Revitalizing the heart: strategies and tools for cardiomyocyte regeneration post-myocardial infarction

Introduction
Heart Failure (HF) is the inability of the heart to pump sufficient blood across the body to meet tissue requirements. Slow health decline poses a significant challenge for patient management and healthcare costs. Although the number of new HF cases in industrialized countries is stabilizing or even decreasing, the overall number of people living with HF is increasing. This is because multiple life-saving treatments support long-term survival and at the same time, the population is aging1.
A leading cause of HF is myocardial infarction (MI), where disrupted coronary blood flow leads to heart muscle death due to oxygen deprivation. Despite various treatments and interventions improving heart function and patient outcomes, they fail to address the root cause: adult mammals, including humans, have a limited ability to generate new cardiomyocytes. This results in permanent loss of millions of these cells. While some treatments can improve outcomes either by increasing cardiomyocyte functionality or supporting compensatory growth, it does not restore the original cell number2. By contrast, various animal species, particularly lower vertebrates such as zebrafish, neonatal mice, opossums, and even 2-day-old pigs, show remarkable self-regenerative capabilities3,4,5. These important discoveries led to a surge in research focused on cardiac regenerative medicine. This holds promise for developing new treatments that could potentially repair damaged hearts in individuals with cardiovascular disease, offering perspectives for more effective treatments or even cures for HF.
Current therapeutic strategies for MI and their caveats
Treatments for ischemic heart disease include revascularization of chronically obstructed coronary arteries (by intracoronary stents or bypass surgery), prompt reperfusion of acute myocardial infarction using emergent angioplasty or thrombolytic therapy and pharmacological compounds, all of which aim to protect the heart against further damage. Moreover, electrical, or mechanical support therapies, such as cardiac resynchronization and left ventricular assist devices (LVADs), respectively, have significantly improved the quality of life of end-stage HF patients. In some cases, reducing the load on the heart can have a regenerative effect6. However, heart transplant remains the only definitive solution and this is limited by the scarcity of donors7.
Cell therapy emerged as a potential solution to replenish the pool of functional cardiomyocytes. Various cell types have been tested, from non-cardiac cells harvested from skeletal muscle, bone marrow, adipose tissue or Wharton’s jelly of umbilical cord to cardiac-committed cells isolated from myocardial biopsy specimens or differentiated from pluripotent stem cells8. Although myocardial “regeneration” was the historical objective of stem cell therapy, there is now a consensus that at most, this therapy supports some degree of cardiac repair through indirect (paracrine signalling) mechanisms (such as limiting inflammation, fibrosis or increasing angiogenesis) but has overall failed to trigger the generation of new functional and electro-mechanically coupled cardiomyocytes (reviewed by Menasché et al. 8,9,10).
Another approach has involved direct in situ reprogramming, or transdifferentiation, of somatic cells into cardiomyocytes. Reprogramming cardiac fibroblasts (CFs) into cardiomyocytes could improve heart contractility while reducing cardiac fibrosis. Studies in mice have shown that this cardiac reprogramming in vivo can be as efficient as that in vitro (10–15%)11 or result in lower percentages of reprogrammed cells12, but still yield comparable improvements in cardiac function and scar size reduction. However, these studies used retroviruses to introduce the required transgenes into the infarct region, a method generally regarded as unsafe for clinical practice. Furthermore, translating these findings to humans has proven challenging primarily due to low reprogramming efficiency and different, as well as more, reprogramming (transcription) factors required for human CFs than for mouse11,12. Subsequent research has focused on improving the efficiency of cardiac reprogramming in human cells. This includes optimizing the overexpression of key transcription factors and cardiogenic genes, as well as adding miRNAs or using non integrative approaches such as small molecules. Efficiencies of up to 10% have been reported as a result13,14,15. Another major challenge is fine-tuning the regulation of fibroblast conversion so that neither too many nor too few cardiomyocytes are formed to ensure both safety and efficiency.
An alternative direct strategy would be to leverage the inherent capacity of cardiomyocytes to divide during fetal development and possibly just after birth, restoring this activity in adulthood in a controlled manner16,17,18. This cardiac regeneration approach is based on three pillars: firstly, the adult mammalian heart possesses the necessary cellular machinery for cardiomyocyte division; secondly, this potential for division is lost soon after birth; and finally, it is technically feasible to reactivate this dormant process by modulating pathways involved in cardiomyocyte proliferation18.
Potential molecular therapeutics for cardiac regeneration
Despite the challenges of developing regenerative strategies for the adult mammalian heart, considerable progress has been made through various approaches including oxygen level modulation, epigenetic regulation, hormone administration, and regulation of cell-cycle or proliferative signalling pathways (Fig. 1; Supplementary data 1, an online version is available at https://abois.shinyapps.io/table/).
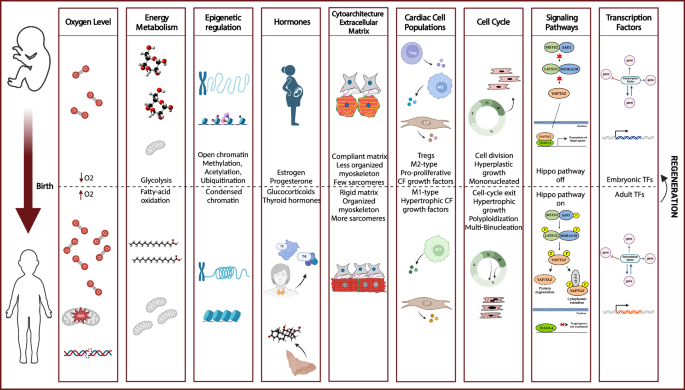
The transition from fetal to adult stages encompasses several changes, including modulation of oxygen levels, shifts in energy metabolism, regulation by epigenetic factors and hormones, modifications in cytoarchitecture and extracellular matrix components and rigidity, adjustments in cardiac cell populations, control of the cell-cycle, activation or deactivation of signalling pathways, and the roles played by transcription factors. The understanding of this fetal-to-adult cardiac transition has highlighted numerous potential molecular strategies for cardiac regeneration. Created in BioRender. Axelle, B. (2021) BioRender.com/n07c558.
