From lignin self assembly to nanoparticles nucleation and growth: A critical perspective
Introduction
The interest in the valorisation of lignin has become a pivotal focus in advancing the sustainable circular economic system1. Due to the chemical heterogeneity of lignin and the different extraction and fractionation strategies used to isolate this biopolymer, its valorisation is still challenging.
Upgrading lignin to value-added products has historically proceeded with difficulty mainly due to two intrinsic factors, namely its wide heterogeneity and diversity2,3. Lignin heterogeneity arises from the inherent lack of a defined primary structure, the occurrence of several different and randomly distributed interunit bonding motifs, along with botanical differences4,5,6. Additional major contributors to its variability and heterogeneity are the nature of wood or plant species and the process and intensity of the process used for its isolation7,8,9,10. The three-dimensional character of the lignin polymer inherently produces a highly polydisperse polymeric mixture composed of variable amounts of oligomeric and polymeric materials at different ratios depending on the extent of degradation the material is subjected to11,12,13,14.
The behaviour of lignin in solution has been the subject of voluminous literature with diverse opinions amplified by its complex, intricate, and variable behaviour whether in aqueous and/or organic media. For example, within a given lignin sample one can identify the presence of various phenolic, carboxylic acids, and aliphatic groups15,16,17, all in different amounts and of different acid dissociation constants18. Added to this, the presence of carbonyl groups and of aromatic rings6, creates centres of additional inter and/or intramolecular interactions of varying thermodynamic stabilities depending on the specific environment19. This creates a situation where at a given pH20, ionic strength21, or dielectric constant of the medium22, the molecular species present respond in a concerted fashion to reach an energy minimum. This eventually causes molecular shape alterations, and the accompanying associative and repulsive effects manifest themselves in the formation of structures that may or may not phase separately at the nano or larger scale, offering colloidal suspensions or larger aggregates.
Systematic advances in our knowledge related to the structure of various lignins, coupled with an understanding of the physical and chemical effects operating within the polymeric and oligomeric lignin species have offered new approaches for creating and modulating lignin at the nano level. An alternative strategy to overcome lignin heterogeneity and multifunctionality lies, in fact, in the exploitation of its tendency to aggregate23: nanosizing is allowing efficient compatibility with other matrix materials associated with tailored orientation of functional groups without the need of chemical functionalization24. The discovery of unique properties in lignin nanoparticles was somewhat unexpected and has prompted intense interest in these materials for their possible utilisation25 as stimuli-responsive drug delivery systems26, food packaging27, antioxidants28, UV-vis shielding barriers29, etc. All these characteristics are a function of nano-lignin size, shape, and hydrophobicity. Present research efforts are focusing on the implementation of these parameters to develop stimuli-responsive materials with enhanced properties.
It is the intent of this effort to offer a perspective of the state of the art in this field with a systematic deciphering of the science that contributes to the numerous factors operating in the creation of these nano-systems.
Lignin inter- and intra-molecular interaction sites
The aggregation behaviour of lignin is the result of intra- and intermolecular interactions. The extraction strategies used for the isolation of lignins have a dramatic effect on their structure, especially as it pertains to the contents of hydroxyl groups and molecular weight distribution. (see Table 1 for typical ranges). These variations impact the overall dissolution/aggregation behaviour in terms of the formation of hydrogen-bonds and/or π-π stacking interactions (Fig. 1).
