Diatom biosilica as a supplementary cementitious material
Introduction
Concrete is the most widely utilized anthropogenic material on earth. More than 4 billion tonnes of ordinary Portland cement (OPC), the key binding material in concrete, are produced annually1 and account for approximately 7% of global CO2 emissions2. Numerous research efforts are underway to reduce CO2 emissions from OPC production. In 2018, the Technology Roadmap for Low-Carbon Transition in the Cement Industry2 published by the International Energy Agency (IEA) classified these decarbonization efforts into four main groups: (1) thermal and electric efficiency improvements, (2) use of alternative fuels, (3) carbon capture and storage, and (4) clinker substitution. Clinker substitution with fillers or supplementary cementitious materials (SCMs) is the most promising strategy to reduce CO2 emissions from OPC production in the near term because of existing economies of scale, optimized processing, availability of raw materials, and market confidence3. SCMs work in conjunction with OPC by contributing to the hardened properties of concrete through hydraulic and/or pozzolanic activity. Partial replacement of OPC with SCMs of industrial (e.g., fly ash, slag, and silica fume) and natural (e.g., calcined clays, zeolites, pumice, and limestone) origin is already widely practiced. The use of SCMs in concrete is associated with lower environmental impact due to the reduction of clinker content, as well as improved durability due to densification of the cement paste microstructure4.
The three most common conventional SCMs are fly ash, ground granulated blast furnace slag (“slag”), and silica fume. Fly ash is a byproduct of the coal industry, slag is a byproduct of the steelmaking industry, and silica fume is a byproduct of the silicon and ferrosilicon alloy industry. Fly ash has been the most used SCM in recent decades. Fly ashes are typically used in dosages of approximately 15–30 wt% by replacement of cement, and they are classified as Class F or Class C according to ASTM C6185. Slag is a byproduct of the smelting of pig iron that exhibits latent hydraulic activity as an SCM in concrete. It is typically used in higher dosages than fly ash, ranging from approximately 30–80 wt% by replacement of cement6,7,8. Silica fume is more of a specialty SCM than fly ash or slag and it is typically used in smaller quantities (i.e., 5–10 wt% cement replacement). Because of its high surface area and high amorphous silica content, silica fume is an extremely effective pozzolanic SCM.
While fly ash was once considered an abundantly available waste material9, sources of suitable fly ash for use in concrete are currently threatened by global decarbonization efforts and the sustained demand for fly ash as an SCM in concrete over the past several decades10. Like fly ash, the supply of slag is also threatened as very high quantities of suitable blast furnace slag are already in use in concrete11. Additionally, many geographical locations exist where limited or negligible amounts of fly ash, slag, and other suitable industrial SCMs are produced12. Considering the marked decline in supplies of today’s most widely utilized SCMs, the market is changing rapidly. Accordingly, there is growing interest in the use of natural pozzolans, processed pozzolans, and manufactured pozzolans as SCM materials13.
Natural pozzolans including volcanic minerals (e.g., tuffs, ashes, pumice, perlites, and zeolites) and sedimentary minerals (e.g., diatomaceous earth) have been used throughout the history of concrete, from ancient times and into the 20th century. However, their usage waned over the latter half of the 20th century due to a lack of widespread availability as compared to the high availability of low-cost, high-quality fly ash and slag at the time14.
Fossilized diatom remains are the defining component of diatomaceous earth. Diatoms, still abundant in today’s oceans, are siliceous marine microalgae that are distinguished by their unique silica cell walls, called frustules15,16. Diatoms are the world’s largest contributors to biosilification17 (accounting for 40–50% of marine primary production today)18,19,20. Diatomaceous earth has experienced a resurgence in popularity in the construction materials literature in recent years12,21,22,23,24. Sierra et al.24 studied the pozzolanic activity of three different diatomaceous earth samples and concluded that even without calcination to increase reactivity, all behaved as pozzolans, reacting with CaO in cementitious systems. Li et al.12 showed that up to 40 wt% of cement could be replaced with diatomaceous earth, and that diatomaceous earth positively impacted the compressive strength and durability of concrete. Although superplasticizer was required to maintain workability even at a relatively high water-to-cementitious ratio of 0.48, it was determined via life cycle assessment (LCA) that substitution of OPC with diatomaceous earth at 30 wt% resulted in over a 30% reduction in the embodied carbon emissions of mortar12.
Due to recent research regarding diatomaceous earth as an SCM12,24, this work explores the use of biosilica extracted from living instead of fossilized diatoms as a novel SCM material. The controlled growth of diatoms to produce biosilica for SCM applications represents an opportunity for enhanced control over the physical and chemical properties of produced SCMs. Growth of SCMs using diatoms would also ensure supply chain consistency, which is a concern for current SCMs and may be a concern if the use of diatomaceous earth is employed at scale. Diatom growth for pozzolanic SCM production also holds the potential for additional CO2 reduction in cementitious systems (depending on biosilica collection and purification techniques used at scale), as CO2 is stored in photosynthetic biomass during diatom growth. As amorphous content is often an indicator of SCM reactivity, diatom biosilica was separated from biological material and calcined to understand heat treatment effects on reactivity. The recently introduced small-scale R3 test (ASTM C1897) bound water method25,26 was implemented to measure the chemical reactivity of diatom biosilica extracted from two different diatom species: T. pseudonana and Phaeodactylum tricornutum. The results were compared to conventional materials, including metakaolin, fly ash, slag, diatomaceous earth, and finely ground quartz (inert control). The mechanisms of chemical reactivity were further elucidated via supporting characterization techniques.
