Research progress on synthesis mechanism and performance evaluation of ball milling biochar-iron based materials
Introduction
Both biochar and iron-based materials are considered to be one of the water pollution remediation materials which are widely available, environmentally friendly, and inexpensive1,2. Among them, biochar is obtained through thermochemical methods based on biomass such as straw, wood chips, and bones under oxygen-limited or anaerobic conditions3. And the iron-based materials include zero-valent iron, iron (hydrogen) oxides, and ferric salts, which have used in reduction, adsorption, and mediating advanced oxidation4. Although with certain recognized advantages for each, researcher have tried to combine the characteristics of the two to further improve the reaction surface area, reactivity and alleviate agglomeration5. In practice, it was discovered that based on the unique or common characteristics of the two, including adsorption, reduction and magnetism, there is a synergistic effect after synthesis, which greatly improves the shortcomings of any single raw material and significantly enhances the removal performance of pollutants. The core of the enhanced performance of biochar-iron based materials can be summarized as follows: (1) strengthen adsorption; (2) improve electron transfer performance; (3) relieve agglomeration and improve migration ability; and (4) enhance magnetism.
Up to now, the preparation methods of biochar-iron based materials include post-impregnation pyrolysis, liquid phase reduction, co-precipitation, ball milling, and hydrothermal carbonization techniques6. The synthesized materials are used to remove various pollutants in water environments through chemical precipitation, reduction, oxidation, and adsorption3,7,8,9. However, it cannot be ignored that the relatively expensive remediation cost and the strict preparation and application conditions of remediation materials3,10. Therefore, exploring sustainable restoration technologies is of great significance to protecting environmental health and development. Among them, ball milling is undoubtedly an efficient and environmentally friendly technology for “top-down” synthesizing biochar-iron based materials5,11. As a robust powder processing technique, it can alter the surface morphology, structure, and composition of both single and mixed materials to varying degrees. This includes reducing particle size to micron or nanometer scales as well as introducing oxygen-containing functional groups and surface oxidation. Multiple studies have proven that ball-milled biochar-iron based materials can effectively remove a variety of contaminants, including chlorinated hydrocarbons12, hexavalent chromium13, antibiotics14, and heavy metals15.
Previous studies have respectively reviewed and introduced the advantages, synthesis methods, characterization technology and removal properties of biochar-iron based composites against different types of pollutants10,16,17. This article focuses on the emerging technology of ball milling and provides a comprehensive analysis of its impact on the structure and composition of biochar-iron based materials, as well as its role in regulating material properties and elucidating the removal mechanism for various types of pollutants. The principle and foundation of ball milling technology have been reviewed, the latest progress in the preparation of iron-based biochar materials is introduced, and suggestions are made for the future application of high-performance materials for groundwater remediation prepared by ball milling.
Ball milling technology
As a practical method of mechanochemical action, ball milling was first proposed by John Benjamin in 197018. It can usually cause particle deformation, lattice defects, local hot spots on the surface and welding between materials during the process, ultimately resulting in products with sizes refined to the nanometer level19,20. Its technical advantage lies in its ability to enable or facilitate catalytic transformations that take place only poorly or not at all in solution21. Ball milling of elemental iron, carbon, or sulfur have been observed to generate new compounds without the need for additional solvents or harsh reaction conditions22,23,24. In organic synthesis, milling medium (milling tank and ball) can be employed as catalysts, exemplified by mechanochemical Sonogashira coupling conducted without a CuI co-catalyst by using a copper-based milling assembly25. This offers a potential avenue for the degradation of pollutants within the ball milling tank. In addition, remarkable stoichiometric control characteristics can be attained, that is, ball milling (mechanical synthesis) enables the direct formation of stoichiometrically distinct products through manipulation of the composition of the reaction mixture21. The reduction in the utilization of chemical reagents and the implementation of a rapid synthesis process enable more efficient energy consumption, resulting in a competitive cost advantage. Compared with traditional solution chemical reactions, ball milling provides a relatively closed, independent and solvent-free or solvent-less reaction environment, and realizes solid-solid and solid-liquid processing which are difficult to occur under conventional conditions26,27. Laboratory-level ball milling methods include oscillating ball milling and planetary ball milling. The former places a single larger milling ball in a small volume milling jar, which is horizontally fixed on the oscillating arm and swings at high speed in the horizontal direction to achieve collision milling. In the latter planetary ball mill, the jar is vertically mounted on the horizontal plane of revolution and keeps moving around the revolution axis while maintaining rotation. Different types of experimental milling jars can be placed vertically or parallel to the horizontal plane. Among the two, milling that imitates “planetary” motion can better simulate the operating principle of large ball mills used in industry, so it is of great significance in researching and simulating the properties of ball milled materials28.