Oxygen level modulation
During fetal development, the uterine environment provides much lower oxygen levels (hypoxemia), than after birth, when the lungs start to function and expose the newborn to ambient oxygen levels (normoxia). This dramatic shift activates oxidative mitochondrial metabolism, leading to the production of reactive oxygen species (ROS), and oxidative DNA damage impacting the regenerative capacity of cardiomyocytes19.
Research by Kimura and colleagues revealed that adult cardiomyocytes capable of division exhibit an “hypoxic signature” and characteristics similar to those seen in the oxygen-poor environment of neonatal cardiomyocytes20. Furthermore, infarcted mice exposed to hypoxemia for one week showed less myocardial fibrosis, improved left ventricular systolic function and more importantly a significant regenerative response compared to non-hypoxic mice21. Using intermittent normobaric hypoxia-hyperoxia training (IHHT) to treat patients with coronary artery disease resulted in lowered blood pressure, reduced glycemia and enhanced left ventricular ejection fraction22. Factors implicated in the regulation of oxygen levels and redox homeostasis like Peroxisome proliferator-activated receptor delta (PPARδ), Hypoxia-inducible factor 1-alpha (HIF1α), Pitx2 and Tnni3k have been shown to have a pro-proliferative effect and can potentially be modulated to achieve heart regeneration23,24,25,26.
Energy metabolism
One hypothesis on why mammalian cardiomyocyte renewal is limited is that metabolism shifts from glycolysis to oxidative phosphorylation postnatally. Neonatal CMs remained proliferative up to 14 days after feeding mice with fatty acid-deficient milk27. Moreover, elevating glucose relative to fatty-acid oxidation, induced by a conditional pyruvate dehydrogenase kinase 4 (PDK4) knockout in mice, decreased DNA damage and enhanced proliferation, while inhibiting fatty acid oxidation enabled heart regeneration in adult mice27,28.
Interestingly, single-cell RNA sequencing revealed that proliferative cardiomyocytes are characterized by upregulated glycolysis and decreased mitochondrial DNA29. Similarly, a cardiomyocyte-specific modified RNA (modRNA) encoding pyruvate kinase muscle isoenzyme 2 (Pkm2), an enzyme that facilitates anabolic glycolytic pathway, such as the pentose phosphate pathway led to cardiomyocyte proliferation, improving cardiac function, and reducing scar size following MI30. Additionally, inhibiting glycogen synthase kinase 3β (GSK-3β), a regulator of Wnt signalling involved in glucose homeostasis, has been shown to enhance cardiomyocyte proliferation post-MI31,32.
Exploring beyond classical metabolic pathways, it has been demonstrated that ceramide synthesis is upregulated in cardiomyocytes 24 h post-MI. Modifying ceramide metabolism through the delivery of a modRNA encoding acid ceramidase (AC), induced cardioprotection post-MI33. Activating the serine synthesis pathway, another metabolic route, also using a modRNA encoding phosphoserine aminotransferase 1 (PSAT1) inhibited oxidative stress, stimulated the proliferation of cardiomyocytes, resulting in improved cardiac function34. Furthermore, modulating the mevalonate pathway, which plays a critical role in cell division, induced cell-cycle reentry in human cardiac organoids (hCO) both in vitro and in vivo. Conversely, inhibiting this pathway with a statin in hCO or blocking succinate dehydrogenase with the competitive inhibitor malonate in vivo attenuated its pro-proliferative effects35,36.
Epigenetic regulation
Distinct transcriptional networks in neonatal and adult muscle cells govern cell-cycle transitions and metabolism37. Epigenetic mechanisms are critical for the transition of cardiomyocytes from neonatal to adult stages, affecting cell-cycle transitions and metabolism. The ability of fully matured cells to divide is often hindered by changes in chromatin compaction and the regulation of cell-cycle genes37,38. Research has highlighted how dynamic changes in DNA methylation patterns, from the addition of methyl groups in embryonic stem cells to the modulation of these processes in adult cells, can influence heart development and regeneration39. Removing hypermethylated markers, downregulating methyltransferase (Dnmt3a), and ubiquitin ligase (Cbl, and Itch), demethylase overexpression (ALKBH5), loss of methyltransferase (METTL3) or activation of the chromatin-remodeling protein Brg1 have all been explored to promote heart regeneration39,40,41,42,43,44,45,46. Additionally, the interplay between enhancers and repressors fine-tune gene expression for cellular responsiveness and is essential for response to damage. For instance, zebrafish-derived tissue regeneration enhancer elements (TREEs) delivered by AAV directed pro-regenerative gene expression in injured cardiac tissues in mice and pigs47. This showcases the potential of transferring regenerative capabilities across species since these processes are conserved.
Non-coding RNAs are gaining recognition for their diverse roles in regulating gene expression, cellular development, and various aspects of physiology. Long non-coding RNAs (lncRNAs), for example AZIN2-sv and GAS5, influence cardiomyocyte proliferation post-MI by acting as miRNA “sponges”48,49,50 while Long Intergenic noncoding RNA predicting CARdiac remodeling (LIPCAR) has been highlighted as a valuable diagnostic and prognostic marker for cardiac conditions51. On the other hand, circular RNAs (circRNAs) can regulate cardiac function as exemplified by the beneficial effects of AAV-mediated cardiac overexpression of circFndc3b in post-MI hearts that stimulated neovascularization, decreased cardiomyocyte apoptosis, and improved cardiac function52.
