Accelerometer-based sedentary time and physical activity with MASLD and liver cirrhosis in 2684 British adolescents
Introduction
Among adolescents, steatotic liver disease (SLD) or metabolic dysfunction-associated SLD (MASLD) is the most prevalent (7.6%) chronic liver disease globally and the prevalence could increase fivefold in populations with obesity1,2,3. The infiltration of at least 5% of hepatocytes without significant alcohol consumption may lead to MASLD, which could progress to cirrhosis and hepatocellular carcinoma4,5,6. One in five young adults had steatosis and 2.5% had liver fibrosis around the age of 24 years, thus, the primordial and primary prevention of liver diseases is of public health and clinical significance3,7,8,9. Recent long-term longitudinal studies have shown that increasing physical activity (PA) and reducing sedentary time (ST) may lower the risk of fat mass obesity, dyslipidaemia, insulin resistance, inflammation and vascular stiffness among children, adolescents, and young adults10,11,12,13,14,15,16,17. In clinical trials, PA has been associated with a reduced risk of MASLD among adults3,18. A recent longitudinal study of 50-year-old adults reported that questionnaire-based self-reported vigorous PA but not moderate PA was associated with reduced risk of MASLD19. However, studies on long-term patterns of accelerometer-based movement behaviours, such as ST, light PA (LPA) and moderate-to-vigorous PA (MVPA), in relation to MASLD and liver fibrosis in the young population are lacking3,8,10,11,20,21.
Evidence suggests that triglycerides and inflammation are higher in adolescents and young adults with MASLD, and the prevalence of MASLD worsens with obesity, but smoking, socioeconomic status and alcohol intake were not associated with MASLD1,2,3,8,22,23. Higher liver enzyme concentrations such as alanine aminotransferase, aspartate aminotransferase and gamma(γ)-glutamyl transferase but not insulin resistance have been positively associated with liver fibrosis in the presence of MASLD in youth23. Nonetheless, the potential explanatory roles of these metabolic factors in the relationships of movement behaviour with MASLD, liver fibrosis and liver enzymes remain unclear1,2,3,8,9,22,23,24. Since children and adolescents accumulate more time engaging in LPA than MVPA, a potential long-term lifestyle intervention strategy for lowering the risk for MASLD is an urgent research priority3,8,9,10,11,12,13,14,15,16. The present study examined (1) the longitudinal associations of objectively measured ST, LPA and MVPA, from ages 11 to 24 years with the risk of MASLD and liver fibrosis in adolescents (age 17 years) and in young adults (age 24 years) measured with transient elastography which has high detection accuracy in reference to liver biopsy25, (2) the mediating effect of triglycerides, inflammation, fat mass and lean mass, on the relationships of movement behaviour with MASLD, liver fibrosis, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase from ages 17 to 24 years (adolescence through young adulthood), using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, England, UK.
Results
Cohort study characteristics
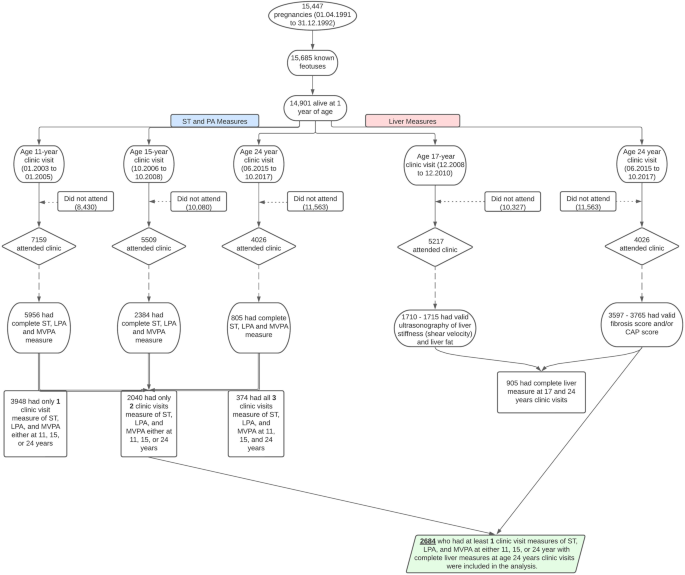
Altogether, 2684 (57% female) participants who had at least one-time measures of ST, LPA and MVPA during the ages 11–24 years observation period and complete assessment of liver fibrosis and steatosis at the age of 24 years clinic visit were studied (Fig. 1). Females had higher fat mass, higher ST and lower MVPA, at baseline and follow-up compared to males (Table 1). Males had a higher proportion of advanced or severe liver steatosis than females both at baseline (age 17 years) and at follow-up (age 24 years). In the total cohort, the average prevalence of liver steatosis at ages 17 years and 24 years was 2.6% and 20.5%, respectively, a circa 8-fold increase within a 7-year growth period. Males had a higher mean liver fibrosis staging score compared to females. In both males and females, ST increased from 6 h/day in childhood to 9 h/day in young adulthood, while LPA decreased from 6 h/day in childhood to 3 h/day in young adulthood (Table 1). MVPA had a U-shaped increase from childhood through young adulthood. Males had higher concentrations of alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase than females both at baseline and follow-up. Other characteristics are described in Table 1.