Methods
Biomass harvested from a 20 L culture of the marine-centric diatom T. pseudonana (CCMP 1335) was obtained from the Bigelow Laboratory for Ocean Sciences. P. tricornutum biomass harvested from open pond cultures was obtained from the National Renewable Energy Laboratory (Golden, CO, USA).
Deionized water (DI-H2O) was used for all experiments. Potassium sulfate (K2SO4) and potassium hydroxide (KOH) were obtained from Fisher Scientific. A potassium solution was prepared by dissolving 4 g/L (71 mM) KOH and 20 g/L (115 mM) K2SO4 in DI-H2O. The potassium solution was allowed to equilibrate at ambient temperature for at least 1 h prior to experimentation. Reagent-grade calcium hydroxide (i.e., portlandite) was obtained from Sigma-Aldrich. Reagent-grade calcium carbonate (CaCO3) was obtained from Research Products International.
Metakaolin (MK) was obtained from BASF. Ground granulated blast furnace slag (SL) (Grade 120) and a Type F fly ash (FA) were provided by US Concrete (San Jose, CA, USA). Diatomaceous earth (DE) was supplied by PF Harris (Cartersville, GA, USA). In accordance with previous studies27,28,29, inert quartz (Q) powder provided by Sigma-Aldrich was used as a negative control for the chemical reactivity experiments.
SCM material preparation
Conventional SCM materials were used as obtained from suppliers; no further treatments were applied to MK, SL, DE, FA, or Q. To test diatom biosilica as a novel SCM, inorganic mineral frustules were obtained from dried T. pseudonana (DB-Tp) and P. tricornutum (DB-Pt) biomass. Each source of biosilica was treated to remove organic biomass for the fundamental experiments conducted herein using nitric acid digestion at 90 °C for 1 h, followed by washing with DI-H2O and calcination at 700 °C for 1 h. Wu et al.30 used a similar protocol to prepare shape-stabilized phase change materials from freshly cultured diatom frustules.
Recent studies have shown that heat treatment of diatom biosilica can induce mineralogical changes towards a more ordered crystalline state. The amorphous titania present in Pinnularia sp.31 and F. solaris32 silica-titania frustules were converted to anatase by heating at 550 °C and 500 °C, respectively. Wu et al.30 found that calcining diatom frustules at 800 °C might have induced partial crystallization of quartz. Arasuna & Okuno33 studied the effect of calcination on the mineralogy of C. calcitrans and found that both unheated diatom frustules and frustules calcined at 600 °C were both completely amorphous by X-ray diffraction (XRD) analysis. However, frustules calcined at 800 °C showed weak diffraction peaks indicating crystallization, and frustules calcined at 1000 °C and 1200 °C exhibited even sharper peaks mainly matching cristobalite33. Because of these recent reports, the crystallinity of diatom biosilica used here was analyzed to understand if the 700 °C calcination induced significant crystallization effects, which may have in turn affected the overall reactivity when used as an SCM.
Experimental safety considerations
Finely ground silica (SiO2) is the basic component of many SCMs, including the diatom biosilica analyzed in this study. Finely ground silica poses several health and safety risks, especially for inhalation34; care should be taken to avoid inhalation or exposure to finely ground silica materials through the use of fume hoods and N95 masks while handling. The purification protocol selected herein to remove organic material from cultured diatom frustules uses nitric acid. Nitric acid is a strong oxidizer, highly corrosive, and decomposes more rapidly upon heating35,36,37. All appropriate PPE and safety precautions included in a safety data sheet (such as the one referenced here in ref. 37) should be followed when using this chemical.
Characterization of SCM sources
The microstructure and morphology of DB-Tp, DB-Pt, MK, SL, DE, FA, and Q were examined using a Hitachi SU3500 scanning electron microscope (SEM). Approximately 20 mg of sample were mounted on carbon tape and sputter coated with 15 nm of platinum to ensure conductivity. SEM was conducted at a magnification of 1500× using an accelerating voltage of 10 kV and a working distance of 8–10 mm. Energy-dispersive X-ray spectroscopy (EDS) was performed using an Oxford Instruments Ultim Max EDS detector. EDS spectra were collected at five randomly selected sites on each sample within a single sample frame. The chemical composition obtained by the EDS spectra for each sample was averaged to obtain an average chemical composition of each sample.
To analyze the crystalline phase content of each SCM, a Bruker D8 Advance X-ray Diffractometer (Billerica, MA, USA) with Cu Kα radiation (wavelength 1.5406 Å) was used to collect XRD data. Approximately 20 mg of sample was mixed with isopropyl alcohol and drop cast onto a silica zero background plate; isopropyl alcohol was then allowed to evaporate prior to testing. Data were collected from 10° to 40° 2θ with a step size of 0.02° and a dwell time of 2 s per step. The current was 40 mA and the voltage was 40 kV. Crystalline phases were identified using Bruker DIFFRAC.EVA software and the International Center for Diffraction Data (ICDD) PDF-4 AXIOM 2019 database38.