Some features of cell therapy have been replicated using extracellular vesicles rather than cells themselves; certain benefits have been linked to specific miRNAs being delivered by the exosomes. Thus, exosomes containing miRNAs, such as miR-210, showed cardioprotective effects resembling those reported for cell-based therapies, while the administration of miR-146a emulated benefits reported for cardiosphere-derived cell (CDC) exosomes53,54. These outcomes led to clinical trials exploring miR-199a and the miR-17-92 cluster to induce cardiomyocyte proliferation, illustrating the potential of miRNAs in cardiovascular regenerative medicine55,56,57.
Cytoarchitecture and extracellular matrix control
Postnatal cardiomyocyte development involves critical structural maturation, shaping cardiac adaptability after birth. The concomitant increase in cardiomyocyte size, sarcomeric transitions, marked by aligned Z-lines and isoform shifts herald the loss in regenerative potential, revealing an intimate link between cellular architecture and proliferative capacity58. Simultaneously, variations in extracellular matrix stiffness decisively influence cardiomyocyte behavior, as compliant microenvironment supports dedifferentiation and cytokinesis by influencing the organization of the myoskeleton59.
Mechanical signalling pathways, intricately governed by cytoskeletal components and gatekeeper proteins, tightly regulate cardiomyocyte proliferation, as demonstrated by the cardiac regeneration and reduced mitochondrial content that follow prolonged mechanical unloading6. The role of the extracellular matrix has further been highlighted by Tzahor et al., who showed that the extracellular matrix protein agrin promotes heart regeneration in mice and improves cardiac repair in pigs60,61.
Exploring connections between the assembly and disassembly of the cytoskeleton with the regulation of cell division could lead to innovative strategies that would stimulate cardiac regeneration.
Hormone administration
Studies across species highlighted the significance of endocrine signalling as a key regulator of cardiomyocyte proliferation following injury. During the prenatal stage, an increase in glucocorticoids prepares the fetus for postnatal life. This hormonal surge is accompanied by an increase in glucocorticoid receptors (GR) in early postnatal cardiomyocytes, which play a crucial role in heart development. In line with this, the ablation of GR triggers cardiomyocyte proliferation after MI suggesting potential regenerative capabilities62,63.
Interestingly, research in non-mammalian species highlighted the role of cortisol. In zebrafish heart regeneration, for example, stress-induced cortisol secretion limited tissue replacement; this was replicated with pharmacologically-induced stress64.
Sex steroids contribute to heart repair, as evidenced by gender-related cardiovascular differences. Heart regeneration is influenced for example by the induction of estrogen as an inflammatory response to cardiac injury, while progesterone enhances both neonatal and adult cardiomyocyte proliferation through the upregulation of yes-associated protein (YAP) expression65,66.
The decline in mammalian heart regenerative capacity involves cardiomyocyte cell-cycle arrest and polyploidization. Across 41 species, diploid cardiomyocyte abundance inversely correlates with metabolic rate, body temperature, and thyroxine levels. In mice, adult cardiomyocytes with defects in thyroid hormone receptor activation retain significant proliferative and regenerative potential, while administering external thyroid hormones impedes zebrafish heart regeneration67,68,69.
These findings reveal the intricate ways in which hormones influence heart repair and regeneration, offering potential therapeutic targets for recovery of the heart following injury.
Interactions between cardiac cell populations
The adult heart is composed of various cell types, with cardiomyocytes, making up about 30% of the total cell population70.
Paracrine signalling between the endocardium and epicardium is necessary for proper development of the heart; this involves the Wnt, fibroblast growth factor (FGF), and retinoic acid signalling pathways, among others70,71,72. While intracoronary administration of rFGF2, for example, to patients resulted in short-term symptomatic improvement, exercise tolerance was not significantly enhanced73,74. Conversely, in a pig model of ischemia-reperfusion, intramyocardial injection of microparticles containing both neuregulin 1 (NRG1) and FGF1 enhanced left ventricular function by promoting angiogenesis and reducing ventricular remodeling75. Additionally, injecting recombinant NRG1 alone in adult mice induced cardiomyocyte cell-cycle activity and promoted myocardial regeneration. This has led to a phase I, single ascending dose study of Cimaglermin Alfa (Neuregulin 1b3) intravenously administered in patients with systolic dysfunction and HF (NCT01258387)76,77; this resulted in a dose-dependent improvement in left ventricular ejection fraction lasting 90 days following infusion77. Moreover, in a similar model the sustained delivery of Insulin-Like Growth Factor-1/Hepatocyte Growth Factor through an ureido-pyrimidinone (UPy) hydrogel stimulated endogenous cardiac repair78. To highlight potential pro-proliferative epicardial-derived signals, Ieda et al. co-cultured primary mice embryonic or adult CFs together with cardiomyocytes. Remarkably, embryonic CFs stimulated cardiomyocyte proliferation through the secretion of fibronectin, collagen, and heparin-binding EGF-like growth factor (HBEGF), whereas adult CFs induced a hypertrophic response79.
To counteract this fibrotic response, the effects of various growth factors have been studied. For example, recombinant human bone morphogenetic protein 7 (BMP-7) or a pigment epithelium-derived factor (PEDF) inhibitor of the endothelial-mesenchymal transition showed promise in attenuating fibrosis and ameliorating cardiac function post-myocardial injury80,81.
Angiogenesis is crucial for cardiac regeneration, as new vessels supply the heart with nutrients and oxygen82. This principle was demonstrated through the administration of synthetic mRNA encoding vascular endothelial growth factor A (VEGF-A), known as AZD8601, which enhanced the regenerative response following injury by promoting angiogenesis. Consequently, a phase 2a clinical trial assessing the safety, tolerability, and efficacy of epicardial injections of AZD8601 in patients undergoing coronary artery bypass grafting commenced in 2018 (NCT03370887). At the 2021 American Heart Association scientific meeting, the trial was reported to have met its primary endpoint of safety and tolerability but efficacy outcomes were not significant83. Furthermore, the results were difficult to interpret because of the confounding effect of the concomitant coronary artery bypass. AstraZeneca has chosen not to proceed with this treatment84,85.