CAP controlled attenuation parameter, ST sedentary time, LPA light-intensity physical activity, MVPA moderate to vigorous–intensity physical activity.
Longitudinal associations of ST, LPA and MVPA with liver cirrhosis and severe steatosis
The increase in ST from ages 11 to 15 years was associated with a higher risk of advanced liver stiffness but a lower risk of liver steatosis at age 17 years after full adjustments for cardiometabolic and lifestyle factors (Table 2). The cumulative increase in ST from ages 11 to 24 years was associated with a higher risk of liver cirrhosis at age 24 years, which became statistically non-significant after full adjustment (Table 2). The cumulative increase in ST from ages 11 to 24 years was associated with a higher risk of severe liver steatosis at age 24 years after full adjustment.
Cumulative LPA from ages 11 to 15 years was associated with a lower risk of advanced liver stiffness and liver steatosis at age 17 years after full adjustments for cardiometabolic and lifestyle factors (Table 2). Cumulative LPA from ages 11 to 24 years was associated with a lower risk of liver cirrhosis at age 24 years after full adjustment (Table 2). Cumulative LPA from ages 11 to 24 years was associated with a lower risk of severe liver steatosis at age 24 years but was statistically non-significant after full adjustment.
Cumulative MVPA from ages 11 to 15 years was associated with a lower risk of advanced liver stiffness and liver steatosis at age 17 years after full adjustments for cardiometabolic and lifestyle factors (Table 2). Cumulative MVPA from ages 11 to 24 years was not associated with a lower risk of liver cirrhosis at age 24 years before or after full adjustment (Table 2). Cumulative MVPA from ages 11 to 24 years was associated with a lower risk of severe liver steatosis at age 24 years after full adjustment.
The prevalence of severe liver steatosis of ≥275 dB/min cut point at age 24 years was 11%. Sensitivity analyses with this cutpoint revealed that cumulative ST (ages 11 through 24 years) was directly associated with an increased risk of severe liver steatosis [odds ratio 1.001 (95% confidence interval 1.001–1.002); p < 0.001]. Cumulative LPA was associated with a reduced risk of severe liver steatosis [0.996 (0.996–0.997); p < 0.001]. Cumulative MVPA had no statistically significant association with reduced risk of severe liver steatosis [1.000 (0.999–1.002); p = 0.528] after full covariate adjustment.
Mediation analyses of ST, LPA and MVPA with liver indices and enzymes
Increased lean mass partially mediated (33.3% mediation) the longitudinal associations of increased ST with lower liver steatosis CAP score at age 24 years after full adjustments for covariates (Table 3). Increased fat mass partially suppressed (18.2–28.4% suppression) the longitudinal associations of increased ST with higher liver fibrosis score and lower liver steatosis CAP score at age 24 years (Table 3). Increased lean mass partially suppressed (2.9–22.9% suppression) the longitudinal associations of increased ST with increased alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Increased fat mass partially mediated (0.7–6.7% mediation) the longitudinal associations of increased ST with increased alanine aminotransferase and aspartate aminotransferase from ages 17 to 24 years. Increased high-sensitivity C-reactive protein partially mediated (13.3–15.5% mediation) the longitudinal associations of increased ST with higher liver fibrosis score and lower liver steatosis CAP score at age 24 years but partially suppressed (1.5–2.9% suppression) the associations of increased ST with increased alanine aminotransferase and aspartate aminotransferase. Increased triglycerides and insulin resistance had no statistically significant mediating effect on the associations of increased ST with liver fibrosis, steatosis, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase (Table 3). Liver steatosis CAP score at age 24 years partially suppressed (50% suppression) the non-significant associations of increased ST with liver fibrosis score, while liver fibrosis score partially suppressed (14.8% suppression) the negative associations of increased ST with liver steatosis CAP score (Fig. 2).