To examine the local structure of silicon in certain samples, solid-state 29Si direct-polarization magic angle spinning (DP-MAS) nuclear magnetic resonance (NMR) spectra were obtained using a Varian INOVA 400 MHz NMR spectrometer. The magnetic field was 9.39 T, the operating frequency was 79.50 MHz, and the MAS speed was 8 kHz. Approximately 50 mg of powdered sample was packed into 4 mm zirconia rotors sealed at either end with Teflon plugs. Spectra were collected using a broadband probe equipped with a 4 mm MAS spinning module (Revolution NMR, Fort Collins, CO, USA). 29Si chemical shifts were determined using a reference of DSS (2,2-dimethyl-2-silapentanesulfonate) at 1.46 ppm. The spectra were acquired through a Bloch-decay experiment with 1600 scans using a pulse recycle delay of 45 s, a 90° pulse width of 4.5 μs, and an acquisition time of ~20 ms.
Analysis of the collected NMR data was performed in Mestrenova software, and quantitative peak deconvolution was performed in Origin39. Gaussian peak profiles were used to deconvolute the 29Si MAS NMR spectra40. The minimum number of peaks possible were fitted41. The isotropic chemical shift (δiso), peak intensity (integrated area), and peak full width at half maximum (FWHM) were determined and reported for each peak in each sample. Qn notation, where n is the number of Si–O–Si oxo-bridges (i.e., 0, 1, 2, 3, and 4), was used to categorize each peak42.
Chemical reactivity (ASTM C1897)
Chemical reactivity testing was conducted in accordance with a validated small-scale R3 (ASTM C1897) method previously developed by the authors25. Briefly, small paste samples were prepared with SCM-to-portlandite, SCM-to-limestone, and potassium solution-to-solids ratios of 1:3, 2:1, and 6:5, respectively, in accordance with ASTM C189743. The samples were mixed in microcentrifuge tubes by pulse vortex for 2 min. The freshly mixed pastes were sealed and cured in an oven at 40 °C for 7 days. Following curing, the pastes were removed from the oven and placed in a vacuum desiccator for at least 24 h prior to characterization. After drying, thermogravimetric analysis (TGA) was performed using a TA Discovery 5500 (TA Instruments, New Castle, DE, USA) in an inert nitrogen atmosphere. Approximately 10 mg of reacted paste was placed in a platinum pan and allowed to equilibrate at 40 °C. Then, the temperature was ramped to 500 °C at a rate of 10 °C/min. Bound water content was determined by calculating the difference between the initial mass of the sample at 40 °C and the final mass after heating to 350 °C. Portlandite content was measured by determining the difference between the mass of the sample at 350 °C and the mass of the sample at 450 °C and calculating according to the known stoichiometry of portlandite decomposition in this temperature range26. Each sample was tested in triplicate. XRD was also collected on each paste, using the same method as described in the section “Characterization of SCM sources” (results shown in Supplementary Information).
Results
Morphology and chemical composition of diatom biosilica and conventional SCMs
SEM images of DB-Tp and DB-Pt are displayed in Fig. 1. SEM images of the conventional SCMs (i.e., MK, SL, DE, FA, and Q) are also shown in Fig. 1 for comparison. It is evident that the ultrastructure of the diatom frustules in both DB-Tp and DB-Pt was lost during the chemical and physical treatments that were applied to separate the organic biomass from the inorganic matter (i.e., biosilica). Wu et al.30 described a similar alteration, noting that the acid treatment resulted in fractured frustules. Figure 1 illustrates that the DB-Tp and DB-Pt biosilica both exhibit slightly larger particle sizes as compared to the conventional SCMs. Notably, MK and SL exhibit the smallest particle sizes.
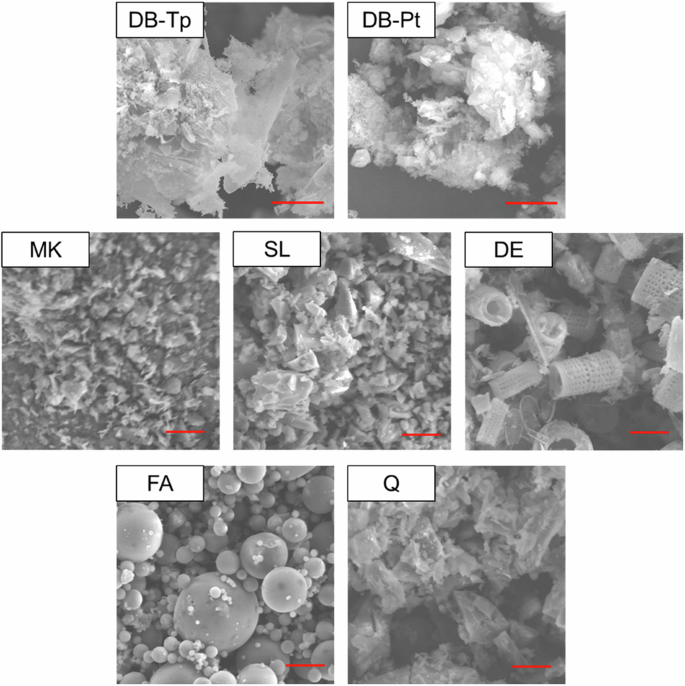
SEM images of diatom biosilica extracted from T. pseudonana (DB-Tp) and P. tricornutum (DB-Pt), metakaolin (MK), slag (SL), diatomaceous earth (DE), fly ash (FA), and quartz (Q). Scale bar = 10 μm.