The immune system plays a dual role in maintaining cardiac homeostasis and facilitating repair. Type 1 monocyte-derived macrophages (M1) are cardioprotective by promoting fibrosis during the early stage of cardiac repair, while type 2 resident cardiac macrophages (M2) act at later stages by promoting cardiac remodeling and angiogenesis86. Injecting regulatory T cells (Tregs) reduced infarct size and increased the number of proliferating cells, while the transfer of adult IFN-γ-producing T-cells into neonates contributed to impaired cardiac regeneration and promoted irreversible structural and functional cardiac damage87,88,89. Depleting B cells in adult mice suppressed tissue inflammation, inhibited myocardial fibrosis, and improved cardiac function90.
Another promising direction to consider is intervening in cardiac innervation. In neonatal mice, a chemical sympathectomy impaired cardiac regeneration, indicating the crucial role of sympathetic innervation in neonatal regenerative capacity91.
Direct regulation of the cell-cycle
The precise regulation of cardiomyocyte cell-cycle dynamics relies on the interplay among cyclins, cyclin-dependent protein kinases (CDKs), CDK inhibitors (CKIs), and CDK-activating kinases (CAKs). Efforts to stimulate cardiac regeneration have focused on manipulating cell-cycle regulators, with the combination of Cyclin B1, Cyclin D1, CDK1, and CDK4, named “4 F” exemplifying such attempts. While the 4 F strategy showed promise in rats, leading to sustained improvements in cardiac function over four months compared to controls, its results in pigs were less conclusive. Four weeks post-injection treated pigs exhibited a 25% reduction in scar size. However, no statistically significant improvements in ejection fraction (EF) were observed from baseline (p = 0.12 for Echo, p = 0.11 for MRI), with only a trend toward increased EF that did not reach significance92,93.
The activity of CDK2 in G1 phase is context dependent. When CDK2 is overexpressed it leads to an increase in the proportion of smaller mononuclear cardiomyocytes in adult mice, while its chemical inhibition reduced DNA synthesis in neonatal cardiomyocytes94,95. Moreover, during S phase of the cell-cycle, the combination of A-type cyclins with CDK2, promoted cell-cycle entry, while combining them with CDK1 facilitated M phase entry96,97,98,99,100. Cyclin A2 alone, when injected into the peri-infarct myocardium of a pig model using an adenovirus promoted cardiomyocyte mitosis, reduced fibrosis, and improved cardiac function. Shapiro et al. employed a novel sarcomere labeling method with time-lapse microscopy to assess cytokinesis in adult porcine cardiomyocytes. By monitoring cytokinetic events over 72 h within defined regions of interest, they quantified the cytokinetic index, observing a ~ 15-fold increase in experimental cells compared to controls98.
Additionally, cyclin G1 plays a significant role in cardiomyocyte polyploidization. Its overexpression in primary neonatal rat cardiomyocytes increased DNA synthesis and delayed mitosis, while its inactivation in mice accelerated cardiomyocyte withdrawal from the cell cycle, resulting in fewer polynucleated cells101.
Cyclin D2 is a promising candidate for heart regeneration. Cardiomyocyte-specific overexpression of cyclin D2 induced cardiac regeneration, decreased infarct size, and enhanced cardiac function in mammalian hearts following MI16,102. Finally, the co-expression of cyclin T1 and Myc, a pivotal transcription factor for tissue regeneration post-injury has been shown to drive proliferation in the adult mouse heart103.
Proliferative signalling pathways modulation
Exploring pivotal cardiac signalling pathways such as Hippo, JAK-STAT, MAPK, TGFβ, mTORC, Akt, and Wnt, has revealed potential targets for regenerative therapies.
In fact, the discovery of the Warts (Wts) gene laid the foundation for understanding cardiac regeneration links to the Hippo pathway104. Shortly after birth, the activation of Hippo signalling initiates the inactivation of one of its critical downstream effectors, YAP, which undergoes phosphorylation and remains sequestered in the cytosol. This sequestration prevents YAP from translocating into the nucleus, thereby hindering its ability to activate genes necessary for cell growth and division105.
In a recent advancement, gene therapy utilizing an adeno-associated virus 9 (AAV9) has been used to selectively knockdown the Hippo pathway gene Salvador (Sav) in border zone cardiomyocytes in a pig model of ischemia/reperfusion-induced MI. This innovative approach resulted in remarkable enhancement of left ventricular ejection fraction by 14.3%. This improvement was attributed to the generation of new cardiomyocytes, accompanied by reduced fibrosis and increased capillary density106.
Moreover, a study that examined translated RNAs in zebrafish cardiomyocytes during heart regeneration revealed the dynamic induction of several members of the Jak1/Stat3 pathway following injury107. Later, the administration of Rln3, a secreted protein induced by injury in a Stat3-dependent manner, was reported to promote cardiomyocyte proliferation107.
The Ras family of small Guanosine Triphosphate (GTP)‑binding proteins (G proteins) is required for normal cardiac growth but are also critically involved in the development of cardiac hypertrophy and HF108. Kinase pathways such as MAPK, p38, and SAPK displayed a low level of activation in hypertrophy, whereas they were highly activated in HF. However, many questions remain as K-Ras leads to hyperplasia, while H-Ras has hypertrophic effects108,109. Along the same line, despite the numerous potential benefits of p38 inhibitors, clinical trials have not demonstrated improvements in cardiac outcomes following ischemia–reperfusion, likely due to the absence of isoform-specific inhibitors110,111.
Notch activation appears to precisely regulate the balance between proliferation and differentiation of stem and progenitor cells in various tissues, including the heart. Furthermore, as people age, there is a decline in the number of Notch1-positive cells in the heart. Administering an adenoviral vector expressing the Notch intracellular domain in infarcted hearts enhanced hemodynamic function compared to control mice after 4 weeks, indicating the involvement of Notch signalling in a cardioprotective capacity following cardiac injury112,113,114,115.
