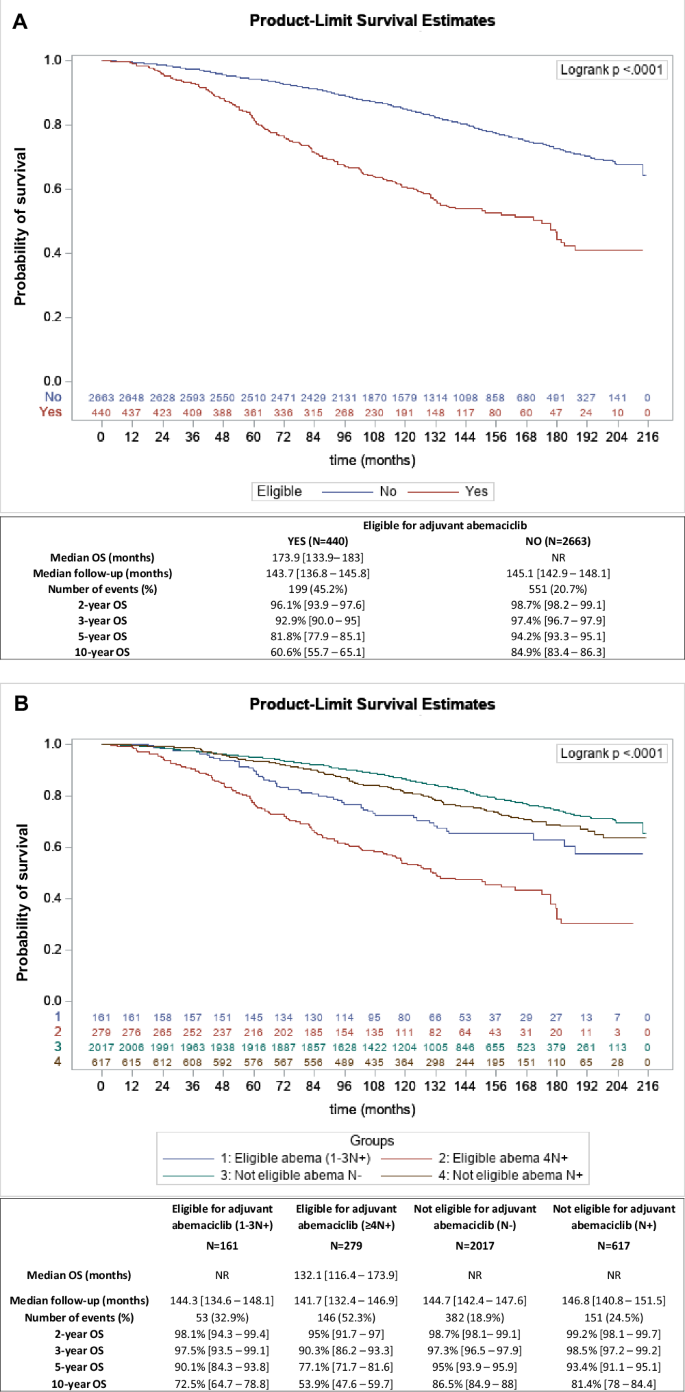
A Kaplan-Meier estimates for overall survival (OS) according to eligibility (red curve) or non-eligibility (blue curve) for adjuvant abemaciclib (according to the monarchE risk-based inclusion criteria). B Kaplan-Meier estimates for overall survival (OS) according to eligibility or not for adjuvant abemaciclib (according to the monarchE risk-based inclusion criteria), and axillary lymph node status: patients eligible for adjuvant abemaciclib with ≥4N+ (red curve), patients eligible for adjuvant abemaciclib with 1-3N+ (blue curve), patients not eligible for adjuvant abemaciclib with 1-3N+ (brown curve), and patients not eligible for adjuvant abemaciclib without node involvement (green curve). Median OS, median follow-up, number of events (deaths), and OS at 2, 3, 5, and 10 years are shown in the table below each survival curve.