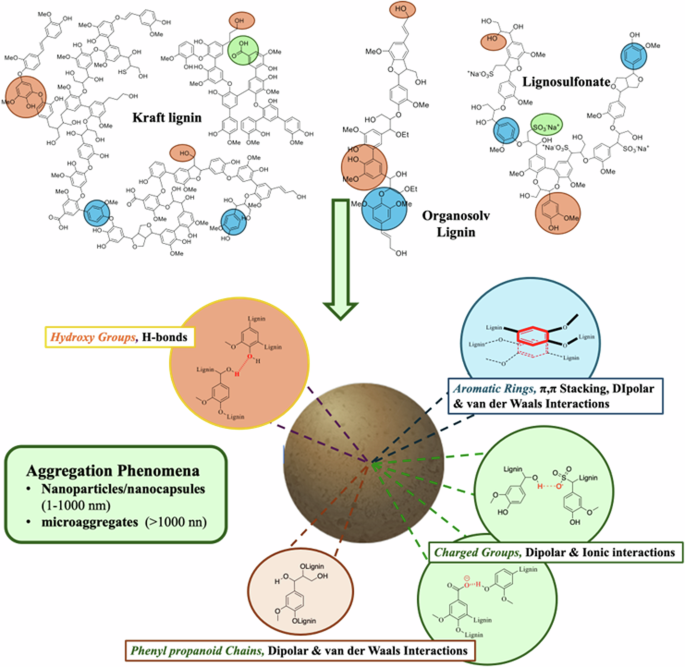
The highlighted areas in each structure show the sites for inter/intramolecular interactions. The lower part of the figure attempts to display various major interactions among the sites.
The interunit bonding patterns of native lignins are: aryl-glycerol-β-aryl ether (β-O-4’)11,30,31,32, phenyl-cumarane (β,5’)11,33, pinoresinol (β,β’)11,34, dibenzodioxocyn (DBDO)11,35,36, diphenyl (5-5’)11,37,38 and diaryl ether (4-O-5’)11,37. Aliphatic hydroxy-groups are the most abundant (Table 1) functional motif, while the content of phenolic hydroxy groups range from 0.9 to 2.0 mmol/g and the carboxylic acid group content is limited (0.0.1–0.05 mmol/g). Their weight average molecular weight ranges (Mw) roughly from 1000 to 50,000 Da.
The technical lignins produced by the kraft process have been heavily modified from that of the starting wood and new functional groups have been introduced in it while other structural motifs have been dramatically reduced. The new groups introduced are aryl-enol-ethers, stilbenes, carboxylic acids, condensation sites (1,4’), ethoxy- and thio-moieties12. On the other hand aryl-glycerol-β-aryl ether (β-O-4’) interunit linkages are heavily depleted12. Generally speaking, an increase in the content of free-phenolics and carboxylic acids along with a decrease of aliphatic hydroxy groups is also characteristic of kraft lignin as compared to the lignin from the original wood39,40,41,42,43. Organosolv lignin displays a molecular weight distribution narrower than kraft lignin while the majority of the alkyl-aryl ether bonds present in the original wood, especially β-O-4’ structures, are preserved44,45,46. Organosolv lignin is therefore, anticipated to be less hydrophilic than kraft lignin. Unlike kraft and organosolv lignins, lignosulfonates are characterised by their highly hydrophilic nature. This is due to the presence of significant amounts of sulfonic groups on the aliphatic chains47. The molecular weight of lignosulfonates is higher than in other lignins.
Self-assembly processes in Lignin
Overall, the various lignin domains can be differentiated according to their polarizability (Fig. 1). Polar groups are phenolated, hydroxylated, carboxylated and sulfonated moieties, while polarisable groups are mostly the aromatic rings. Non-polarisable domains are mostly manifested on the phenyl propanoid alkylated chains.
The so described system of bonding patterns and functional groups inherently allows the creation of long-range (van der Waals, electrostatic & hydrophobic forces) and short-range interactions (H-bonds and π-π interactions), all of them being responsible for the various types of aggregations phenomena in lignin48.
Hydroxy groups, which can be of aliphatic, phenolic, and carboxylic type, can generate hydrogen-bonds, or dipolar electrostatic interactions when they appear in their deprotonated form. The presence of polar groups, such as sulfonates, can favour the formation of dipolar and ionic interactions. Alternatively, aromatic rings, whose quadrupole moments can favour the establishment of quadrupolar interactions are responsible for the formation of π-π stacking interactions21,48,49,50,51.