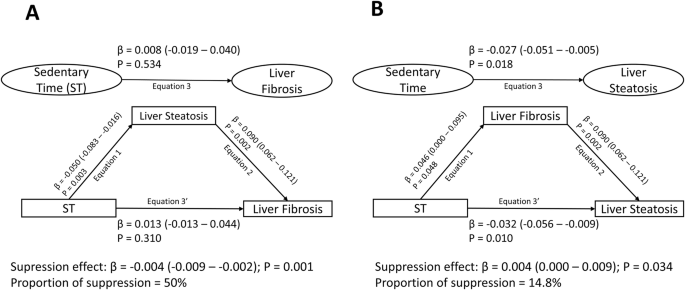
Mediating or suppressing role of liver steatosis and on the longitudinal associations of cumulative sedentary time from ages 11 through 24 years with liver fibrosis (A) and the mediating or suppressing role of liver fibrosis and on the longitudinal associations of sedentary time with liver steatosis (B) in 2684 participants. When the magnitude of the longitudinal association between the predictor and outcome is increased upon inclusion of a third variable, a suppression is confirmed, However, when decreased it is mediation. Mediation structural equation model estimating natural direct and indirect effects was adjusted for sex, family history of hypertension/diabetes/high cholesterol/ vascular disease and socioeconomic status, in addition to time-varying covariates such as age, high-sensitivity C-reactive protein, heart rate, systolic blood pressure, smoking status, sedentary time, light physical activity, high-density lipoprotein cholesterol, low-density. lipoprotein cholesterol, triglyceride, lean mass, fat mass, glucose and insulin. β is a standardised regression coefficient. Two-sided p-value < 0.05 were considered statistically significant. Liver fibrosis was computed from a controlled attenuation parameter score (dB/m) during transient elastography, whilst liver steatosis was computed from METAVIR scoring system (kPa).
Increased fat mass partially mediated (7.1–16.7% mediation) the longitudinal associations of cumulative LPA with lower liver fibrosis score and higher liver steatosis CAP score at age 24 years and increased γ-glutamyl transferase from ages 17–24 years (Table 4). Increased lean mass partially mediated (1.6–2.2% mediation) the longitudinal associations of cumulative LPA with decreased alanine aminotransferase and aspartate aminotransferase. Increased fat mass partially suppressed (0.4–3.5% mediation) the longitudinal associations of cumulative LPA with decreased alanine aminotransferase and aspartate aminotransferase from ages 17 to 24 years. Increased high-sensitivity C-reactive protein partially mediated (2.8–5.9% mediation) the longitudinal associations of cumulative LPA with decreased alanine aminotransferase and aspartate aminotransferase. Increased triglyceride and insulin resistance had no statistically significant mediating effect on the associations of cumulative LPA with liver fibrosis, steatosis, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase (Table 4).
Increased fat mass partially mediated (9.4% mediation) the longitudinal associations of cumulative MVPA with higher liver fibrosis CAP score at age 24 years (Table 5). Increased fat mass partially suppressed (41.4–63.6% suppression) the longitudinal associations of cumulative MVPA with higher liver steatosis CAP score at age 24 years, and increased aspartate aminotransferase and γ-glutamyl transferase from ages 17 to 24 years. Increased lean mass partially mediated (66.7% mediation) the longitudinal associations of cumulative MVPA with higher liver fibrosis score but partially suppressed (20.6–35.3% suppression) the longitudinal associations of MVPA with lower liver steatosis CAP score, decreased alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase (Table 5). Increased high-sensitivity C-reactive protein partially mediated (42.9% mediation) the longitudinal associations of cumulative MVPA with increased aspartate aminotransferase and γ-glutamyl transferase. Increased triglycerides partially mediated (29% mediation) the longitudinal associations of cumulative MVPA with decreased alanine aminotransferase. Insulin resistance had no significant mediation effect on the associations of cumulative MVPA with liver fibrosis, steatosis, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase (Table 5).