Similar trends were observed for patients eligible for adjuvant ribociclib, compared to non-eligible patients. Overall, eligible patients had worse 10-year OS than non-eligible patients (71.3% (95%CI [68.4–74]) versus 86.9% (95%CI[85.2-88.3]); p < 0.0001) (Fig. 3A). Estimated net survival for these 2 groups also showed a significant difference, with excellent 5- and 10-year survival (100% for both) among patients not eligible for adjuvant ribociclib, compared with eligible patients (94.4% (95%CI [92.1–96.8]) and 84% (95%CI [80-88.2]) respectively) (Supplementary Fig. 3A). We also analysed OS according to nodal status (N+ or N0), thus separating patients eligible for adjuvant ribociclib between N+ and N0 patients, for comparison with N0 non-eligible patients: Kaplan-Meier curves from these 3 groups differed significantly (p < 0.0001), but 10-year OS of patients eligible for adjuvant ribociclib did not differ between those with (N+) and without (N0) axillary lymph node involvement (71% (95%CI[67.9-73.9]) vs 73.9% (95%CI[64.4-81.1]), respectively) (Fig. 3B).

A Kaplan-Meier estimates for overall survival (OS) according to eligibility (red curve) or non-eligibility (blue curve) for adjuvant ribociclib (according to the NATALEE risk-based inclusion criteria). B Kaplan-Meier estimates for overall survival (OS) according to eligibility or not for adjuvant ribociclib (according to NATALEE risk-based inclusion criteria), and axillary lymph node status: patients eligible for adjuvant ribociclib with N+ status (blue curve), patients eligible for adjuvant ribociclib with N- status (red curve), and patients not eligible for adjuvant ribociclib (green curve). Median OS, median follow-up, number of events (deaths), and OS at 2, 3, 5, and 10 years are shown in the table below each survival curve.
Concerning the population of patients not eligible for adjuvant ribociclib, there was no difference in 10-year OS between patients without axillary lymph node involvement (N0), and patients (N = 98) with micrometastatic lymph node involvement (T0 N1mi, and T1 N1mi stage, who were excluded according to the NATALEE inclusion criteria) (Supplementary Fig. 4).
Finally, we compared long-term OS of N+ patients, who were eligible for both adjuvant abemaciclib and ribociclib, vs patients who were only eligible for adjuvant ribociclib, vs patients who were not eligible for any adjuvant CDK4/6 inhibitor. Ten-year OS was significantly different across these 3 populations (60.2% (95%CI[55.3–64.8]) vs 78.9% (95%CI[75.4–82]) vs 86.8% (95%CI[85.2–88.3]) respectively; p < 0.0001 (Supplementary Fig. 5A)). Net survival estimates also found statistically significant differences between these populations, with 10-year net survival estimated at 70.8% (95%CI[64.2–77.9]) vs 93% (95%CI[88.3–98]) vs 100% respectively; p < 0.0001 (Supplementary Fig. 5B).
As chemotherapy prescription is lower in patients eligible for adjuvant CDK4/6 inhibitors (compared with monarchE and NATALEE), it may represent a confounding factor (potentially explaining more early deaths in these patients). To answer this question, we analyzed OS in patients alive from the 5th year after diagnosis (which should no longer be affected by the effect of chemotherapy): This analysis shows that even with this methodology, the OS of these 3 categories of patients remains very significantly different (Supplementary Fig. 6).
Discussion
We report here, for the first time to our knowledge, epidemiological data from a European population-based database describing the population of patients treated for HR+/HER2− eBC who would now be eligible for adjuvant treatment with a CDK4/6 inhibitor. To analyze this population, we used the inclusion criteria of the monarchE and NATALEE clinical trials (based on different levels of relapse risk)21,22, which showed a statistically significant and clinically relevant benefit (in terms of iDFS) of adjuvant abemaciclib and ribociclib, respectively21,22. In the exhaustive population of patients from a period of over 10 years in our study, we showed that patients eligible for adjuvant abemaciclib and ribociclib constituted respectively 14.2% and 34.4% of all patients treated for HR+/HER2− eBC during this period.