Lignin solubility
The dissolution of lignin can occur either in organic solvents or in alkaline water with different solvation behaviour. Lignin solubility can be conveniently studied by the Hildebrand and Hansen parameters. The Hildebrand parameter corresponds to the energy required to break the intermolecular interactions within a substance in order to favour its solvation (δt), while the Hansen theory, considers the sum of contributions deriving from dispersive (δD) and dipolar forces(δp) and of hydrogen bonds (δh)52,53,54. Hildebrand and Hansen parameters for typical lignin solvents are reported in Table 2.
Solvents characterised by a Hildebrand parameter close to 22.5 MPa1/2 have been reported as a best fit for lignin dissolution53. This is the case of dimethyl-sulfoxide (DMSO), whose Hildebrand parameter is 26.7 MPa1/2. However, the Hildebrand parameter is not always sufficient; in fact, DMSO is a better solvent for lignin than 1-butanol, which is characterised by a Hildebrand parameter closer to the target value of 22.5 MPa1/2 (1-butanol δt = 23.2 MPa1/2). Hansen parameters show that in DMSO the larger contribution to dissolution emerges from the polar interactions (δP) rather than from the formation of H-bonds (δH).
Interestingly, the polarity of solvents cannot be directly correlated with a beneficial lignin solubilisation effect; in fact, it was demonstrated that the intermediate polarity of DMSO, if compared to other solvents, resulted in the greatest unfolding of the polymeric chains17,48,55,56,57.
The behaviour of lignin in solution can be partially explained by the Flory-Huggins theory22. More specifically, it is possible to correlate the radius of gyration (Rgyr), to the degree of solvation of a polymer, with its molecular weight (MW) according to the following relation:
Where a represents the efficiency of lignin solvation in the solvent considered. Under Ɵ conditions, a tends to 0.5 where the intermolecular interactions of lignin are counterbalanced by the solvent effects; resulting in a situation where the solvent and the polymer mix freely. When a < 0.5, polymer dissolution results; if a > 0.5, good solvation occurs leading to a polymer with a more exposed surface to the action of the solvent.
With the aid of molecular simulation, it is possible to apply the Flory-Huggins theory to lignin and identify the best solvent for its dissolution under specific conditions. DMSO appears to be an optimal solvent due to the fact that it offers the greatest degree of unfolding within the lignin structure. This promotes more of the lignin surface to be exposed to the solvent. This model was investigated and documented with different types of lignins (spruce, birch and miscanthus) and was related to their structural features22. Water is characterised by the smallest a, leading to compact structures. A pictorial scheme of the aggregation behaviour of lignin in DMSO and water is depicted in Fig. 2.
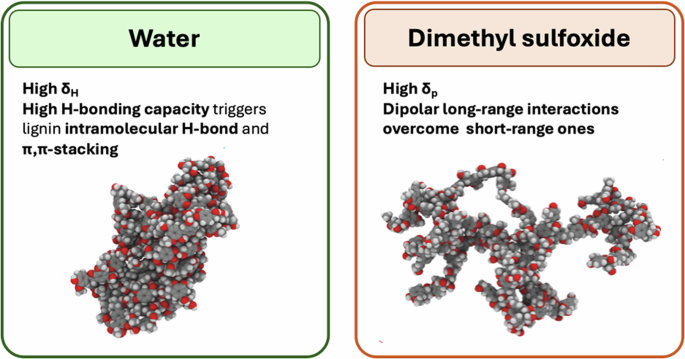
The high δH of water corresponding to high short-range H-bond capacity increases lignin intramolecular π−π stacking and H-bonding, thus generating a collapsed structure. On the contrary, the high δP value of DMSO allows the formation of long-range dipolar interactions with the solvent and triggers the elongated conformation. Structures reprinted with permission by ACS Journals.
Radial distribution functions (RDFs) represent other fundamental factors for quantifying the interactions between a solvent and a solute. More specifically, different chemical moieties contribute to the interactions of the solvent-solute pair, to different extents. The hydroxy groups interact with the solvent through hydrogen bonds, causing the formation of a hydration shell within specific segments in lignin chains resulting in reducing the frequency of intramolecular interactions. Concomitantly, free OH groups can promote intramolecular hydrogen bonds. Overall, the different types of hydroxy groups and their position in lignin chains need to be considered in order to elucidate the lignin-solvent interactions22.