Discussion
In the largest and longest follow-up study to date, accelerometer-measured cumulative ST, LPA and MVPA from childhood through young adulthood were independently but differently related to the various liver indices and enzymes during growth from adolescence to young adulthood. Specifically, cumulatively increased ST from childhood through young adulthood was associated with an increased risk of advanced liver stiffness or cirrhosis in adolescence and severe liver steatosis in young adulthood. Cumulative LPA from childhood through young adulthood was associated with a decreased risk of advanced liver stiffness in adolescence and liver cirrhosis in young adulthood and a decreased risk of liver steatosis in adolescence. Similarly, cumulative MVPA from childhood through young adulthood was associated with a decreased risk of advanced liver stiffness in adolescence and a decreased risk of liver steatosis in adolescence and severe liver steatosis in young adulthood, with body composition and inflammation having differing mediation effects.
There is a scarcity of longitudinal studies in children and adolescents that have examined the relationships between accelerometer-measured ST and liver steatosis and fibrosis3,11,20,21. A recent cross-sectional study of 667 middle-aged adults from the Netherlands with a MASLD prevalence of 34.3% reported that accelerometer-measured ST was not associated with higher odds of ultrasound-assessed liver steatosis after full adjustment for cardiometabolic factors26. Similar results were observed in a Japanese adult population where ST was not associated with ultrasound-assessed liver steatosis27. In the present study, each 1 min/day increase in ST from childhood was positively associated with a 1 in 1000 odds of advanced liver stiffness in adolescence and severe liver steatosis in young adulthood after full adjustments for cardiometabolic and lifestyle factors. In the present cohort, the prevalence of ultrasound-measured advanced liver stiffness in adolescence is <1%, and transient elastography-measured severe liver steatosis is ~10% in young adulthood. Inflammation partly mediated the association of ST with severe liver steatosis and cirrhosis28, while triglyceride and insulin resistance had no significant mediating effect in contrast with previous evidence3,4,21,28. Although ST may independently increase fat mass, fat mass suppressed the relationship between ST and severe liver steatosis and fibrosis, suggesting that fat mass in an apparently healthy cohort may not be deleterious to liver health, akin to a report on vascular health12,14,29. It was also observed that liver steatosis and liver fibrosis may have bi-directional positive relationships and either seem to suppress the associations of the other with ST, nonetheless temporal analyses of repeated transient elastography measures are warranted3. ST increased from 6 h/day in childhood to 9 h/day in young adulthood and was cumulatively associated with progressively increased alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase, which are surrogates for liver injury, inflammation and potential liver damage1,3,4,5.
In the latest health guideline, there was no recommendation on LPA due to the scarcity of evidence on device-measured LPA in youth, and it has been established that most youths do not meet the recommended average of 60 minutes of MVPA per day but may accumulate a substantial amount of LPA minutes per day10. Several emerging longitudinal studies are reporting that LPA might yield more significant health benefits than MVPA, such as reducing systolic blood pressure, dyslipidaemia, fat mass obesity, insulin resistance, inflammation and vascular stiffness12,13,14,15,16,30. Longitudinal studies of accelerometer-based LPA and liver indices in the young population are scarce3,8,9,10,11,19,21. In the present study, although LPA decreased from 6 h/day to 3 h/day, it was cumulatively associated with a 1–11 in 1000 decreased odds of advanced liver stiffness and liver steatosis in adolescence and liver cirrhosis in young adulthood. These results were consistent in the mediation analyses where LPA was cumulatively associated with decreased alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase, with lean mass having a partial mediating effect. Neither triglyceride nor insulin resistance had any significant mediating effect on LPA and liver indices, as previously reported23. In the cross-sectional study of 667 Dutch middle-aged adults, LPA was not associated with lower odds of ultrasound-assessed liver steatosis after full adjustment for cardiometabolic factors26.