Interestingly, patients eligible for adjuvant treatment with CDK4/6 inhibitors in our series appear to have a distribution of lymph node risk similar to that of the populations included in the monarchE and NATALEE trials. Accordingly, in the population eligible for abemaciclib, 63.4% of these patients in our real-life series had ≥4 N+ and 36.6% had 1-3 N+ and an additional risk factor, while in the monarchE trial, the proportions were 59.6% and 40.2% respectively21. The same was true for lymph node risk stratification in ribociclib-eligible patients: 89.1% N+ and 10.9% N- in our series, versus 84.2% and 15.6% in the NATALEE trial population22. We can therefore assume that enrollment in these 2 trials is a good reflection (in terms of lymph node risk) of the population that will now be eligible for these treatments in clinical practice. Data from specialized cancer centers or registries of non-European populations have recently been reported24,25,26, showing results in line with ours, with 17.5–19.4% of patients eligible for adjuvant abemaciclib, but with a slightly higher proportion (41-43%) of patients eligible for adjuvant ribociclib (with a higher proportion (27.8%) of N- disease in this population of patients24).
We also report the long-term prognosis (with a median follow-up of over 12 years for our cohort) in these different patient populations. We clearly show major differences in overall survival (OS) at 10 years, with a major prognostic role for the extent of lymph node invasion. Here again, our survival data are in line with similar results from a recently published Australian registry reporting 10-year DFS of 66% and 77%, and 10-year OS of 67% and 78% for patients eligible to adjuvant abemaciclib and ribociclib respectively26. These long-term OS results are important because, for the moment, the median follow-up of patients included in the monarchE and NATALEE trials is rather short, and OS data from these clinical trials are still immature21,22. We show that the 10-year OS of patients considered “at risk” of relapse in the 2 trials, and therefore eligible for adjuvant treatment with a CDK4/6 inhibitor, is poor (60.6% for patients eligible for abemaciclib and 71.3% for patients eligible for ribociclib). Our net survival results are exactly in line with these findings, suggesting a high mortality rate associated with metastatic breast cancer relapse. These data therefore confirm the relevance of the clinico-biological criteria taken into account in the monarchE and NATALEE trials for selecting a population of patients at risk of relapse, and therefore, in whom additional adjuvant treatments are necessary. Our long-term data show that the overall survival of these patients deteriorates markedly after 5 years (which is well described in the HR+/HER2− eBC subtypes)4. We can therefore speculate from these data that if the benefit currently reported for iDFS in the monarchE and NATALEE trials translates into an OS benefit, this will only be seen with relatively extended patient follow-up. To assess the long-term prognosis of patients in our cohort in the absence of breast cancer relapse data available in our registry, we report reliable OS data derived from mortality data from the French National Institute of Statistics and Economic Studies (INSEE). We also estimated net survival for each patient group, which is the survival that would be observed if the only possible underlying cause of death was breast cancer27. Here, we used the relative survival (which is robust to non-comparability in the estimation of background mortality28), and OS was compared to the survival that patients would have experienced if they had had the mortality of the general population from which they were drawn29.