The transforming growth factor β (TGFβ) superfamily plays key roles during heart development. While some TGFβ ligands influence cardiomyocyte proliferation, their release alone falls short of inducing proliferation effectively116,117,118,119. Moreover, TGFβ superfamily signalling is extremely pleiotropic, impacting processes well beyond proliferation. It is important to understand the intracellular signalling pathways and interaction between TGFβ signalling and pro-proliferative stimuli to uncover potential therapeutic approaches120. Cardiac hypertrophy, commonly associated with cardiac fibrosis, represents one of the risk factors for HF. Both are controlled by master regulators mTOR complex 1 (mTORC1) and TGFβ, respectively. Accordingly, targeting the mTOR pathway, through modRNA encoding phosphatidylinositol-5-phosphate 4-kinase type 2 gamma (Pip4k2c), a known mTORC1 regulator, showed promise in reducing cardiac hypertrophy and fibrosis121,122,123,124,125.
AKT signalling influences vital physiological functions, including survival, energy metabolism and adaptation to stress. Distinct AKT isoforms play specific roles in cardiac function: AKT1 induces cardiac hypertrophy, AKT2 knockout leads to insulin resistance and diabetes, and AKT3 overexpression results in maladaptive hypertrophy126,127,128,129,130. The intricate interplay between AKT and other signalling pathways, such as PI3K-AKT and MEK1-ERK1/2 pathways working in concert to mediate cardioprotection highlights the complexity of biological processes131,132,133,134,135.
Research in zebrafish, mice, and human embryonic stem cells illustrates the dual role of Wnt/β-catenin signalling in heart development. Depending on the stage of embryogenesis, Wnt/β-catenin signalling exhibits antagonistic effects on cardiac specification and differentiation. Nevertheless, inhibiting Wnt signalling appears to be advantageous for cardiac wound healing and functional recovery following injury32,136,137,138,139,140,141,142,143.
Lastly, the Hedgehog (Hh) pathway, a crucial angiogenic regulator, shows regenerative potential, with Shh agonists promoting cardiomyocyte proliferation. This pathway could help identify routes to new regenerative therapies if explored144,145,146,147,148,149.
Transcription factors
Intricate transcriptional regulation orchestrating cell-cycle and metabolism differs in neonatal and adult muscle cells. For example, GATA4 expression is high in embryonic and early neonatal cardiomyocytes but downregulated following postnatal cell-cycle arrest. The adenoviral gene transfer of GATA4 significantly improved cardiac regeneration after cryoinjury at P7150.
The process of cardiac regeneration is dynamic and time-sensitive, necessitating deep understanding of how transcription factors (TFs) change over time. Research by Nunes et al. identified 135 TFs that play crucial roles in regeneration of the zebrafish heart. Among these, TFs such as Hand2, Nkx2.5, Tbx20, Fosl1, Fosb, Junb, Vdr, Wt1, and Tcf21 have been noted for their importance in tissue regeneration151. Tbx20 has been further validated for its ability to promote adult cardiomyocyte cycle re-entry and reducing scar size post-MI152,153,154. Interestingly, cell type–specific expression of pluripotency factors (Oct4, Sox2, Klf4, and c-Myc) dedifferentiated adult cardiomyocytes to a state that resembles fetal cardiomyocytes, enabling adult cardiomyocytes to reenter mitosis155.
Other transcriptional networks, including AP-1, SRF, Myc, Nrf1, ZEB2, NFYa and NFE2L1, are promising therapeutic targets for heart disease and post-injury cardiac regeneration103,156,157,158,159,160. The intricate interplay of TFs could also reveal novel interventions for cardiac regeneration.
Cell-cycle assays: demonstration of true cardiomyocyte division
The quest for the best method to measure cardiomyocyte division has been a focal point in the exploration of potential myocardial regeneration strategies. This is due to the need for rigorous assessment of cell-cycle progression and division across both in vivo and in vitro assays (Table 1). Different methods have been used but, in many studies, claims of “cardiac regeneration” have lacked conclusive evidence of cell division and have simply relied on using S-phase markers (PCNA, 3H-thymidine, EdU, BrdU). These are, however, not capable of distinguishing endoreplication (nuclear division) from mitosis (cell division leading to more cells). Ki-67, which marks all cell-cycle phases, yields no insights into division, polyploidization, or binucleation. Phosphohistone H3 (pHH3) which cannot distinguish endomitosis from mitosis, and sarcomere disassembly, vital for cardiomyocyte proliferation, is not reliable. Even Aurora B-kinase, considered a “gold standard” for identifying cell division, faces challenges in detecting cells that divide without separating (acytokinetic mitosis); however this can be improved by additionally staining for anillin161,162,163.
To overcome these challenges, several transgenic reporter mice have been developed. These include lines referred to as BrainBow/Confetti mice, which can identify cells that have previously divided as having the same colour, or αDKRC labelling Ki67 positive cardiomyocytes to identify clonal cell-cycle events. One of the gold standards, the Mosaic Analysis with Double Marker (MADM) mice, marks cells that undergo mitosis. However, the MADM model has notable limitations, including sensitivity in detecting proliferative events and dependence on recombination efficiency161. Other reporters that allow quantification of actively cycling cells include the Fluorescent Ubiquitination-based Cell-Cycle Indicator (FUCCI) reporters and its last version FUCCI2a to assess cell-cycle stages, (eGFP)-Anillin mice, which label the contractile ring during cytokinesis, and Aurora kinase B (Aurkb)-ER Cre/+ mice, where Aurkb localizes at the centromeres and midbody during mitosis and cytokinesis164,165,166,167,168.