A systematic review and meta-analysis of 14 clinical trials (1231 youths) concluded that short-term, aerobic exercise was associated with lower intrahepatic fat and γ-glutamyl transferase9. Another systematic review and meta-analysis concluded that supervised short-term exercise reduced liver steatosis compared to the control groups in children and adolescents aged 6–19 years8. Moreover, another systematic review and meta-analysis of 19 studies involving 923 children and adolescents aged 6–18 years concluded that short-term, aerobic exercise and dietary intervention decreased aminotransferase levels and reduced liver steatosis risk31. In consonance with previous studies, it was observed that accelerometer-based MVPA from childhood was associated with a 1–5 in 1000 decreased odds of advanced liver stiffness and liver steatosis in adolescence and severe liver steatosis in young adulthood. These results were consistent in the mediation analyses where MVPA was cumulatively associated with decreased alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase, with fat mass having >40% suppression effect. MVPA effect on lowering liver steatosis may be significantly suppressed (>60% suppression) by increased fat mass28 similar to recent studies where increased fat mass attenuated the effect of MVPA on decreasing inflammation and dyslipidemia13,14. It was earlier reported that obesity may be the strongest predictor of steatosis in youth23. In the present study, it was observed that MVPA from childhood was paradoxically associated with higher fibrosis score, which was mediated (67% mediation) by increased lean mass. This seems a physiologic adaptation of the liver smooth muscle cells to MVPA as previously noted with progressive increased left ventricular mass and vascular wall thickness, but MVPA was not associated with an increased risk of liver cirrhosis16,32,33.
The strength of the study includes a well-phenotyped large birth cohort (ALSPAC) with extensive repeated assessments from childhood through young adulthood, a comprehensive array of repeatedly measured covariates such as device-measured body composition such as fat mass, lean mass and other lifestyle factors, smoking, socio-economic status and family history of cardiovascular and metabolic diseases, which enhanced the investigation of the independent relationships of movement behaviour with liver indices. A few limitations are that the gold standard method of liver biopsy was not ethically viable in this large cohort, but transient elastography is recommended in the guidelines for SLD and MASLD management34,35. The analyses of movement behaviour and liver steatosis and fibrosis at age 17 years and age 24 years were conducted separately due to the disparities in equipment (ultrasonography versus transient elastography), measurement indices, and cut points employed at various clinic visits36. Residual biases such as not accounting for sleep time, dietary intake and grams of alcohol consumed may not be excluded since the measures were not available. The accelerometer was worn for 7 days, which may not be sufficient to reveal the habitual lifestyle of adolescents. The differences in Actigraph models AM7164 and GTX3 models employed at different follow-up time points might introduce residual bias, however, the different movement behaviour cutpoints for adolescents at ages 11 and 15 years, and at young adulthood (age 24 years) might reduce potential variabilities37. The possibility of a Hawthorne effect cannot be excluded where participants modify their behaviour based on their awareness of being observed. Nonetheless, the device measured PA and ST is superior to self-reported lifestyle behaviour, which is fraught with recall and report bias38. Also, the participants were mostly Caucasian. Thus, findings may not be generalisable to other ethnicities.
In conclusion, the prevalence of liver steatosis increased 8-fold during growth from adolescence (age 17 years) to young adulthood (age 24 years); however, increasing LPA and MVPA and decreasing ST may independently attenuate the risk of severe liver steatosis and liver cirrhosis in adolescence and young adulthood. Specifically, engaging in at least 3–4 h/day of LPA from childhood through young adulthood may protect 11 out of 1000 youths from the risk of liver cirrhosis and 4 out of 1000 youths from severe liver steatosis (SLD, MASLD and MASH). Similarly, engaging in at least 50 min/day of MVPA from childhood through young adulthood may protect 3 out of 1000 youths from premature severe liver steatosis (SLD, MASLD and MASH).
Methods
Study cohort
Details of the ALSPAC birth cohort have been published earlier39,40,41,42. The ALSPAC birth cohort investigates factors that influence childhood development and growth. Altogether, 14,541 pregnancies from women residing in Avon, southwestern England, UK, who had a total of 14,676 foetuses, with an expected date of delivery between April 1, 1991, and December 31, 1992, were enrolled. When the oldest children were approximately 7 years of age, an attempt was made to bolster the initial sample with eligible cases who had failed to join the study originally resulting in 913 additional pregnancies. The total sample size for analyses using any data collected after 7 years of age was 15,454 pregnancies, resulting in 15,589 foetuses. Of these, 14,901 were alive at 1 year of age. Regular clinic visits of the children commenced at 7 years of age and are still ongoing. Study data at 24 years were collected and managed using REDCap electronic data capture tools43. For this analysis, 2684 participants were included who had at least one timepoint measure of ST, LPA, MVPA during clinic visits at 11, 15 or 24 years and complete measure of liver steatosis and liver fibrosis at age 24 years (Fig. 1). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