Beyond the population-based representation of patients now eligible for these two CDK4/6 inhibitors and their long-term prognosis, we also report here interesting real-life data on how these patients are treated in routine clinical practice. Interestingly, a significant proportion of patients eligible for abemaciclib (21.7%), and even more so for ribociclib (32.1%), did not receive (neo)adjuvant chemotherapy at the time of treatment. Our registry data preclude understanding why clinicians chose not to give adjuvant chemotherapy for individual patients, despite having clinicopathological risk factors for relapse. Thus, our real-life data contrast with those of the monarchE and NATALEE trials, in which 98% and 88.1% respectively received (neo)adjuvant chemotherapy21,22. Our data show that, in daily routine practice, patients potentially eligible for adjuvant CDK4/6 inhibitors will differ from those included in the pivotal trials, particularly with regard to the indications or possibilities for (neo)adjuvant chemotherapy that will be selected in practice by clinicians. The results we report here are supported by other real-world data, in particular from German centers, which show that over 50% of patients eligible for adjuvant ribociclib, and almost 49% of patients eligible for adjuvant abemaciclib, did not receive (neo)adjuvant chemotherapy24,25. Furthermore, this lower prescription of chemotherapy in real life has a potentially negative impact on the survival data we report in these patients at risk of relapse, and must be taken into account when interpreting our OS results. These data also raise the question of the real-life magnitude of the benefits of adjuvant CDK4/6 inhibitors that will be seen in clinical practice in eligible patients, a significant proportion of whom may not have received additional chemotherapy. A number of clinical trials are currently investigating the extent to which adjuvant treatment with a CDK4/6 inhibitor could make chemotherapy unnecessary in certain patients considered to be at significant risk (clinical or genomic)30,31,32.
Some limitations of our work include the study period (2005-2015), during which clinical practices may have been slightly different from today. Nevertheless, we believe that the management of HR+/HER2- eBC during that period was quite similar to that of today, particularly in terms of indications for surgery, chemotherapy, radiotherapy and hormone therapy. Furthermore, we chose this study period in order to have sufficient hindsight to study long-term OS, bearing in mind that many relapses and deaths are late in these subtypes of breast cancer4. Nevertheless, our study suffers from some limitations: for example, the duration of adjuvant endocrine therapy, and compliance with it, is unknown for the patients we studied, and the influence of this parameter on observed OS cannot therefore be studied.
Moreover, during this period, genomic prognostic signatures now routinely used to assess the risk of relapse and the indications for adjuvant chemotherapy were not performed in France. However, in the NATALEE trial, patients could be included with T2N0 stage, with a grade 2 tumor if they had a “high risk” genomic signature. We were therefore unable to identify such patients in our series, but believe that they are quite rare in practice (in the NATALEE study, they represented only 4% of N0 patients)33. Similarly, in our series, a number of patients did not have Ki67 evaluation of the tumor at that time, and thus, we may have missed patients who had T2N0 grade 2 tumor stage, and were eligible for adjuvant ribociclib. Retrospective data from 2 large German centers, and obtained more recently (2018-2020), seem to confirm that a very limited number of patients could receive ribociclib solely because of a high genomic risk (0.1% in this series). On the contrary, in this publication, the proportion of patients eligible for adjuvant ribociclib due to an elevated Ki67 was 9.5% (4% of all patients with HR+/HER2- eBC).
Finally, we did not study the population of men with ER+/HER2- eBC. Interestingly, a recent study conducted on a German registry reported specifically in this population, 21.2% and 47.6% of patients eligible for adjuvant abemaciclib and ribociclib respectively34. These results highlighted that more men with eBC are at clinical high risk compared to women, most likely due to more advanced stages at initial diagnosis in men.
In conclusion, despite these limitations, we provide concrete insights into the prevalence, clinicopathological characteristics and long-term prognosis of HR+/HER2- early breast cancer patients who would now be eligible for adjuvant treatment with CDK4/6 inhibitors in daily practice. These data may help clinicians to consider the use of the new adjuvant therapeutic options available to prevent relapse of HR+/HER2- breast cancers with different levels of recurrence risk.
Methods
The findings reported here follow the ESMO guidance for reporting oncology real-world evidence (ESMO-GROW)35.