The planetary ball milling process is controlled by a number of parameters, including milling rotational speed, milling time, and ball-to-material ratio, which all affect product performance. Most studies use fixed milling parameters to prepare single biochar or iron materials, and biochar-iron based composites, while a few studies explored preparation conditions with the aim of maximizing pollutant removal performance. Involving ball milling times ranging from 0.5 to 36 h, rotational speeds ranging from 300 to 3000 rpm, and ball-feed ratios ranging from 20:1 to 170:114,23,29,30,31,32,33. Lyu et al. compared the effects of iron salt-impregnated biochar in the milling range of 3 to 24 h and pointed out that the optimal ball milling time is 12 h based on the Ni(II) removal performance and the economic principles of the preparation process15. Wang et al. found that the hexavalent chromium removal performance of synthetic biochar-iron (Fe0) composites linearly increased with milling time during the longest milling period of 48 h31. The rotational speed used in planetary ball milling methods was mostly in the range of 300 to 500 rpm, while in a few literature mentioned the method of using 3000 rpm, and the ball-to-material ratio in the rang of 20:1 to 170:1 with 100:1 was the most commonly used12,23,24,34,35. A central composite experiment design for parameter optimization of ball-milled pine biochar compared 20 parameter conditions to obtain the optimal milling time of 1.6 h, rotation speed of 575 rpm and a ball-to-material ratio of 4.5:136. It is generally believed that higher ball milling speed, longer ball milling time, and larger ball-to-material ratio mean that more energy is delivered to the material particles to cause changes in the morphology and composition. However, a decrease in milling efficiency was found during continuous energy input, such as particle agglomeration caused by the formation of excessive surface energy, and the pollutant removal performance also decreased accordingly in this process37,38. At the same time, it should be pointed out that actual condition is often hard to meet extreme preparation parameters, and production efficiency needs to be taken into consideration to meet mass production.
In addition to ball milling parameters, other conditions during the preparation process also have an important influence, such as the atmosphere during milling, dry or wet milling environment and milling aids can impact on the properties of the ball milled product. In particular, atmospheric conditions can introduce the oxygen-containing functional groups on the surface of biochar and cause the oxidation of iron-based materials which lead to changes in material properties. Nitrogen is the most common atmosphere to ensure anaerobic conditions during the milling process. Xu et al. studied the effect of milling under air, nitrogen and vacuum conditions on material particles and pointed out that vacuum and nitrogen conditions are more conducive to the formation of materials with smaller particle sizes, but ultra-fine particles will lead to agglomeration and inhibit material properties39.
Propylene glycol, quartz sand and NaCl were added into the ball mill jars as milling aids, which were designed to reduce contact with oxygen during the milling process and accelerate the crushing of material particles23,29,37. In fact, it has been pointed out that in the traditional soild-soild milling process, the particles often formed the adhesion layer on the inner wall of milling jars or the surface of the milling balls, which was related to the introduction of defects or lattice deformation on the solid particles surface and the increase of the particles surface free energy27. The wet ball milling with liquid milling aid can improve this situation. Kotake et al. revealed that wet ball milling could obtain particles with smaller particle size than dry milling under the same conditions, and the viscosity of the slurry during the process determined the milling efficiency40. Li et al. investigated the reducing properties of ball milled zero-valent iron with water, ethanol, ethylene glycol and Na2S solution as milling aids, respectively, and found that the wet milling environment effectively alleviated the oxidation of zero-valent iron41. When milling with Na2S solution, a sulfide layer was formed on the surface of iron particles at the same time to enhance the electron transport ability and electron selectivity. However, in the high-speed milling process, the safety and use cost of aids must be considered, and whether there is the possibility of causing secondary pollution. The various milling conditions employed in the aforementioned literature are summarized in Table 1 to facilitate a more comprehensive and intuitive comparison.