Softwood kraft lignin displayed a solubility behaviour that was opposite to the polarity of the solvent used when solubility in ethanol, acetone and THF, whose relative polarities were 0.654, 0.355 and 0.207 respectively, were studied58,59.
Different sources of lignin contain different amounts of phenylpropanoid units that influence intra- and intermolecular interactions6. For example, the G-type of structure of softwood lignin contains a single methoxy group per aromatic ring60 significantly affecting the abundance of intramolecular hydrogen bonds, resulting in an enhancement of the solvation state48,51. Based on these assumptions, it is possible to confirm and rationalise the solubility of kraft lignin as studied by Zwilling58. In different solvents, lignin reveals different degrees of compactness with the decrease in the polarity of the solvent. The dissolution process is not only governed by the type of lignin used, (previously attributed to the difference in its propensity to interact via solute-solvent interactions), but also on the characteristics of the solvent used such as its polarity, its protic or aprotic character, and its ability to interact with the polar regions of the lignin.
Lignin aggregation
The driving force of the self-assembly processes of lignin is attributed to weak inter- and intra-molecular forces between hydroxylated moieties and aromatic rings. A convenient method to describe their balance is represented by the Derjanguin–Landau–Verwey–Overbeek (DLVO) theory, which relates the electrostatic potential repulsion energy of aggregates with their radius, surface potential and the distances amongst them via the use of the Debye length58,61.
The balance between long range attractive (van der Waals) and repulsive (electric double layer, EDL) interactions determines lignin nanoparticles size and stability. Additionally, the entanglement of lignin molecules plays a key role in the three-dimensional conformation of lignin; in fact, a variation in the content of the various types of bonding patterns results in a modification of its supra-molecular and self-assembly behaviour. For example, the content of aryl-glycerol-β-aryl ethers, has been successfully correlated with the aggregation characteristics48.
Lignin aggregation phenomena proceed via a primary nucleation step and a growth step. According to the classical nucleation theory (CNT), nuclei which are considered as macroscopic objects such as crystalline phases, separate from the bulk environment in which they were dissolved. Solute molecules in an oversaturated solution reversibly form small aggregates, called clusters. Once their size reaches a critical value, they become thermodynamically stable, and their growth is favoured62,63. For lignin, the first step is molecular interactions caused by electrostatic forces and resulting in the generation of nanoaggregates. Subsequently, the aggregation proceeds via the layer-by-layer deposition of additional macromolecules on the newly formed nano-aggregates20,55,58,64,65.
Notably, the core of the aggregates is formed by the high molecular weight fraction of lignin. After a sedimentation step, the low molecular weight lignin fractions are deposited onto the external part of the aggregates58,64. After the first nucleation step, governed by the nature of lignin, the growth stage occurs according to the relationship between lignin and solvent.
A more compact structure promotes the intramolecular interactions between lignin chains such as the π-π stacking of aromatic rings. G-units are capable to generate stronger π-π stacking interactions than S-units. As such, softwood lignins offer smaller aggregates than hardwood ones. This is due to the higher content in G-units that cause stronger interactions creating more compact aggregated structures51,64,66,67.
The specific nature of lignin affects not only the particle size of colloidal dispersions but also their shape51. It was demonstrated that hardwood lignin forms long packed rod-like aggregates while softwood lignin cylindrical and rod-like aggregates.
Overall, our understanding of vital correlations between structural differences in lignin and the interaction with the solvating environment needs to be significantly enriched and diversified.
Recent developments have shown that kraft lignin interacts with ethylene glycol only via H-bonds, while its interactions with DMSO promote dipolar stacking, causing quadrupolar interactions to predominate20,64. Variations on the solvation environment may favour the formation of lignin-lignin interactions; for alkali lignin, altering the nature of the anti-solvent added to a lignin solution favours the aggregation phenomena via π-π stacking interactions. Figure 3 shows the range of solvents used that favour the self-assembly of lignin via π-π stacking interactions as calculated for arylglycerol-β-aryl ether dimers67.