Movement behaviour measures
ST, LPA and MVPA were assessed with MTI Actigraph AM7164 2.2 (LLC, Fort Walton Beach, FL, USA) accelerometer worn for 7 days at 11- and 15-year clinic visits, whereas at 24 years movement behaviour was assessed using ActiGraph GT3X+ accelerometer device worn for four consecutive days12,13,14,15,16,17. An accelerometer was worn around the waist and detects acceleration and deceleration in a vertical plane as a combined function of movement frequency and intensity. There is a strong absolute agreement between the ActigraphTM models (intraclass correlation coefficient 0.99 (95% CI = 0.98–0.99), thus making it acceptable to use different models within a study, but newer models require additional extension to improve sensitivity to low-intensity activities37,44. The device was worn during waking hours on at least 2 week days and one weekend day. A valid day was defined as providing data for at least 10 h per day (excluding sequences of 10 or more minutes with consecutive zero counts) and children were only included in the analyses if they provided at least 3 valid days of recording. The devices capture movement in terms of acceleration as a combined function of frequency and intensity. Data are recorded as counts that result from summing postfiltered accelerometer values (raw data at 30 Hz) into 60 s epoch units. Data were processed using Kinesoft software, version 3.3.75 (Kinesoft), according to established protocol. Activity counts per minute (cpm) threshold validated in children and adolescents were used to calculate the amount of time spent in different movement behaviours42,45,46. At ages 11 and 15 years, the following validated cutpoints were employed: ST, 0 – <100 cpm; LPA, 100–2296 cpm; and MVPA, >2296. For young adults at age 24 years follow-up visit, slightly lower cutpoints were used viz, ST, 0 – <100 cpm; LPA, 100–2020 cpm; and MVPA, >2020 cpm42,45,46. The Evenson cutpoint used in stratifying activity threshold has shown the best overall performance across all intensity levels and was suggested as the most appropriate cut point for youth47,48.
Liver enzymes, structure and function measures
All participants attending the 24-year clinic visit were asked to fast overnight, or for a minimum of 6 h, before blood tests and subsequent transient elastography. Assessing liver steatosis and fibrosis with transient elastography has 0.70–0.89 accuracy in reference to liver biopsy25. Serology analyses for liver function tests for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase were conducted using an automated analyzer with enzymatic methods. Measurements were assayed between three and nine months after samples were taken, with no previous freeze-thaw cycles. All assays were completed in the same laboratory at the University of Glasgow. A non-invasive liver scan (transient elastography) was performed using FibroScan 502 Touch; Echosens, Paris, France. Participants were excluded from liver scans if they were pregnant, had an active implant (e.g., pacemaker), or if they suffered from liver ascites (excessive accumulation of fluid in the abdominal cavity). The scan provides two results. One is a measure of liver stiffness as a quantification of fibrosis. The other is a measure of a controlled attenuation parameter (CAP) as a quantification of steatosis (or fat retention within the liver cells). Ten valid readings were required to derive a CAP score and fibrosis result. The M probe was used initially unless the machine indicated the use of the XL probe. In line with manufacturer instructions, the cut-off values used for the M probe and XL probe were the same25. Cut-off values for CAP score for different grades of steatosis (S0–S3) were derived from a meta-analysis on CAP technology: S0 was defined as a score of less than 248 dB/m (<10% steatosis); S1 as a score of 248 to less than 268 dB/m (10%– < 33% steatosis [mild]); S2 as a score of 268 to less than 280 dB/m (33%– < 66% steatosis [moderate]); and S3 as a score of 280 dB/m or more (≥66% steatosis [severe])49. CAP scores of 248 dB/m or greater (≥S1) were considered as suspected steatosis. Participants’ CAP scores were considered eligible for analysis if ten valid readings (100–400 dB/m) could be obtained49,50.
Considering that MASLD is more common than alcohol-related liver disease in adolescents and young adults with MASLD present in >25% of the global adult population6, transient elastography cut-off values for metabolic dysfunction-associated steatohepatitis (MASH) related to the METAVIR scoring system6 were used for fibrosis staging (F0–F4: F0–F1, <7.9 kPa; F2, 7.9 to <8.8 kPa; F3, 8.8 to <11.7 kPa; and F4, ≥11.7 kPa)51,52. Participants with a transient elastography value between 7.9 kPa and <11.7 kPa were considered to have clinically significant or advanced fibrosis (F2–3), whereas participants with ≥11.7 kPa were considered to have cirrhosis (F4)34. If a participant’s median fibrosis result was greater than 7.1 kPa, the IQR to median ratio had to be less than 30% to be considered valid53.