It should be pointed out that most of the existing studies focus on the pollutants removal mechanism by ball milled products, while ignoring the impact of ball milling on the structure of the material itself. But the ball milling process always controls the pollutants removal behavior of ball milled materials in the form of a “black box”. The milling parameters used lack sufficient support, and there were certain contradictions in the conclusions of different studies. The dynamic accumulation properties and synergy between parameters of ball milling effect need to be systematically studied.
Performance of ball milling on biochar-iron based materials
The pretreatment of ball milled biochar-iron based materials includes: (1) impregnating iron salt solution; and (2) direct mixing. The former initially immerses the original biomass in an iron salt solution prior to pyrolysis, then the produced biochar followed by ball milling. The latter does not treat the biomass, and directly ball mill the biochar after pyrolysis. Obviously, the impregnation of iron salts involves additional steps of dissolution, impregnation, and drying, which also result in excess chemical residue. On the other hand, direct ball milling is a one-step method that can enhance the load capacity42. However, certain studies suggest that direct ball milling may hinder the adsorption of anionic pollutants due to the formation of numerous negatively charged acidic oxygen-containing functional groups during ball milling processing15,20,29,31,43. It is worth noting that there are no comprehensive studies comparing these two pretreatment methods. Nevertheless, it is undeniable that the properties of disparate pretreated products after ball milling differ significantly.
The physical properties of biochar-iron based materials after ball milling are reflected in the morphology, specific surface area, porosity and particle size. The performance of single raw material and mixed raw material differs after ball milling. Notably, the specific surface area of biochar has garnered significant attention in the field of environment or agriculture. A study was conducted to investigate the effects of ball milling biochar derived from three different biomass sources (sugarcane bagasse, bamboo, and hickory wood chips) after pyrolysis at temperatures of 300 °C, 450 °C, and 600 °C, respectively15. The results revealed that while ball milling had a limited impact on the surface area of high or low temperature pyrolyzed biochar, which significantly enhanced the surface area of biochar produced through medium temperature. Taking hickory wood chips biochar pyrolyzed at 300, 450, and 600 °C as an example, its surface area increased from 0.8, 9.8 and 222 m2·g−1 to 5.6, 309 and 270 m2·g−1 after ball milling. Wang et al. observed the same phenomenon with corn straw biochar pyrolyzed at 300 and 500 °C, the specific surface area of 700 °C pyrolyzed biochar decreased after ball milling31. The study by Li et al. showed that the surface area of biochar increased after ball milling, while that of activated carbon decreased34. Iron nanoparticles have been widely utilized in various fields such as catalysis, magnetism, and adsorption due to their elevated surface atomic ratio44. In this context, ball milling emerges as a pivotal technique for the fabrication of micro-nano iron materials. Zhang et al. obtained zero-valent iron particle with a size of 20 nm after 8 h milling, and the surface area of 39.00 m2 ∙ g−1 was a key factor for efficient dechlorination38. Selvaraj and Xu et al. only obtained iron particles of 0.49 and 2.10 m2 ∙ g−1 after milling for 3 and 10 h, respectively45,46. Fan et al. successfully achieved a reduction in the size of iron particles from 30 μm to 5 μm flakes through 15 h of ball milling47. The inherent ductility of zero-valent iron led to its fragmentation after initially forming thin sheets during the ball milling process. Furthermore, previous research has reported an almost linear decrease in particle size with increasing milling time38. However, another study observed a gradual decline in the rate of size reduction, suggesting the existence of a minimum limit for particle size45. It can be observed that the physical properties of iron-based materials exhibit inconsistency after ball milling, whereas alterations in surface area and porosity following biochar ball milling are found to be correlated with pyrolysis temperature, which will be elaborated upon below.
As previously stated, the pretreatment techniques of biochar and iron-based composites will yield distinct impacts on the material’s physical characteristics. Due to the great difference in the surface properties of the original materials, the biochar obtained by pyrolysis after impregnation with iron salt mainly reflects the properties of biochar during the physical modification process of ball milling. Direct co-milling with iron-based materials is more complicated. The schematic diagram of the two pre-processing methods is depicted in Fig. 1.