Despite these advances, most of these transgenic models have limitations. For instance, the ability to detect all actively cycling cells depends on the timing of tissue analysis and whether the model allows the ubiquitous expression of the marker. In such cases, all cycling cells are labelled, requiring careful co-localization of these markers with cardiomyocytes161. Moreover, certain experiments necessitate Tamoxifen administration to ensure the induction of Cre recombinase, which may complicate the labelling process and potentially impact heart function. In Rainbow/BrainBow/Confetti mice, the efficiency of Cre-mediated recombination events is crucial for precisely quantifying proliferative activity161,169. Importantly, accurately measuring proliferating cardiomyocytes involves detecting cytokinesis and daughter cells resulting from division, as cycling cardiomyocytes can also undergo endoreplication and multinucleation. Thus, an important limitation is the inability of some transgenic models, to distinguish proliferation from endoreplicative events169,170,171. The most direct solution to confirm cardiomyocyte division is live cell imaging. By using a FUCCI cardiomyocyte-specific cell line, combined with a membrane dye, it would be possible to track the formation of a membrane between two daughter cells167.
In summary, while these tools have significantly advanced our understanding of cardiomyocyte cell-cycle dynamics, refining these models and developing new methods are essential for unraveling the complexities of heart regeneration.
Current experimental models and overcoming their limitations
A good model system in the context of heart regeneration is ideally cost-effective, easy to handle, simple to replicate, and ethically sound while closely mirroring human disease processes. While no single model meets all these criteria, various models have been developed to study various aspects of development, regeneration, and disease. Some excel in deciphering cardiac regeneration mechanisms, while others are better suited for evaluating regenerative therapies (Fig. 2)172.
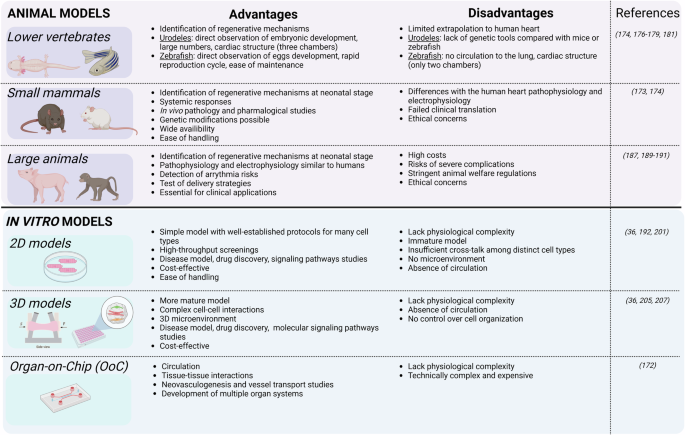
Advantages and disadvantages of animal and in vitro models for cardiac regenerative studies. Created in BioRender. Axelle, B. (2021) BioRender.com/u90h698.
Lower vertebrates
In vivo models are irreplaceable for understanding the intricate dynamics of the heart within a living organism. Fish and amphibians, for example, offer a window into natural cardiac regeneration processes (reviewed by Weinberger, M., et al. 173). Thus, lower vertebrates are commonly used to identify mechanisms linked with regenerative potential. Species such as zebrafish and axolotl have been useful to explore endogenous cardiomyocyte proliferation174,175, fibrosis176,177,178 and neovascularization175,179,180, all key interacting processes that facilitate heart regeneration.
Urodeles are particularly valuable since their embryonic development is external, allowing easy observation, and they are available in large numbers. Their cardiac structure is more complex than that of zebrafish, with three chambers (two atria and one ventricle) instead of two (a single atrium and ventricle) in zebrafish. In contrast to mammalian hearts which have many multinucleated cardiac muscle cells, 98% of urodele heart cells have single nuclei and are diploid, which may enhance their regenerative capability and simplify histological analysis172. However, despite their remarkable regenerative abilities, evolutionary divergence limits extrapolation of urodele findings to human hearts. Moreover, genetic tools are scarce compared to those available for mice or zebrafish.
Zebrafish stand out for their rapid reproduction cycle and ease of maintenance, producing large numbers of transparent eggs that allow direct observation of cardiac development and regeneration181. The main limitation as a model for human is that there is no circulation to the lung and the heart thus, only has two chambers. Structurally, the zebrafish heart resembles the embryonic mammalian heart, with smaller, mononucleated cardiac muscle cells lacking certain features found in mammalian hearts. Thus, teleost hearts seem better suited for growth and regeneration, while mammalian hearts appear more specialized for strong, sustained contractile force to meet the demands of higher metabolic rates and larger body sizes181.
Small mammalian models
Small mammals, such as mice, offer physiologically relevant systems for cardiac research, bridging the gap between simpler organisms like fish and amphibians and more complex human biology. Their widespread availability, ease of management, and cost-effective care make them indispensable tools for preclinical studies172.
Research has shown that human hearts undergo slow renewal, with about 1% of cardiomyocytes being replaced annually at age 20, a rate that decreases to 0.3% by age 75. These findings are based on analysis of 14C levels in DNA from individuals exposed to Cold War nuclear tests. When compared to MHC-nLAC mice, where only 0.0005% of heart cells divide over 4 h, the annual turnover rate is estimated at 1.095% in adult mice, closely aligning with human data182. Despite these similarities, significant differences exist in heart rate (mouse: 500 beats per minute (bpm); human: 60–100 bpm), electrocardiogram duration (mouse: 50 to 100 ms; human: 450 ms), cardiac repolarization currents, phospholamban regulation of calcium homeostasis (crucial for myocardial relaxation), and the expression of surface and structural genes. These differences limit the direct application of findings from animal studies to human cardiac disease183. Additionally, ninety percent of drug candidates tested in mice fail to achieve clinical approval, particularly in cardiovascular research184.