EDS was performed on each SCM material to estimate elemental composition. The composition of oxides, which was averaged from EDS spectra from five randomly selected sites on each sample, is displayed for DB-Tp and DB-Pt, as well as the conventional SCMs (MK, SL, DE, FA, and Q) in Fig. 2. For a detailed discussion on the oxide composition of the conventional SCMs, see our recent work25. DB-Tp contained predominantly SiO2 (97.6 wt%) with trace amounts of Al2O3 (2.4 wt%). DB-Tp is most chemically similar to the conventional materials DE and Q. Each of these materials contained >90 wt% SiO2 with Al2O3 as the second most abundant oxide. The elemental composition of DB-Pt is more heterogeneous. DB-Pt contained predominantly SiO2 (65.4 wt%) with higher quantities of Al2O3 (14.5 wt%) and TiO2 (10.6 wt%), as well as trace amounts of CaO (0.9 wt%), Fe2O3 (1.7 wt%), MgO (2.4 wt%), K2O (2.6 wt%), and Na2O (1.8 wt%). DB-Pt was most chemically similar to FA and, to a lesser extent, MK. With these calculations, it is important to note the limitations of EDS analysis for elemental quantification. Standardless EDS quantification, as used here, is typically accurate to ±2–5% for major identified elements, although it may be worse for rough particles, as analyzed here44. Elements with atomic numbers greater than 9 can be detected down to approximately 0.1%, indicating that element identification is relatively accurate. EDS point analysis also results in relatively small amounts of data from which to make definitive quantitative conclusions. The exact quantitative values calculated here are thus estimates/comparisons and should be taken as such.
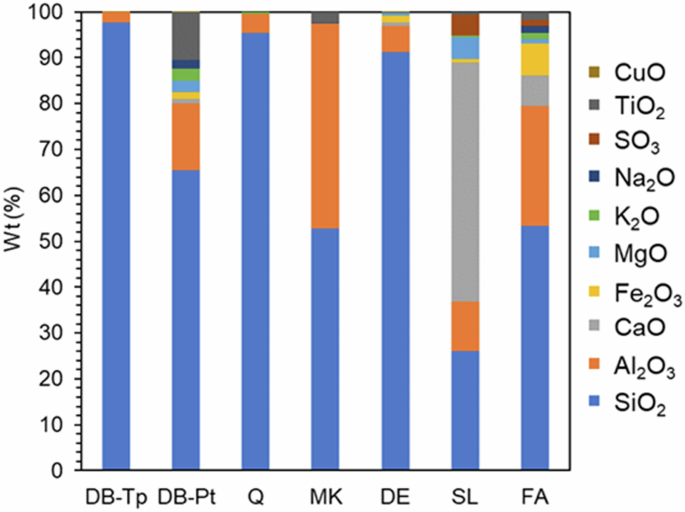
Average oxide composition of diatom biosilica extracted from T. pseudonana (DB-Tp) and P. tricornutum (DB-Pt), and conventional SCMs metakaolin (MK), slag (SL), diatomaceous earth (DE), fly ash (FA), and quartz (Q) determined via EDS (average of five measurements).
Within these constraints, DB-Tp, DB-Pt, MK, DE, and Q each meet the chemical requirements for classification as a Class N natural pozzolan in accordance with ASTM C6185 (SiO2 + Al2O3 + Fe2O3 ≥ 70 wt%, SO3 = 4 wt%). FA can be characterized as a Class F fly ash and can be expected to exhibit pozzolanic activity. EDS results of SL indicated a CaO content of ~50 wt%, indicating it would behave as a latent hydraulic material instead of a pozzolanic material.
The differences between the chemical compositions of DB-Tp and DB-Pt could be attributable to numerous factors. First, the centric T. pseudonana has a very tightly regulated silicon metabolism and, like most diatoms, silicon is an obligatory requirement for growth. The pennate P. tricornutum, on the other hand, has a highly variable silicon metabolism and is the only known diatom species where silicon is a non-obligatory requirement for growth45, thus explaining, at least in part, the lower silicon content of the DB-Pt compared to DB-Tp. Second, P. tricornutum is pleiomorphic, meaning it can exist as one of three different morphotypes (i.e., oval, fusiform, triradiate)46,47. It has been postulated that the pleiomorphism of P. tricornutum is facilitated by the plasticity in the cell wall, because it is only poorly silicified compared to other diatom species47,48. Again, this is an indication that silicon contents in P. tricornutum frustules would perhaps be lower than those for T. pseudonana. Finally, T. pseudonana biomass, from which DB-Tp was extracted, was grown in a well-controlled laboratory environment while P. tricornutum biomass, from which DB-Pt was extracted, was grown in outdoor open ponds. Therefore, P. tricornutum was likely exposed to more environmental stressors and variable conditions during growth which could have led to increased chemical variability in DB-Pt biosilica. For example, nitrogen and phosphorus limitations have been shown to greatly influence element accumulation in P. tricornutum49,50,51.
The presence of aluminum in both DB-Tp and DB-Pt was expected as aluminum incorporation into diatom frustules has been observed extensively both in nature52,53,54,55,56 and in laboratory studies57,58,59,60. In a study by Kohler et al.57, it was also observed that as the concentration of Al in the frustules increased, so did the concentration of metal cations abundant in seawater such as Na+, K+, Ca2+, and Mg2+. The authors attributed this to the incorporation of Al into the silica tetrahedra network structure, leading to a negative framework charge that is then counterbalanced by the metal cations57. A similar trend was also evident in the current study. DB-Tp contained approximately 2.35 wt% Al2O3 without other metal cations as detected by EDS analysis. However, DB-Pt contained a much higher concentration of 14.5 wt% Al2O3. With higher Al2O3 content in DB-Pt, measurable quantities of Na2O (1.8 wt%), K2O (2.6 wt%), CaO (0.9 wt%), and MgO (2.4 wt%) were detected, as expected to counterbalance metal framework charge57.