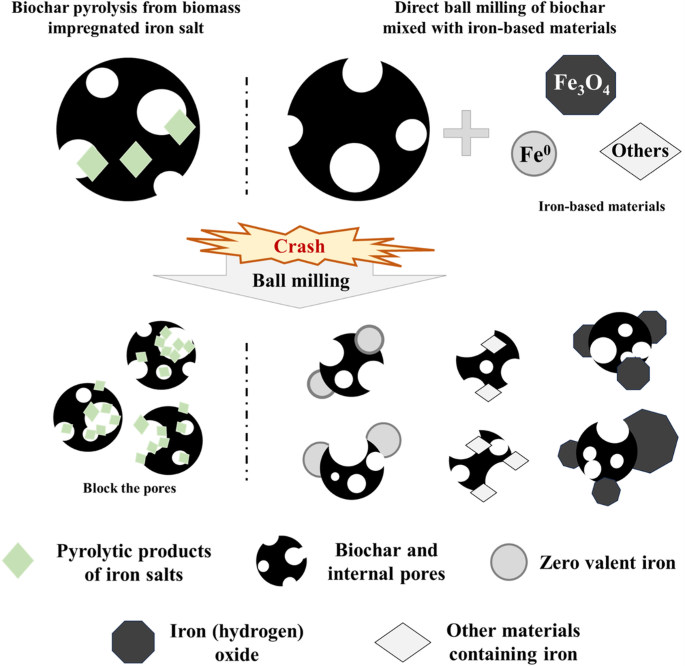
Ball milling effect of different biochar-iron based materials.
After ball milling biochar obtained by pyrolyzing corn straw impregnated with FeCl3 at 800 °C, Zhang et al. discovered that the specific surface area and pore volume were 3.77 and 1.98 times those before ball milling, respectively35. Feng et al. found that the biochar pyrolyzed at 600 °C with a 2:1 mass ratio of hickory chips to FeCl3 during impregnation had the best removal performance in removing reactive red after ball milling29, and the surface area of the original carbon increased from 24.9 to 48.3 m2·g−1. However, this increment was entirely realized by the expansion of external surface area, accompanied by a decrease in porosity after milling. Two studies using biochar and activated carbon co-ball milling with magnetite to prepare magnetic adsorption showed that milling would destroy or block the original pores of activated carbon, while promoting the opening of biochar pores (especially for micropores)14,34. In addition, biochar can be used as a carrier for iron-based materials to alleviate the agglomeration of iron after ball milling11. In fact, this effect can be summarized as the “carrier effect”. Ultrafine particles provide a larger surface for the load to bind, which can effectively alleviate the stacking and agglomeration, and this concept has been preliminarily applied in the study of ball milling biochar loaded hydrotalcite48.
It has been observed that there seems to be a certain correlation between the specific surface area and porosity of biochar-iron based materials and the pyrolysis temperature of biochar, which has been abstracted into a schematic shown in Fig. 2. Furthermore, due to notable disparities in content or initial surface area between the two components, the surface area properties of the composites are predominantly determined by biochar. Specifically, pyrolysis within the medium temperature range (approximately 450 °C) proves more favorable for augmenting the specific surface area and porosity of biochar subsequent to ball milling, whereas low-temperature or high-temperature pyrolysis does not exhibit significant alterations or is impeded after ball milling.
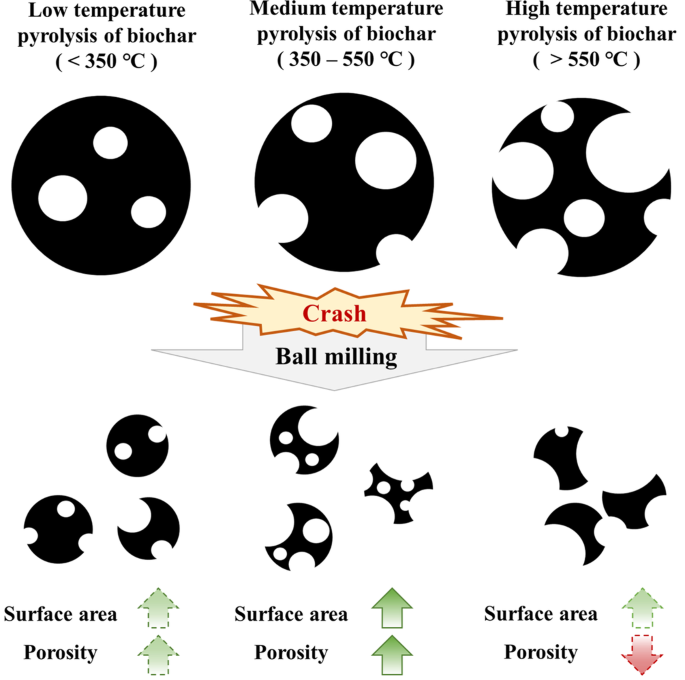
The performance difference of biochar with different pyrolysis temperature in ball milling process.