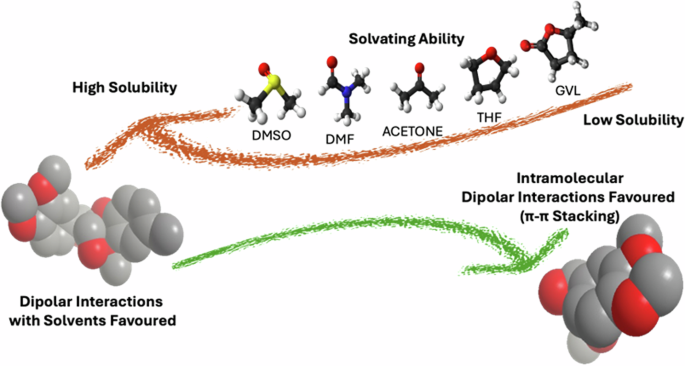
Accordingly, when the solvating efficiency is low, lignin intramolecular π−π stacking and H-bonds are favoured. The relative lignin conformation in solution is thus compact and, upon addition of water as antisolvent, the formation of nanoparticles is favoured (right).
Ultimately, these differences in interactions with the solvent result in different aggregation mechanisms and eventually promote the formation of different materials.
Colloidal lignin is mostly prepared by the solvent/antisolvent methodologies either by addition of an antisolvent to a lignin solution or by reducing the pH of an alkaline aqueous solution23,68,69.
Interestingly, the polarity of the anti-solvent used promotes its π-π stacking interactions48,64,67. Water has been identified as the best antisolvent due to its limited lignin solvation ability. This supports previous observations related to water favouring the formation of compact aggregates of a well-defined structure. Therefore, a useful approach for the synthesis of lignin nanoparticles is using water as an antisolvent48,64,67.
The addition of water was carefully investigated by Salentinig in analysing the formation of aggregates in THF70. In particular, it was observed that in pure solvent the interactions mostly arise from hydrogen-bonds, binding carboxylic and phenolic hydroxy groups as well as quadrupolar π-π stacking interactions. SAXS measurements identified the minimum size of the aggregates formed under the examined conditions (6.4 nm). The sudden water addition, in a final ratio 1:9 (water/THF) resulted in a self-assembly of lignin molecules generating aggregates of a larger size than those obtained in pure THF. This is in agreement with a previous report71, in which the aggregation was attributed to the layer-by-layer deposition of lignin molecules deriving from the modifying effect of water on the π-π stacking pre-existing in pure THF. Similar results were also reported for the preparation of lignin nanoparticles via dialysis of THF and ethylene glycol lignin solutions20. In particular, lignin aggregates dispersed in water, after extensive dialysis offered particles of homogeneous spherical morphology.
Concentration is another factor playing a pivotal role in lignin aggregation20,48,51,58,66. An increase in lignin concentration corresponds to an increased collision frequency and interactions among the lignin molecules, favouring the growth mechanism. Consequently, larger aggregates are formed together with nucleation, resulting in a polydisperse system20,66.
A critical concentration, ranging from about 1 to 3 g/L is needed to start the nucleation process from different solvents58. It depends on the nature of lignin and its structural characteristics and increases with its hydrophobicity. The dimensions of the aggregates decrease with increasing lignin concentration (5, 10 and 15 wt/vol%) and specific structure51.
More specifically, the stronger π-π stacking interactions of G- with respect to S-units, results in the formation of smaller aggregates from softwood than from hardwood lignin.
Furthermore, SANS analysis suggested that with increasing concentration lignin nanostructures generate fractal aggregates51.