At age 17 years, a pseudo-random sample of 1887 ALSPAC participants had an ultrasonography scan of the upper abdomen by one of four trained sonographers using Siemens Acuson S2000 ultrasonography scan system22,54. The presence or absence of liver steatosis was assessed by echogenicity during deep inspiration as but participants with mild (S1) steatosis category were classified in the absence of liver steatosis category22,54. Thirteen participants who reported continuous harmful drinking over the year prior to the ultrasound scan were excluded. Harmful drinking was defined in the ALSPAC cohort with Alcohol Use Disorder Identification Test for Consumption (AUDIT-C) of ≥5 cut-point to assess drinking in the past 1 year55. Using the same ultrasound scan, an acoustic radiation force impulse imaging of the right lobe of the liver was used to measure liver stiffness [shear velocity in metres per second m/s] as an indicator of liver fibrosis54. The extent of fibrosis was categorised as follows; none, <1.31 m/s as mild; 1.31 to ≤1.39 m/s as significant; 1.39 m/s to ≤2.25 m/s as severe; and ≥2.25 m/s as advanced22,54.
Anthropometric, cardiometabolic and lifestyle factors measures
Anthropometry (height and weight) of participants at ages 17 and 24 years was assessed by observing standard protocols and body mass index was computed as weight in kilograms per height in metres squared. Heart rate and systolic and diastolic blood pressure were measured at ages 17 and 24 years using an Omron 705-IT as previously detailed29,42,56. Fasting blood samples at ages 17 and 24 years were collected, spun, and frozen at −80 °C and later assayed for lipids, glucose, insulin, and high-sensitivity C-reactive protein as detailed previously42,56,57,58. Homoeostatic model assessment of insulin resistance (HOMA-IR) was estimated from (fasting plasma insulin × fasting plasma glucose/22.5)59. Total fat mass and lean mass were assessed using a dual-energy X-ray absorptiometry scanner at 17-year and 24-year clinic visits. Questionnaires to assess smoking behaviour were administered at the 17-year and 24-year clinic visits. Question related to smoking in the last 30 days was used as a marker of current smoking status. At the 17-year clinic visit, participants were briefly asked about their personal and family (mother, father and siblings) medical history, such as a history of hypertension, diabetes, high cholesterol and vascular disease. The participant’s mother’s socioeconomic status was grouped according to the 1991 British Office of Population and Census Statistics classification60.
Statistical analysis
Cohort descriptive characteristics were summarised as means and standard deviation, medians and interquartile ranges or frequencies and percentages. Sex differences were explored using independent t-tests, Mann–Whitney U tests or chi-square tests for normally distributed, skewed or dichotomous variables, respectively. Multicategory variables were analysed using a one-way analysis of variance. Normality was assessed by histogram curve, quantile-quantile plot, and Kolmogorov–Smirnov tests. A logarithmic transformation of skewed covariates was conducted, and normality was confirmed prior to further analysis.
The separate associations of the 4-year cumulative ST, LPA and MVPA progression (ages 11 through 15 years) with each of advanced liver fibrosis and the presence of liver steatosis at age 17 years were examined using generalized linear mixed-effect models. Then, separate associations of the 13-year cumulative ST, LPA and MVPA progression (ages 11 through 24 years) with each of liver cirrhosis (F4) and severe liver steatosis (S3) at age 24 years were examined using generalized linear mixed-effect models. The relationship between movement behaviour and progression in liver damage from ages 17 to 24 years was not examined due to significant differences in measures of liver indices and threshold cut points. The generalized linear mixed-effect model is robust for handling highly correlated variables such as ST and LPA with a Pearson correlation of (−0.70). The optimal model was one with sex and predictor as a factor and a random intercept modelled on the subject level. Moreover, the best model fit was accepted for the model with the lowest Bayesian Information Criteria. A random effect variance component type was selected and the effect of the predictor trajectory on the outcome variables was determined. To test for fixed effects and coefficients, robust covariances estimation was selected to handle violations of model assumptions. The analysis strategy accounted for baseline ST, LPA, MVPA predictors, and covariates and their repeated measures. Model 1 was unadjusted. Model 2 was adjusted for sex and other time-varying covariates measured at both baseline and follow-up, such as age, low-density lipoprotein cholesterol, insulin, triglyceride, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, heart rate, systolic blood pressure, glucose, fat mass, lean mass, smoking status, family history of hypertension/diabetes/high cholesterol/vascular disease and socioeconomic status, including additional mutual adjustment for ST, LPA or MVPA depending on the predictor. Sequential Sidak correction- was applied to correct for multiple comparisons. Covariates were selected based on previous studies7,19,22,23,54. A sensitivity analysis was performed with the 13-year cumulative ST, LPA and MVPA progression (ages 11 through 24 years) with severe liver steatosis of ≥275 dB/min cutpoint at age 24 years in line with clinical practice guideline35.