A speculation suggests that the observed disparity is likely attributed to the mechanical characteristics of biochar, an aspect that has received limited attention within conventional fields like agriculture or environment49. The raw biomass is rich in lignocellulose with a honeycomb structure, which is preserved throughout the pyrolysis to confer a certain of strength and stiffness upon the resulting biochar49,50,51. Elevated pyrolysis temperatures promote improved crystallinity and carbonization, thereby enhancing the modulus of biochar49,52,53. However, at a medium temperature of 450 °C, there are limitations in terms of both crystallinity and carbonization, and the formation of open pores due to volatile gas and carbon defects that result in decreased structural integrity49,54. The study of Kumar et al. showed a similar trend55, which the crushing and impact strength of acacia and eucalyptus biochar experienced a decline until reaching a minimum point at 600 °C before, and subsequently increasing. Therefore, the enhancement of biochar’s specific surface area and porosity with the medium pyrolysis temperatures following ball milling can be attributed to its mechanical characteristics. It is imperative to conduct a systematic investigation into the interplay between mechanical properties and ball milling. The advantage of the complex is also reflected in the change of the surface charge and polarity of the material. The incorporation of iron-based materials will enhance the surface electronegativity and electrostatic interaction, thereby profoundly affecting the processes of adsorption and complexation14,15,29,38.
In addition, it is noteworthy that the study conducted by Maziarka et al. has highlighted a potential error in the commonly employed traditional method for specific surface area testing of pyrolyzed biochar at 650 °C, which utilizes N2 as an adsorbent and the BET model56. Similar studies were summarized in the review by ref. 57. The presence of micropores within the material significantly influences its surface area, particularly when N2 was utilized as an adsorbent. There is no doubt that this may also affect the results obtained in surface area tests of biochar-iron based materials after ball milling. Using CO2 as an adsorbent and employing a DFT model for isotherm calculation appears to offer a viable solution to rectify this error, thereby holding significant importance in accurately comprehending the material properties pre- and post-ball milling.
The chemical properties of biochar and iron-based materials are primarily manifested through phase changes (such as surface oxides and new components) and alterations in surface functional groups. The occurrence of frequent high-energy collisions provides a sufficient energy foundation for reactions, particularly to complete solid-solid reaction that are typically challenging to achieve via traditional solution-based reactions.
During ball milling of biochar, there are typically no restrictions on the milling atmosphere, in contrast to the strict inert gas protection required for iron-based materials. This disparity can lead to alterations in the types and quantities of oxygen-containing functional groups present on the surface of biochar, ultimately affecting its surface charge properties, pH levels, and pollutant removal capabilities. It has been demonstrated that the elevated temperature leaded to a reduction in the quantity of functional groups present on the biochar surface3,58,59. Simultaneously, multiple reports have indicated that ball milling can enhance the content of surface functional groups in biochar produced through pyrolysis at any given temperature15,33,36. Lyu et al. determined the functional groups by Boehm titration and found that the content of total acidic functional groups on the surface of bagcane biochar increased from 0.8 to 2.5 mmol·g−1 after ball milling, including carboxyl group, phenolic hydroxyl group and lactone15. Zhang et al. demonstrated that the dominant functional groups of rice straw biochar changed from carboxyl groups to phenolic hydroxyl groups after ball milling, and the total number of acidic functional groups increased35. The reason for this change may be due to the introduction of oxygen during the milling process, or to the exposure of the original oxygen-containing functional groups inside the biochar accompanying the formation of new surface39. The simultaneous occurrence of a lower pH in biochar is also believed to contribute to this phenomenon, enhancing particle polarity (hydrophilicity) and augmenting the negative surface charge. Additionally, the milling process further enhances the development of amorphous carbon and graphite structures within biochar, with the latter believed to facilitate efficient electron transport31,38.