DB-Pt studied here also contained 10.6 wt% TiO2. Concentrations of ~1.0 wt% TiO2 have been reported in the literature for commercially cultivated diatoms61. TiO2 has been metabolically incorporated into diatom frustules in laboratory studies with the goal of imparting photocatalytic activity32,62,63. Most of these studies involve doping the culture medium with water-soluble Ti-precursors such as titanium (IV) bis(ammonium lactate) dihydroxide (TiBALDH), although none have studied the TiO2 uptake of diatom P. tricornutum specifically. In the biosilica studied here, it is hypothesized that outdoor culture conditions/stressors may have led to increased TiO2 uptake by P. tricornutum, although additional studies are needed to substantiate this hypothesis.
Mineralogy (XRD) of diatom biosilica and conventional SCMs
XRD patterns for DB-Tp, DB-Pt, and the conventional SCMs are shown in Fig. 3. Crystallinity and amorphous content are important characterizations of potential SCMs, as amorphous materials typically exhibit a much higher chemical reactivity in cementitious systems compared to crystalline materials, regardless of exact chemical composition. The mineralogy of conventional SCMs was analyzed in our previous work25; therefore, only the mineralogy of DB-Pt and DB-Tp is discussed in detail here.
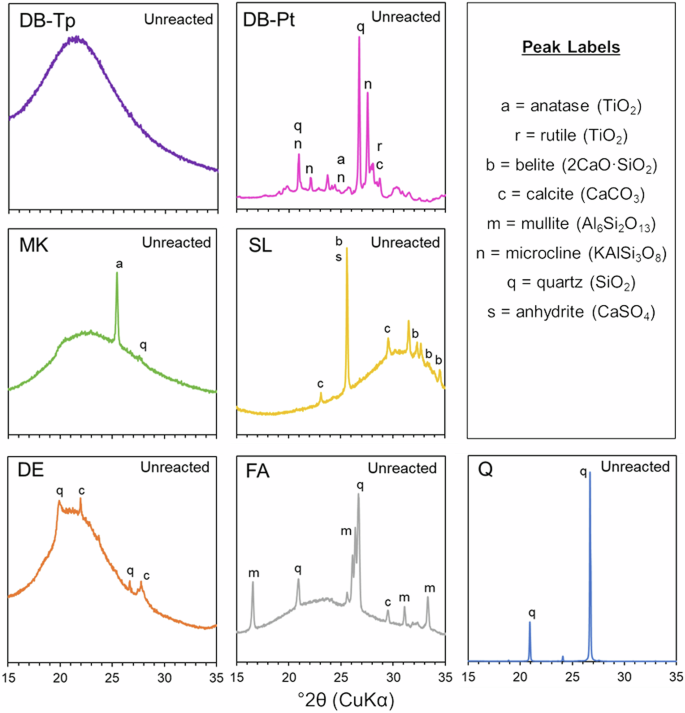
XRD patterns for diatom biosilica extracted from T. pseudonana (DB-Tp) and P. tricornutum (DB-Pt), and conventional SCMs metakaolin (MK), slag (SL), diatomaceous earth (DE), fly ash (FA), and quartz (Q).
According to the XRD results, DB-Tp is composed of a highly amorphous structure, while DB-Pt exhibited partial crystallinity with peaks matching those of microcline, calcite, anatase, rutile, and quartz. Microcline (KAlSi3O8), which has been detected in diatom frustules by XRD in the literature61, was likely formed due to the substitution of aluminum into the silica tetrahedron network followed by charge-balancing by potassium or other similar metal cations. This finding is supported by the EDS results presented in the section “Morphology and chemical composition of diatom biosilica and conventional SCMs”, where both Al2O3 and K2O (and similarly, Na2O, CaO, and MgO) concentrations were elevated in DB-Pt as compared to DB-Tp. The presence of anatase and rutile in DB-Pt was also supported by EDS results, since larger quantities of TiO2 were measured. Q was fully crystalline, FA and SL were semi-crystalline, and DE and MK were mostly amorphous with a small amount of crystalline character. The crystalline anatase peak seen in the MK diffractogram indicates that the TiO2 content as estimated by EDS is a crystalline chemical impurity and is not incorporated into the amorphous alumina-silica structure.
In the current work, both DB-Tp and DB-Pt were calcined at 700 °C for 1 h. After calcination, DB-Tp was completely amorphous as indicated via XRD (Fig. 3). Although no biosilica was tested without calcination, it appears that the heat treatment did not induce crystalline phase transitions in DB-Tp. It is possible that calcination enhanced crystallinity in DB-Pt, as the crystallization of both quartz and anatase induced by heat treatment in diatom biosilica has been reported in the literature30,31,32,33,61.
Silica coordination (29Si MAS NMR) of diatom biosilica and conventional SCMs
29Si MAS NMR is a well-established and widely utilized tool that is highly sensitive to the local chemical environment of the silica tetrahedra in a sample. One of the most important parameters to consider is the chemical shift, or the line position on the spectrum, which is highly influenced by the immediate structural surroundings of Si atoms (i.e., resonance atoms). In the current study, the chemical shift is described by Qn notation, where n is the number of Si–O–Si oxo-bridges42. Therefore, a high-field chemical shift corresponds to a higher degree of SiO4 polymerization (n = 4), while a low-field shift corresponds to a lower degree of SiO4 polymerization (n = 0). For aluminosilicate materials, a low-field shift is typically encountered due to the substitution of Al within the silica framework64. Such NMR analysis can be used to further understand the atomic order and potential reactivity of the SCM materials studied here.