A particular study on miR199a for cardiac regeneration in infarcted mice, highlighted both the potential and pitfalls of using small mammalian models. While the treatment improved heart function and reduced scar size in mice, when delivered to pigs using AAV-mediated gene transfer, the treatment led to sudden arrhythmic death in most of the treated animals. In these experiments, evidence for cardiomyocyte proliferation was primarily based on BrdU and Ki67 labelling, the limitations of which were outlined above, and there was only “occasional” demonstration of cytokinesis57,185. Moreover, delivering the therapy at the same time as inducing cardiac injury is common in studies, but this does not reflect current clinical practice where the success of early reperfusion of myocardial infarctions make it challenging to demonstrate efficacy of any superimposed therapeutic strategy. After an infarct, the heart undergoes inflammatory, proliferative, maturation, and scaring phases. Therefore, fibrosis could limit the regenerative potential of therapies186.
Large animal models
A systematic review and meta-analysis conducted in 2022 revealed that cardiac stem cell treatments showed more pronounced effectiveness in small animal models of MI than in large animals187. Additionally, recent reports of cardiac arrhythmias in non-human primates and pigs following human pluripotent stem cells (hPSCs)-derived cardiomyocyte transplantation were not seen in small animals, possibly due to their higher heart rate188,189,190. Thus, it remains crucial to reevaluate the effectiveness of therapies using large animals like rabbits, dogs, pigs, sheep, and non-human primates, given their advantage of sharing similar pathophysiology and electrophysiology with humans191. However, the adoption of large animal models is not without challenge. High costs, stringent animal welfare regulations, and the risk of severe complications like fatal ventricular arrhythmias, leading to high mortality rates during and after procedures, can impact sample size and outcomes of these studies. Moreover, replicating the complex human physiology influenced by factors like medications, other health conditions, age, gender, and genetics in these animal models is challenging. Nonetheless, despite these obstacles, preclinical studies using animal models remain essential for advancing research in cardiac regeneration and ensuring successful clinical applications. These large animal models are also mandatory to test delivery strategies at a clinical scale.
For researchers, the selection of a suitable animal model involves careful consideration of several factors, including the physiological similarity to humans, availability, cost, and ethical considerations. This meticulous approach is essential for developing robust disease models that can yield reliable and translatable insights into cardiac disease and treatment.
In vitro models
The last two decades have seen significant advances in stem cell research, particularly with hPSCs. Breakthroughs here have transformed drug discovery, disease modeling, and personalized medicine by introducing a “human model in a dish”192. Cardiomyocytes derived from hPSCs (hPSC-CMs), typically grown in 2D culture, have been used to investigate molecular signalling pathways, evaluate drug-induced cardiotoxicity, and assess gene- and cell-based therapy strategies193,194,195. Kasamoto et al. used a FUCCI reporter line for high-throughput screening and identified Am80, a retinoic acid receptor (RAR) agonist, as a potent cell cycle activator in hPSC-cardiomyocytes. Similarly, Diez-Cuñado M et al. screened a whole genome collection of human miRNAs and identified 96 miRNAs that enhance proliferation (DNA synthesis and cytokinesis) in hPSC-CMs196,197.
However, hPSC-CMs are immature compared to primary adult cardiomyocytes in terms of their morphology, metabolism, contractility, and electrophysiology198,199.
To address this, 3D cardiac models have emerged which create microenvironments in which hPSC-CMs can mature200,201. Models like 3D engineered heart tissues (EHTs) have emerged that enhance the maturity of hPSC-CMs and also facilitate the measurement of contractile force202. EHTs are formed when cardiac cells self-organize into beating muscle strips around flexible anchoring points in the presence of ECM proteins. These structures exhibit well-developed sarcomeric organization, alignment, and abundant mitochondria, characteristics of mature cardiac muscle203,204. Moreover, when cells in EHTs contract against a restraint, they undergo a metabolic shift, reducing glycolysis and increasing fatty acid oxidation (FAO), resembling the metabolic profile of adult cardiac muscle cells203. Additionally, EHTs replicate the typical responses of adult cardiomyocytes to various physiological and pharmacological stimuli, making them valuable for studying heart function204. This model has been miniaturized, requiring only 16,000 cells per data point, enabling precise measurements of tissue contractile properties205. Comparing non-human primate PSC-CMs cultured in both monolayer (2D) and EHT (3D) formats revealed similar hypoxic responses to in vivo ischemia. The 3D format showed elevated key gene expression profiles and corresponding pathway activation, highlighting its superiority in emulating physiological conditions and signalling pathways206. Applied to cardiac regenerative studies, Mills and colleagues developed a high-throughput platform, which provides functional contractile tissue with biological characteristics similar to native heart tissue. This includes mature cardiomyocytes that are arrested in the cell-cycle36. A drug screen using this platform identified pro-proliferative compounds for cardiomyocytes and highlighted the necessity of the mevalonate pathway for their proliferation36.
Furthermore, other innovative models incorporate hPSC-CMs alongside other cardiac cell types, such as cardiac endothelial cells (ECs) and CFs, also normally present in the heart to form 3D cardiac microtissues (cMTs)200,207. By including these other cell types, more accurate representations of the complex cell interactions and dynamics within the heart have been created, enhancing understanding of cardiovascular physiology and mechanism of disease as well as drug discoveries207. hPSC-CMs in cMTs with CFs improved sarcomeric structures with T-tubules, enhanced contractility, and calcium handling, and increased mitochondrial respiration. Furthermore, cardiomyocytes in these cMTs demonstrate electrophysiological maturity compared to those in cMTs without CFs200. A recent step forward has been the development of 3D cMTs to model an infarcted heart. The interplay of oxygen diffusion and chronic adrenergic stimulation resulted in a gradient characterized by an “apoptotic center-dysfunctional interior-functional edge” pattern, reminiscent of the “infarct-border-remote zones” observed in infarcted hearts208.
As the field evolves, finding the right balance between model complexity, experimental throughput, and cost per data point stays crucial. While simple 2D and 3D systems provide a solid foundation for high-throughput screening and basic research, the more complex organ- or human-on-a-chip technologies offer in-depth insights into cardiac mechanisms, offering a new era of cardiac research that bridges the gap between traditional in vitro studies and clinical realities.