Data collection
The Côte d’Or breast and gynecological cancer registry is the only population-based cancer registry in France to focus on breast and gynecological cancers. It has been collecting data on all cases of breast and gynecological cancers occurring in residents of the Côte d’Or (a French Department) since 1982. The registry catchment area has approximatively 500 000 inhabitants, 54% of whom are women. Information about clinical characteristics, tumors, treatments, and vital status for patients recorded in the registry was obtained from various sources (pathological reports, medical records, and the National Institute of Statistics and Economic Studies (INSEE)). The Côte-d’Or breast and gynecological cancer registry, hosted at the Georges-François Leclerc Center, Dijon (CGFL) is approved by the French national data protection authority. Moreover, the CGFL complies with the reference methodology governing the processing of personal data for studies based on existing data (N°: 2203771).
Patient selection for adjuvant abemaciclib and/or ribociclib eligibility
Between 2005 and 2015, a total of 3103 patients underwent surgery (conservative surgery, mastectomy and axillary lymph node evaluation) for localized HR+/HER2- breast cancer. Tumors were classified according to the staging criteria of the American Joint Cancer Committee (AJCC), tumor grade, and Ki67 result (if available). Two different populations were studied, namely:
i) The population of patients corresponding to the monarchE inclusion criteria21: In this study, the definition of “high risk” was based on the presence of ≥ 4 positive lymph nodes (≥pN2 stages) or 1-3 positive lymph nodes and at least 1 of the following criteria: primary tumor size ≥5 cm and/or tumor grade III.
ii) The population of patients corresponding to the NATALEE inclusion criteria22: In this study, the definition of “high risk” was based on AJCC tumor stage III or IIB (irrespective of nodal status). Patients with AJCC tumor stage IIA were also eligible if they had at least one lymph node involved. Patients with IIA tumor stage but without nodal involvement were also eligible in case of grade 3 tumor, or a grade 2 tumor associated with a Ki67 proliferation index of at least 20%, or who were considered to be in a high genomic risk group (according to a genomic signature).
For patients treated with neoadjuvant chemotherapy, eligibility was assessed on the basis of both initial clinical characteristics and surgical findings: patients were considered eligible if they fulfilled the inclusion criteria of the monarchE and/or NATALEE studies at either of these points in their management. Concerning tumor hormone receptor (HR) status, according to French recommendations, tumors were defined HR-negative (HR-) if estrogen and progesterone receptors were expressed in <10% of tumor cells, and HR-positive (HR+) if expressed in ≥10% of tumor cells. HER2 status was assessed using standard antibodies and FISH techniques, and HER2 scoring was assessed according to the ASCO/CAP guidelines in force at the time of the patient’s recruitment36,37,38. During this period, germline BRCA mutation (gBRCAm) screening was carried out by clinicians according to recommendations in force at the time39,40,41.
During this study period, the use of prognostic genomic signatures was not routinely performed in France, which explains why none of the patients in our series benefited from this evaluation.
Statistical analysis
Treatments (chemotherapy, endocrine therapy, radiotherapy, lymph node dissection) were recorded. Chemotherapy and endocrine therapy were categorized as none, neoadjuvant, adjuvant, or both, and radiotherapy as yes/no. Lymphadenectomy was categorized as sentinel lymph node, node dissection, or both. We also collected data on BRCA mutation, histological subgroups, and menopausal status.
Quantitative data are presented as mean ± standard deviation, or median (quartile (Q)1, Q3), and qualitative data as number and percentage. The chi square and Fisher’s exact test were used to compare frequency data and the Wilcoxon test for quantitative data. Missing data have been described in terms of numbers, and have not been included in the calculation of percentages for each of the variables studied. Overall survival was estimated using the Kaplan-Meier method, and survival curves were compared using the log rank test. Median follow-up was estimated using the reverse Kaplan-Meier method.
Overall survival (OS) was defined as the time between the date of diagnosis and the date of death from any cause. Vital status was updated using INSEE (French National Institute of Statistics and Economic Studies) data. The cut-off date for OS was December 31, 2022. Net survival was estimated using the Pohar Perme method23 and curves were compared using the equivalent of the log rank test for net distributions42.
The significance level for all analyses was set at 5%. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.5.2.


Responses