Spectra from 29Si MAS NMR are shown for DB-Tp and DB-Pt in Fig. 4 alongside those obtained for the conventional SCMs (MK, DE, FA, and Q). Both DB-Tp and Q are composed of a majority of Q4 silica species, with peaks centered at −112.8 ppm and −110.4 ppm, respectively. As discussed previously, both DB-Tp and Q were composed almost entirely (>95 wt%) of SiO2 with only trace amounts of Al2O3 (section “Morphology and chemical composition of diatom biosilica and conventional SCMs”).
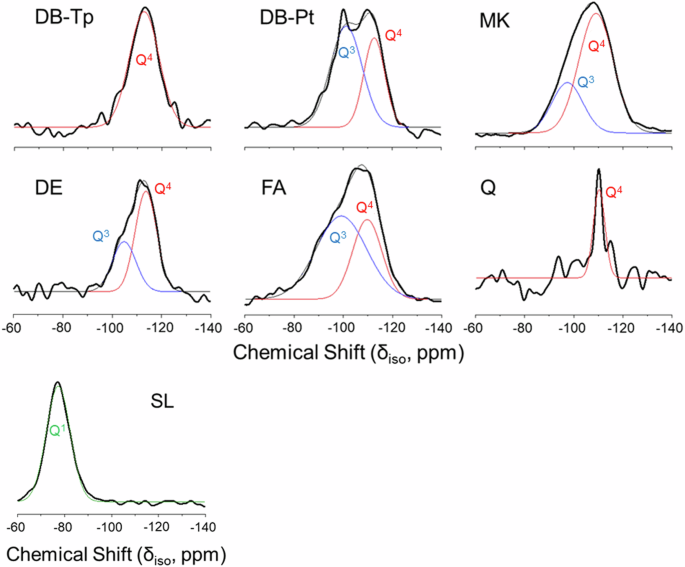
29Si MAS-NMR spectra for diatom biosilica extracted from T. pseudonana (DB-Tp) and P. tricornutum (DB-Pt), and conventional SCMs metakaolin (MK), slag (SL), diatomaceous earth (DE), fly ash (FA), and quartz (Q).
DB-Pt, MK, DE, and FA each contained both Q3 and Q4 silica species. It was determined in the section “Morphology and chemical composition of diatom biosilica and conventional SCMs” that each of these materials contained >5 wt% Al2O3. Therefore, it is likely that the Q3 species in these materials resulted from the substitution of aluminum into the silica framework, corresponding to a decrease in the degree of SiO4 polymerization. Following peak deconvolution, the chemical shifts for the Q3 peaks for DB-Pt, MK, DE, and FA were centered at −101.3 ppm, −97.4 ppm, −104.8 ppm, and −99.2 ppm, respectively. The chemical shift for the Q4 peaks for the same samples were centered at −112.7 ppm, −109.1 ppm, −113.7 ppm, and −109.7 ppm, respectively.
While DB-Tp, DB-Pt, MK, SL, DE, FA, and Q are all predominantly siliceous (or aluminosiliceous) materials, ground granulated blast furnace slag consists primarily of depolymerized calcium silicate glasses, as well as minor amounts of poorly crystalline phases. Oxide analysis by EDS in the section “Morphology and chemical composition of diatom biosilica and conventional SCMs” revealed that SL consists of approximately 26 wt% SiO2, while each of the other materials surveyed herein contained >50 wt% SiO2. The 29Si MAS NMR spectra for SL indicate a single peak at −77.3 ppm, which corresponds to disordered, Q1 species. Similar results have been reported for blast furnace slags in the literature, where the chemical shift has been reported to range from −74 ppm to −76 ppm65,66,67.
In addition to the chemical shift, the parameters of peak intensity and linewidth are also utilized to interpret NMR spectra64. These parameters, represented by peak intensity (I) and peak full width at half maximum (FWHM), respectively, were determined via quantitative peak deconvolution (Table 1). The peak intensity, I, is directly related to the number of the respective resonance atoms present in a sample. Therefore, the normalized peak intensities can be used to estimate the relative proportions of Si atoms in various coordination states. As stated previously, both DB-Tp and Q contained only Q4 species so the peak intensity was 100%. Similarly, SL contained only Q1 species with a peak intensity of 100%. The remaining materials (i.e., DB-Pt, DE, MK, and FA) contained both Q3 and Q4 silica species in varying ratios.
FA exhibited the greatest Q3 peak intensity (64.4%) followed by DB-Pt (63.1%) for the predominantly aluminosilicate materials containing both Q3 and Q4 species. This result indicated that FA and DB-Pt had a relatively high rate of aluminum substitution into the silica framework as compared to DE and MK. This finding is supported by XRD results presented in the section “Mineralogy (XRD) of diatom biosilica and conventional SCMs”, where direct evidence of crystalline aluminosilicate phases was only detected in FA and DB-Pt. The main aluminosilicate phases detected by XRD were mullite (Al6Si2O13) and microcline (KAlSi3O8) in FA and DB-Pt, respectively.