As for iron-based materials, the ball milling process inevitably leads to the oxidation of iron, reducing the crystals size and forming cell defects. Several researches have shown that long-term ball milling can cause the conversion of α-Fe to γ-Fe2O3 and then continue to be converted into α-Fe2O3, as well as the transformation process of α-FeOOH to α-Fe2O338,60. However, Zhang et al. observed an increase in the α-Fe signal during the milling process and attributed it to the mechanical activation effect. The surface oxide shell was stripped away to expose the fresh iron core inside38. Unfortunately, there is a dearth of systematic research on the phase transition of iron during the milling process, and it is generally inferred that inadequate or excessive energy input leads to alterations in material properties20.
In the preparation of composite materials, a number of studies using carbon materials including biochar, activated carbon, graphite, carbon nanotubes and iron powder co-ball milling have pointed out that there are crystalline or amorphous FexC phases in the composites23,61,62,63. These ingredients are believed to have well electron transport capabilities. Tang et al. conducted direct ball milling of pine biochar with FeS2 to synthesize iron-carbon-sulfur ternary composites, and highlighted the role of biochar in inhibiting the agglomeration of FeS2 while promoting its exposure30. This enhanced exposure was identified as crucial for improving the reduction and removal efficiency of hexavalent chromium. Ai et al. also provided similar conclusions by preparing composites based on sulfur powder, zero-valent iron powder and biochar22. In addition, Qian et al. prepared four materials, zero-valent iron, zero-valent iron with biochar, zero-valent iron with sulfur, and zero-valent iron with biochar and sulfur, respectively by ball milling, and analyzed the effects of the three components by comparing the reduction performance for cis-dichloroethylene and vinyl chloride12. Among them, zero-valent iron serves as an electron donor, and biochar promotes the corrosion of iron and increases the surface FeS content, which can improve the electron transfer capability and selectivity of the composites. The alterations of biochar and biochar-iron based composites after ball milling are summarized in Fig. 3.
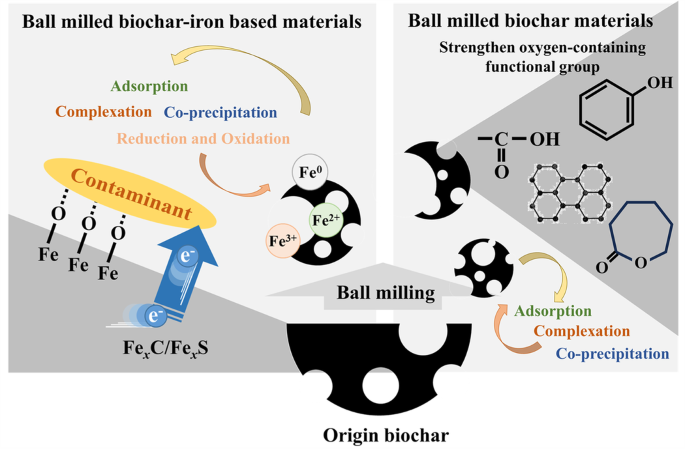
Modification of biochar-iron based materials by ball milling.
Structure-function relationship between ball-milled biochar-iron based composites
Retrospect the applications of biochar-iron based materials prepared by ball milling in the past, primarily in physical/chemical adsorption, chemical reduction, and catalytic oxidation. This section will delve into the distinct synergistic effects exhibited by biochar, iron-based materials, and their composites across various scenarios.