Structural features that impact the size of the obtained nanoparticles include functional groups that can trigger short range intramolecular H-bonds and π−π stacking interactions. Therefore, higher amounts of OH groups and β-O-4 aryl ether bonds favour a compact configuration of lignin in solution thus boosting the formation of smaller nanoparticles upon antisolvent addition. On the contrary, the presence of charged groups induces electrostatic repulsions that induce the formation of bigger nanoparticles. The complex dependence of the size of lignin nanoparticles on the different functional groups present in lignin is synthetized in Fig. 458,64,66,72.
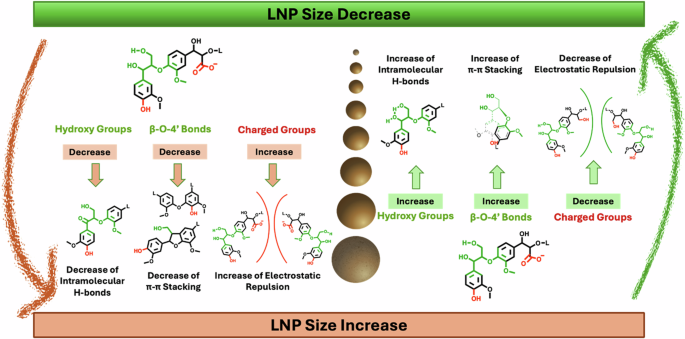
A high content in hydroxy groups undergoes extensive formation of intramolecular hydrogen bonds which, in turn, determines a compact conformation for lignin that causes the formation of small nanoparticles. Analogously, a high content in β-O-4 subunits determines more pronounced π−π stacking and small particles. The stronger π−π interaction occurring in G over S units favours the formation of smaller particles from softwood. On the contrary, the presence of charged groups such as carboxylates increases the electrostatic repulsions and therefore undergoes the formation of big particles. The dependency of lignin particles size on the molecular weight is, instead, the consequence of the different distribution of aliphatic and phenolic OH groups in lignins or lignin fractions58,64,66,72.
The effect of the pH on the aggregation of lignin has also been extensively examined48,55,61,70,73. At pH’s higher than 13.8, lignin is fully dissolved. Under these conditions ionic-dipole and hydrogen bond interactions with the solvent prevail over inter/intramolecular hydrogen bonds and π-π stacking.
Dissociation constant considerations (pKa) allow the modulation of lignin association and de-association phenomena. Controlling the pH of the various functional groups, (which range from 3 to-5 for carboxylic groups, and from 8 to 11 for phenolic hydroxy groups), such a modulation is possible. This approach has been used to design smart lignin-based materials for the controlled release of active ingredients55,73. The balance of both strong short distance interactions (e.g. hydrogen bonds and π-π interactions) and repulsive forces (attributed to the repulsive interactions generated by neighbouring anionic domains) is essential to define the dimensions and stability of the lignin aggregates. Lignin nanostructures are formed only when the pH is lowered from 8-11 to 5-820,74,75.
The deprotonation of phenolic hydroxy groups at pH 8-11 can be seen as a potential source of negative charge on lignin, which would result in a drastic rearrangement of the hydrophilic groups upon protonation (at pH’s 5-8). At such pH values the hydrogen bond tendency of protonated phenolic groups is reduced. Conversely, in the same pH range, carboxylic groups are still in the form of carboxylate anions. Consequently, the repulsive forces in particle-particle interactions are most-likely derived from the interaction of vicinal carboxylate moieties present in lignin61.
In general, the decrease in the pH of a lignin solution favours aggregation due to changes in the external double layer of the particles that is created by the changes in ionisation of the relevant functional groups.
The ionic strength of the medium has a similar effect and is another relevant factor in tailoring the overall process20,74. The thickness of the double layer depends on the presence of ions and as such the aggregation phenomenon increases with ionic strength74. Different ionic species have a specific effect on the formation and stability of colloidal lignin. In this context, the Hofmeister series delineates the following order of ion influence for anions such as: PO43− < CO32− < SO42− < CH3COO− < HPO42− < Cl− < H2PO4− < HCO3– while in the case of cations the order is: Mg2+ < Ca2+ < H+ < Na+ < K+. Kosmotropic ions (early members in the series) reduce the solubility of nonpolar molecules, while chaotropic ions (later members in the series) behave oppositely by promoting solubility. A predictive model, based on the Hofmeister series, can thus be used to rationalize, assess, and facilitate lignin colloidal stability in various environments21,76.