The FWHM, which is principally related to the crystallinity of the sample and may be further affected by dynamic processes and specific spin interactions, was also determined for each peak. The FWHM of the single Q4 species peak for Q, which was the only completely crystalline material by XRD of those surveyed herein, was the narrowest at 5.6 ppm. Contrastingly, the FWHM of the rest of the peaks, which belonged to partially amorphous materials, was all greater than 10 ppm. This suggested that the phases in Q are substantially more crystalline than those in the other in the other samples, which again corroborated the XRD data.
Chemical reactivity (ASTM C1897) using bound water content (TGA)
Representative results for TGA of the reacted paste mixtures containing DB-Tp, DB-Pt, and the conventional SCMs are displayed in Fig. 5. Bound water content can be estimated from this TGA data by calculating the difference between the initial sample mass and the mass of the sample at 350 °C13. Samples containing DB-Tp exhibited the highest bound water content (9.94 ± 0.60 g/100 g dried paste) aside from pastes containing MK (17.18 ± 0.73 g/100 g dried paste), indicating very high chemical reactivity. This result was anticipated, given the high SiO2 content of DB-Tp (section “Morphology and chemical composition of diatom biosilica and conventional SCMs”), its highly amorphous structure (section “Mineralogy (XRD) of diatom biosilica and conventional SCMs”), and its high silica coordination (section “Silica coordination (29Si MAS NMR) of diatom biosilica and conventional SCMs”). For pozzolanic (i.e., non-hydraulic, low calcium) materials, amorphous content is known to correlate particularly well with chemical reactivity. Contrastingly, the partially crystalline and more chemically complex DB-Pt led to a lower bound water content (4.33 ± 0.10 g/100 g dried paste) in pastes containing it as an SCM. The data show that samples containing DB-Pt were similar in terms of bound water content to pastes containing FA (4.02 ± 0.29 g/100 g dried paste). Pastes containing inert control Q had the lowest bound water content (1.38 ± 0.30 g/100 g dried paste), as expected. The bound water contents of pastes containing DE and SL (7.06 ± 0.22 and 8.17 ± 0.34 g/100 g dried paste, respectively), which were both moderately reactive, lie between the two DB samples. More specifically, DE and SL were found to be less reactive than DB-Tp and MK and more reactive than DB-Pt.
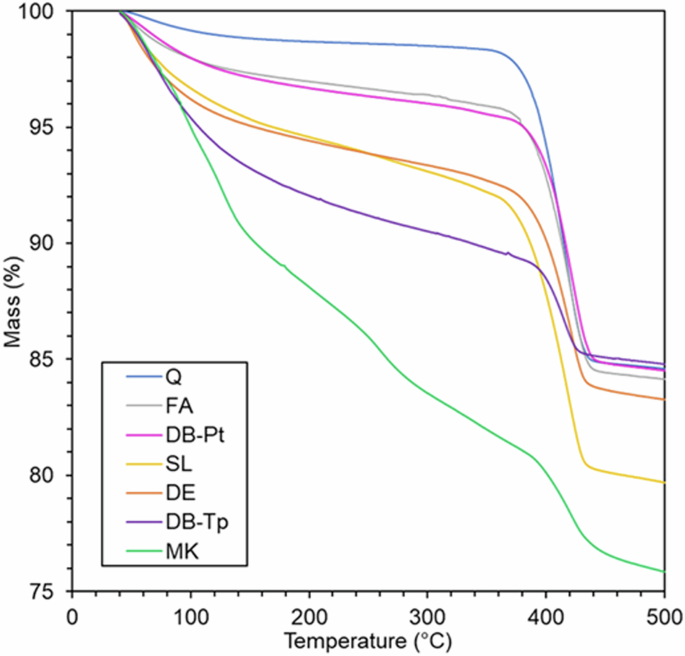
Representative results from thermogravimetric analysis for SCMs studied here: diatom biosilica extracted from T. pseudonana (DB-Tp) and P. tricornutum (DB-Pt), and conventional SCMs metakaolin (MK), slag (SL), diatomaceous earth (DE), fly ash (FA), and quartz (Q). Each sample was tested in triplicate.
Although the R3 bound water content method is rapid, accurate, and convenient, one noted drawback of the method is that it cannot distinguish between pozzolanic reactivity and hydraulic reactivity43,68. The main difference in the mechanisms by which latent hydraulic and pozzolanic SCMs form additional hydration products is that the former generally does not consume portlandite while the latter does. Accordingly, portlandite (CH) consumption, can be compared to bound water estimates to provide more information about the reaction mechanism for each SCM. The estimated CH consumed by each SCM is plotted together with bound water content in Fig. 6. From these data, DB-Tp can be generally classified as pozzolanic, more reactive, while DB-Pt can be classified as pozzolanic, less reactive, similar to FA. The conventional SCMs were classified as expected. Like DB-Tp, MK and DE were also classified as pozzolanic, more reactive due to high bound water content (>6 g/100 g dried paste) and high portlandite consumption (>25 g/100 g dried paste). SL was classified as latent hydraulic, more reactive as it exhibited similarly high bound water content (>6 g/100 g dried paste) but lower portlandite consumption (<25 g/100 g dried paste) than the pozzolanic, more reactive materials (i.e., MK, DB-Tp, and DE). Both the bound water content and portlandite consumption were very low in the samples containing Q, leading to its classification as inert. Portlandite consumption trends were also confirmed using qualitative XRD of small-scale reacted R3 pastes (Fig. S1 in Supplemental Material).