Physical/chemical adsorption is a crucial mechanism for biochar-iron based materials to carry out environmental remediation, enabling the efficient removal of various environmental pollutants such as heavy metals, dyes, antibiotics, and excessive phosphate. Ball milling significantly enhances the surface area of biochar and improves its pore structure in certain studies, thereby providing a larger reactive surface area. Furthermore, the increases of surface acidic oxygen-containing functional groups leads to the formation of hydroxyls, phenols, and lactones; while aromatic C = C/C = O and C-H bonds strengthen π-electron interactions14. On the one hand, it functions as the active reaction site for potential reactions. On the other hand, alterations in surface electronegativity and polarity also impact the material’s performance. The magnetic properties of iron-based materials facilitate easier separation of particles from environmental media, thereby reducing operational costs in remediation systems; however, this may lead to pore clogging in biochar and a subsequent decrease in surface area34. While iron oxides can adsorb pollutants to some extent, they are primarily exercises by biochar29. Furthermore, iron-based materials contribute to further reduction in particle electronegativity. Shan et al. achieved biochar/activated carbon-Fe3O4 composite through ball milling to avoid the formation of Fe2O3 which yields materials with relatively low magnetism caused by heating in other synthesis methods, and the carbamazepine adsorption capacity by the composites dropped from 89.6 mg·g−1 of the original coconut shell biochar to 62.7 mg·g−1 14. Li et al. also used Fe3O4 co-milling to impart magnetism and found that the methylene blue adsorption by ball milled biochar increased by 14 times for biochar34, but the ball milled magnetic biochar was only about 7 times better64. The reason for this change was that Fe3O4 occupies a large portion of the weight of the magnetic biochar but has no adsorption properties. Ball milling promoted the formation of extremely fine nanoparticles from biochar, promotes adsorption kinetics and external mass transfer rate. In the related reports, the adsorption capacity of CH3Hg+ increased from 8.21 to 104.9 mg·g−1 of the ball milled biochar65. Biochar rich in N-functional groups was successfully prepared by co-milling biochar with NH4·OH, resulting in an increased alkalinity of biochar and the N-containing functional groups66. The modification effectively improved the adsorption capacity of acidic CO2. The ball milled biochar is immobilized in calcium alginate by Wang et al. to strengthen the surface oxygen-containing functional group and prevent potential secondary contamination of nano-scale materials67. The maximum Cd(II) adsorption capacity estimated with Langmuir isotherm modeling was 227.1 mg·g−1. The maximum removal amount of Ni(II) after sugarcane bagasse biochar ball milling reached 236 mmol·kg−1, which was much higher than the original 29.6 mmol·kg−1 15. Ai et al. reported that the surface hydroxyl groups of biochar zero-valent iron composites formed O-H-P bonds after the removal of phosphate, and the adsorption was mainly through ligand exchange and achieves more than 12 times improvement22. The change in removal amount controlled by pH indicated that surface functional groups participated in the reaction.
Biochar-iron-based materials are highly effective reductive agents and possess excellent capabilities for removing oxidizing pollutants, such as hexavalent chromium, As(V), and chlorohydrocarbon. Although biochar is generally not considered to be reductive, its substantial surface area allows it to serve as a carrier for iron-based materials, and enhances the enrichment of contaminants on the particle surfaces. The high-temperature pyrolysis promotes the formation of a graphite structure within biochar, which can be further reinforced through ball milling to expedite electron transport as a bridge between iron-based materials and contaminants. Iron-based materials play a crucial role in providing electrons necessary for contaminant reduction during this process. Furthermore, ball milling facilitates the formation of a composite consisting of biochar and iron-based material, resulting in the development of a primary battery that expedites the corrosion of the iron and promoting the production of possible key active components. Zou et al. used ball milling to expand the surface area of biochar-iron based composites to 121% before ball milling, and improved the pores, rapid intramolecular diffusion and electrostatic interaction to increase Cr removal by more than 2 times33. The Fe(II) signal disappeared after the reaction and Cr(III) might precipitated on the particle surface in the form of CrxFe1−x(OH)3 or returned to solution. Sun et al. prepared a ternary composite of FeS2, zero-valent iron, and pine wood biochar to achieve a removal capacity of hexavalent chromium up to 81.5 mg·g−1, which was much higher than the removal capacity of a single component68. The removal rates of cis-dichloroethylene and vinyl chloride significantly increased from almost ineffective to 62.0% and 67.7%, respectively, after adding biochar-iron (zero-valent iron)-sulfur composite materials12. Ball milling achieves a composite effect that is difficult to achieve by simple mixing during the collision and compaction process. On the one hand, it provides a stable electron transport channel, and on the other hand, it promotes the formation of graphite structure carbon to reduce the resistance of electrons in the transfer process. The removal mechanism of above pollutants by biochar-iron based composites is illustrated in Fig. 4.
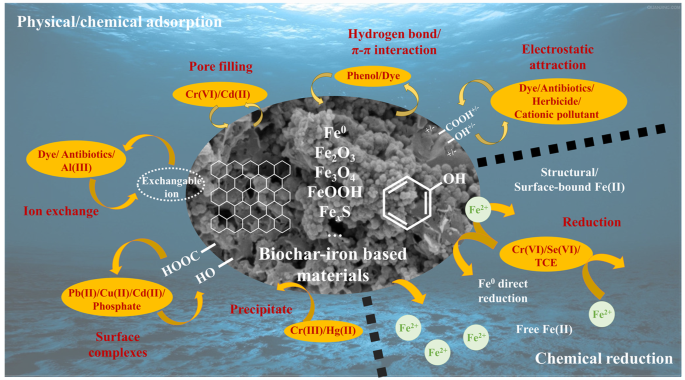
Removal mechanism of pollutants from biochar and iron based materials by ball milling.