Lastly, mediating path analyses using structural equation models separately examined the mediating role of cumulative triglyceride, high-sensitivity C-reactive protein, insulin resistance, total body fat mass and lean mass on the longitudinal associations of cumulative ST, LPA or MVPA with severe liver steatosis and liver cirrhosis at age 24 years, and progressive changes in liver enzymes (alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase) from ages 17 to 24 years. The mediation analysis was conducted in line with the Guideline for Reporting Mediation Analyses of Randomised Trials and Observational Studies (AGReMA)61. Analyses were adjusted for age, sex, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, high-sensitivity C-reactive protein, family history of hypertension and cardiovascular diseases, smoking status, heart rate, systolic blood pressure, sedentary time, light physical activity, moderate-to-vigorous physical activity, total fat mass or lean mass depending on the exposure and mediator. The path models had three equations per regression analysis: the longitudinal associations of cumulative ST, LPA or MVPA with triglyceride, high-sensitivity C-reactive protein, insulin resistance, total body fat mass or lean mass (Equation 1); the longitudinal associations of cumulative triglyceride, high-sensitivity C-reactive protein, insulin resistance, total body fat mass or lean mass with liver indices and enzymes (Equation 2); and the longitudinal associations of cumulative ST, LPA or MVPA with liver indices and enzymes (Equation 3, total effect) and Equation 3’ (direct effect) accounted for the mediating role of cumulative triglyceride, high-sensitivity C-reactive protein, insulin resistance, total body fat mass or lean mass on the longitudinal associations of ST, LPA or MVPA with liver indices and enzymes. The proportion of mediating or suppressing roles was estimated as the ratio of the difference between Equation 3 and Equation 3’ or the multiplication of Equations 1 and 2 divided by Equation 3 and expressed in percentage. A mediating or indirect role is confirmed when there are statistically significant associations between (a) the predictor and mediator, (b) the predictor and outcome, (c) the mediator and outcome and (d) the longitudinal association between the predictor and outcome variable was attenuated upon inclusion of the mediator62. However, when the magnitude of the longitudinal association between the predictor and outcome is increased upon inclusion of a third variable, a suppression is confirmed62. This means that suppression occurs when the mediational path has an opposite effect, i.e. instead of a decrease in the point estimate of the direct effect between an exposure and an outcome in relation to the total effect, there is rather an increase in the direct effect above the total effect’s point estimate62. We considered a statistically significant mediation or suppression of <1% as minimal and ≥1% as partial. Path analyses were conducted with 1000 bootstrapped samples63,64. In the widely known causal mediation model, the direct relationships between a predictor and an outcome usually have the same sign (either positive or negative) with the indirect effect, also known as the mediator effect61. However, when there is an opposite sign such that the direct relationship between a predictor and an outcome is positive and the indirect or mediating effect is negative, it describes a suppression effect62. This suppression effect is also referred to as inconsistent mediation62. A suppressor variable increases the predictive validity of another variable when included in a regression equation where the magnitude of the regression coefficient determines the predictive validity62.
Collinearity diagnoses were performed and accepted results with a variance inflation factor <5. Differences and associations with a 2-sided p-value < 0.05 were considered statistically significant, and conclusions were made based on effect estimates and their confidence intervals (CI). The effect estimates for predictor variable analyses describe a 1 min/day change in ST, LPA or MVPA in relation to the odds of potential liver damage at either age 17 or 24 years. The odds ratio >1 describes the increased risk of an event. Analyses involving 20% of a sample of 10,000 ALSPAC children at 0.8 statistical power, 0.05 alpha and 2-sided p-value would show a minimum detectable difference of 0.062 standard deviations if they had relevant exposure for a normally distributed quantitative variable65. Longitudinal statistical analyses and multiple imputations were performed using SPSS statistics software, Version 27.0 (IBM Corp, Armonk, NY, USA), while mediation analyses from structural equation modelling were conducted using AMOS version 27.0. Chicago: IBM SPSS.
Responses