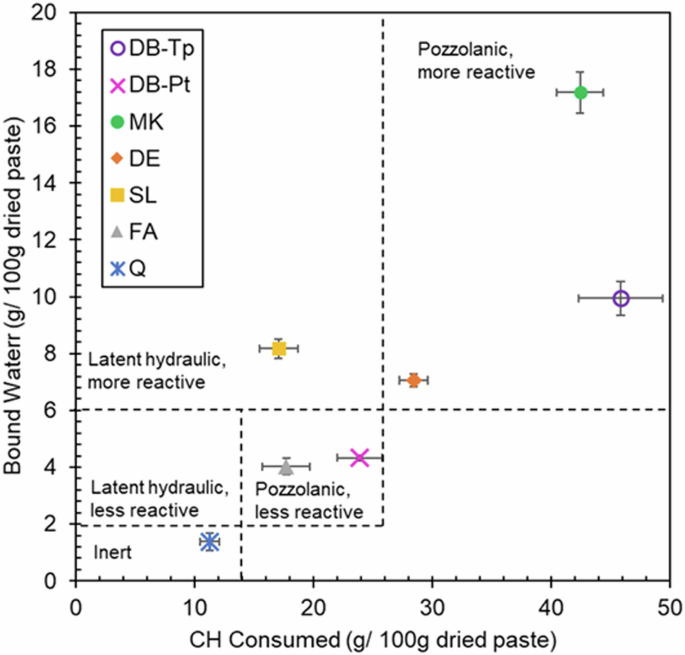
CH consumption vs bound water content for pastes containing SCMs investigated herein (diatom biosilica extracted from T. pseudonana (DB-Tp) and P. tricornutum (DB-Pt), and conventional SCMs metakaolin (MK), slag (SL), diatomaceous earth (DE), fly ash (FA), and quartz (Q)). Boundaries for classification adapted from ref. 70.
The ASTM C1897 R3 bound water contents for DB-Pt and FA were very similar—a result that can be attributed to the similar mineralogy (Fig. 3) and local silicon coordination (Fig. 4) of DB-Pt and FA. DB-Pt contained 4.33 ± 0.10 g of bound water per 100 g of dried paste compared to 4.02 ± 0.29 g/100 g dried paste for FA. As discussed in the section “SCM material preparation”, it is possible that calcination at 700 °C might have enhanced or induced additional crystallinity in the DB-Pt in this work, particularly for titania-bearing phases31,32. Since amorphous content is known to correlate well with the chemical reactivity of SCMs, it is likely that this would have had a negative effect on the reactivity of DB-Pt.
Discussion
This work explored the use of biosilica (DB-Pt and DB-Tp) extracted from non-fossilized diatoms (P. tricornutum and T. pseudonana, respectively) as novel supplementary cementitious materials. Results from these experimental studies not only demonstrated the potential to grow highly reactive diatom biominerals for cementitious materials applications (e.g., DB-Tp) but also the wide variability and potential tunability (e.g., DB-Pt) of diatom biominerals. The results presented here also successfully demonstrated the application of the small-scale R3 SCM screening method designed and validated previously25 for the first time. Extracted from the model-centric T. pseudonana, DB-Tp exhibited the highest chemical reactivity as indicated by ASTM C1897 bound water content (9.94 ± 0.60 g/100 g dried paste), second only to that measured for pure metakaolin. DB-Tp was thus classified as pozzolanic, more reactive. This was expected since DB-Tp was completely amorphous by XRD, and EDS analysis revealed that the oxide composition for DB-Tp was >95 wt% SiO2. DB-Pt, extracted from the pennate P. tricornutum, was classified as less reactive and behaved similarly to fly ash. This was corroborated by XRD and 29Si NMR data which showed evidence of crystalline aluminosilicate phases (microcline) in DB-Pt and EDS data which revealed a higher concentration of chemical impurities for DB-Pt than for DB-Tp.
Considering the positive results of the chemical reactivity screening performed herein for diatom biominerals, further investigation of these materials for use in cementitious materials is encouraged. Future work should take three paths—(1) continuation of comprehensive chemical screening of diatom biominerals, (2) scale-up and further experimental validation of DB as an SCM in blended mortars and concretes (including compressive strength assessments), and (3) techno-economic analyses of growth, collection, and purification of diatom biosilica at the industrial scale. The first of these might involve screening frustules extracted from many other diatom species in conjunction with supporting characterization techniques to elucidate trends in reactivity. Diatoms are incredibly diverse as are the properties of their frustules69, and it is expected that this research would yield first-of-its-kind knowledge regarding the physical, chemical, and mineralogical properties of diatom biosilica, as well as mechanisms of chemical reactivity. Additional studies on diatom processing, including calcination temperature, will also aid in understanding the full range of chemical reactivity achievable for diatom biosilica as an SCM. The second identified path for future work with diatoms would involve the scale-up of diatom cultivation such that appropriate quantities could be obtained for testing and validation in standard mortar and concrete specimens. The third path would involve exploring cost, embodied carbon, and processing techniques needed to produce diatom biosilica at the industrial scale. Such techno-economic analyses would further the understanding of what it would take to produce SCMs on demand through the growth of diatoms and whether such an approach is industrially applicable.
Overall, the data obtained and presented herein highlight the potential to grow highly reactive microalgal biominerals using potentially CO2-sequestering microorganisms (i.e., diatoms). These results also demonstrate the chemical variability and potential tunability of such biominerals for use as SCMs in cement and concrete, as well as in other applications. It is anticipated that this small-scale study will inspire the screening of more non-traditional sources of SCMs so that knowledge and availability of novel material resources can be expanded.
Responses