Ball milling of biochar-iron based materials offers advantages in catalytic oxidation due to their excellent electron transport properties and functional components. This facilitates successfully activated oxidants such as persulfate, peroxymonosulfate, and peroxydisulfate enabling efficient removal of high-risk organic pollutants including phenol69,70, Bisphenol A71, tetracycline72, and sulfadiazine73. The removal mechanism of organic pollutants through the catalytic oxidation of biochar-iron based composites is illustrated in Fig. 5. A higher degree of graphitization is achieved through ball milling to enhance the electron transport ability in both radical and non-radical pathways. This process also results in an increased abundance of oxygen-containing functional group, defect structures, and excellent specific surface area, thereby improving the activation efficiency of oxidizers. The iron-based materials serve as crucial transition metals for catalytic oxidants, encompassing structural, surface-bound or free Fe(II) that play a dominant role in catalytic oxidation. The formation of composite materials combining biochar and iron-based materials effectively mitigates the agglomeration phenomenon of a single material during ball milling and ensures good dispersion. Additionally, this combination exhibits a synergistic effect on adsorbing pollutants and oxidants while accelerating electron transfer and iron-based materials corrosion processes. For instance, the utilization of ball milling on biochar and α-FeOOH demonstrated remarkable efficacy in phenol removal with a capacity of 217 mg·g−1, while maintaining exceptional stability even after four consecutive cycles69. Qu et al. successfully prepared biochar loaded with FeS through ball milling70. The system exhibited rapid and complete degradation of phenol along with excellent adaptability and anti-interference performance across a wide pH range. Moreover, the magnetic biochar-Fe3O4 composite material achieved an impressive 96.73% degradation rate for Bisphenol A in the reaction system by employing both free radical and non-free radical pathways, surpassing the removal rates of single biochar (6.98%) or Fe3O4 (21.59%)71. Similarly, another magnetic biochar-FeS composite fabricated via ball milling employed biochar as an electron transport medium and utilized surf-bonded Fe(II) and S(II) to achieve tetracycline degradation within just 30 min72, yielding comparable results as reported by ref. 72.
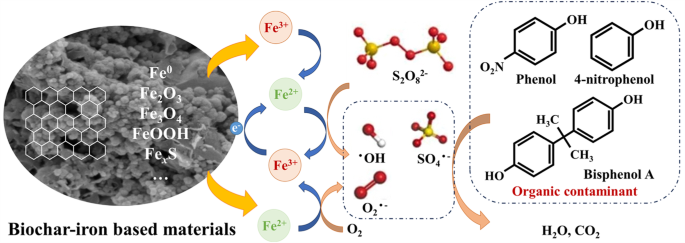
Catalytic oxidation of biochar-iron based materials with activated persulfate as an example.
Conclusion and outlook
Different strategies are taken to remove different types of pollutants for biochar-iron based composites. In this paper, the roles of the two materials and the ball milling process were summarized. Among them, the biochar undertake the function of adsorption, carrier, and electron transport channel. Iron based materials play the role of providing magnetism, introducing key functional components and electron donors. In addition to achieving two material composites, the ball milling has a comprehensive effect in changing the surface appearance of the material particles, the phase composition, and the surface nature. It is noted that the presence of elements such as N, P, S, and other compounds has been observed to have a positive impact on the milling process. This involves a more flexible application of milling products and ball milling methods and should be summarized in future studies. The unclear fields in the existing research has been revealed, including the effect of ball mill parameters on different biochar or iron-based materials, also the connection between pyrolysis temperature and ball milling effect. Finally, the behavior of evaluating the entire life cycle of the material and the corresponding economic benefits are also the key of the application of ball milling synthetic materials. The large-scale production of functional ball milling biochar-iron based materials for environmental remediation still encounters numerous challenges. Moreover, it is crucial to actively explore the application of ball milling technology in soil and groundwater remediation, such as a reactor for pollutant degradation, rather than solely as a synthesis tool for remediation materials. To provide a novel and convenient solution for the corresponding repair needs. This will provide an innovative and convenient solution to address the corresponding repair requirements.
Responses