New regenerative tools
While current treatments can improve heart function and clinical outcomes, they all fail to address the loss of millions of functional cardiomyocytes mentioned earlier. The advent of gene editing technologies, ranging from recombinant proteins to DNA- and mRNA-based therapies, offers new hope for heart regeneration.
Recombinant proteins, despite their potential, face limitations as drugs, especially due to size, stability and the complex manufacturing process required to ensure proper folding and post-translational modifications209,210,211. In contrast, nucleic acid-based strategies circumvent many of these challenges by leveraging the translational machinery of mammalian cells. DNA-based drugs face the challenge of penetrating two membranes (the cytoplasmic and nuclear membranes) to exert their effects. Their entry into the nucleus raises safety concerns, including the potential integration of foreign DNA into host chromosomes and disruption of normal gene function. In contrast, RNA only need to reach the cell cytoplasm, eliminating the risk of chromosomal integration and addressing these safety concerns212,213.
RNA-based treatments, particularly mRNA therapeutics, have gained prominence, partly fueled by their success in COVID-19 vaccines. These therapies, capable of encoding various regenerative factors, offer advantages in safety, versatility, and effectiveness214. Moreover, compared to DNA and recombinant proteins, mRNA offers transient and controlled expression of the proliferative protein, minimizing potential adverse effects like uncontrolled growth215. Innovations in mRNA technology, such as chemical modifications and encapsulation in lipid nanoparticles (LNPs), are enhancing stability and reducing immune responses216, pushing mRNA therapies closer to clinical application. Experimental regenerative therapeutic strategies have shown the potential of mRNA technology. For instance, modified mRNA (modRNA) encoding factors such as VEGFA, FSTL1, PKM2, PSAT1, Myc associated with cyclin T1 and even a combination of STEMIN and YAP5SA have exhibited promising effects in the treatment of MI17,30,34,160,217,218.
One of the key advantages of mRNA over protein therapies is the potential for cell-specific targeting. Techniques using miRNA-mediated positive selection have been shown to enhance cell specificity, refined by technologies like the SMRTs system or a programmable RNA-sensing system219,220. This feature combined with mRNA encapsulation in LNPs that are covered with tissue specific peptides or antibodies will further facilitate mRNA-therapy clinical translation221,222,223. Studies in pigs, employing CCND2 modified mRNA, showcased promising outcomes by influencing left ventricular ejection fraction and encouraging cardiomyocyte proliferation224. Determining the optimal dose and duration of mRNA for therapeutic effects relies on various factors, including the encoded protein function and potency. Delivery methods such as intramyocardial or intravenous injection of mRNA-LNPs constructs have shown promise in directing therapy to the areas of the heart most in need, such as the ischemic regions225,226. However, challenges remain, including understanding the impact of therapy on heart cell connectivity and the mechanical aspects of cell division, such as changes in gap junction protein connexin 43 expression during cardiomyocyte division and transient mechanical consequences during mitosis185. Another still unsettled issue is the choice of the optimal route for delivering the therapeutics: direct intramyocardial administration has the advantage of a controlled on-target delivery but at the cost of an invasiveness which challenges repeated dosing; conversely, intravenous injections address this issue but face the problem of a predominant trapping of the compounds in liver and spleen, thereby requiring additional strategies for escaping the mononuclear phagocyte system and enhancing cardiac targeting of the regenerative treatment. The optimal therapeutic window for mRNA therapy may extend beyond the immediate aftermath of MI, as evidenced by trials involving patients with moderately reduced global left ventricular ejection fraction186,227. Despite the challenges, the potential of mRNA technology for regenerative medicine is immense, offering a new avenue for heart repair that could complement traditional treatments. As research progresses, integrating regenerative therapies with strategies to enhance blood vessel formation and overall heart function could pave the way for comprehensive treatments for HF and other cardiac conditions217.
Conclusions and perspectives
MI results in significant cardiomyocyte loss, which may culminate in HF. The regenerative capacity of the human heart diminishes shortly after birth due to factors like metabolic shifts, epigenetic alterations, and immune system maturation. While interventions for ischemic heart disease primarily target HF prevention, curative options for end-stage cases are limited, with heart transplantation facing donor scarcity228. Alternative strategies, including cell therapy, non-cardiomyocyte reprogramming, and endogenous cardiomyocyte regeneration, have been explored but also face hurdles8,229. To realize the transformative potential of cardiac regeneration, it is imperative to employ models that faithfully replicate the intricacies of human physiological conditions. These meticulously designed models play a pivotal role in advancing understanding of the underlying mechanisms governing cardiac regeneration, thereby paving the way for the fulfillment of its therapeutic promise. As hPSC-derived models become increasingly sophisticated, they are also emerging as valuable tools for advancing understanding of cardiac regeneration36. These models, with their growing complexity, serve as a crucial bridge between theoretical exploration and practical application in regenerative medicine. Furthermore, as we explore the complexities of these models, an equally critical facet emerges- the development of assays that unequivocally prove cell proliferation. Establishing a consensus among experts in the field regarding the specific parameters and assays required becomes vital. This collective agreement would ensure standardized and rigorous approaches, offering clarity on the methodologies necessary to reveal and definitively prove the intricacies and value of cell division. This harmonization should pave the way not only for enhanced scientific rigor but also for promoting a more robust foundation for the transformative promise held within cardiac regeneration research.
Furthermore, unlocking the potential of regenerative therapies for MI demands a nuanced exploration of their effectiveness, delivery strategies, optimal treatment windows, and associated risks. While acute MI can lead to a substantial loss of cardiomyocytes, regenerative interventions do not realistically aim to fully replace all lost cells but to replenish the contractile cell pool to a level that will enhance overall cardiac function. The recent spotlight on mRNA technology, exemplified by successful anti-COVID-19 vaccines, holds promise for regenerative therapy214. mRNA’s transient action, adaptability, and tissue-specific design, alongside advanced LNPs, could reshape regenerative medicine, providing effective and safe treatments230.



Responses