Finally, temperature affects the aggregation phenomena of lignin49,77,78. Increasing the temperature enhances the aggregation tendency, likely by increasing the collision frequency and also by decreasing the surface repulsive forces51,63,79.
Conclusions and perspectives on lignin aggregation
Outstanding advances in the state of the art of lignin self-assembly have been made in the last ten years19,80,81,82. Initial empirical efforts have been replaced by approaches and experimental evidence where the fundamental interactions that occur between and within lignin chains and the environment can now describe configurational aspects of the interacting molecules. This understanding when coupled with the chemical and polymer characteristics of the starting material (i.e. monomeric constituent composition, degrees & identity of hydroxylation and methoxylation, and molecular weight), may pave the way for predictions to be made on the possible properties of the resulting lignin-based nanomaterials.
Unfortunately, the intricate nature of lignin and the ensuing complexity of its aggregation behaviour, coupled with the plethora and diversity of techniques used still require systematic attempts to unify the scattered and at times contradictory data. For example, the methods used to determine lignin structural details, molecular weights and distributions, aggregate sizes etc. need be standardized and unified so as to allow for an overall comparability of the data.
The documented lack of standardization in methodologies used to study lignin structure and behaviour represents a challenge to the field, One avenue that this issue could be advanced is via further developments, advances, and applications in lignin fractionation strategies. Homogeneous lignin products may ensure that the well found inconsistencies outlined by this review are limited in the future.
This effort has attempted to enumerate the various parameters affecting self-assembly of lignins. The DLVO theory has been applied to estimate the role of van der Waals and EDL forces. These are considered as the main attractive and repulsive long distance interactions occurring in the process of lignin aggregation19,61.
What is missing however, is an understanding of the actual contribution for each parameter in controlling the process specially in relation to short distance attractive interactions. A good example of the present limitations in the state of our knowledge is that no precise quantitative models exist that define the role of H-bonds operating between aliphatic, phenolic and carboxylic hydroxy groups in lignin. Possible advances in this area maybe offered by quantitatively delving in efforts that have illustrated and examined nanoparticle formation data from acetylated lignin20. In this case the role of aliphatic and phenolic groups is found to be only marginal, while carboxylic acids (that remained non-acetylated), were held as the responsible driver causing the aggregation via H-bonds. Efforts to quantify the various contributions should also consider the role of molecular weight, steric hindrance, and of π-π stacking, in addition to the content of all hydroxylated moieties that are related to the H-bond contributions. An understanding at this level may eventually define the operational driving-force for the process still remains a matter of debate. In particular, since both attractive and/or repulsive interactions are at play19, a better clarification of their balance would definitely help in planning strategies for optimising lignin aggregation.
The role of the solvents and non-solvents in the aggregation phenomena also deserves, more attention based on an understanding of the efficiency of lignin solvation/desolvation mechanisms and the emergence of ensuing interactions in defining the shape of the final aggregates. Critical correlations involving solvent polarity and different types of lignin preparations (technical or native) may pave the way toward valuable structural correlations. The rate and order of addition of the constituents used to promote aggregation also needs to be carefully considered since it can be a pivotal factor in enhancing or depressing the assembly processes.
Finally, it is to be noted that by creating lignin nanoparticles, major limitations of lignin are addressed avoiding the need of environmentally taxing chemical modifications. It is to be noted, however, that overall, environmental sustainability considerations related to lignin nano-aggregation processes is still a matter of debate. Such considerations are absent from most accounts promoting the use of lignin nano-systems in various applications. Thorough life cycle analyses that consider the yields, the solvents used, and all relevant sustainability aspects of the various processing steps would add further credibility to these efforts and claims.

Responses