Exploring similarities and differences in anti-atherosclerotic potential bioactives among Dendrobium species by UPLC-Q-Exactive Orbitrap MS

Introduction
Cardiovascular diseases are the leading global cause of death, responsible for approximately 18.6 million fatalities each year and significantly contributing to reduced life expectancy. Recent reports suggest that atherosclerosis accounts for nearly two-thirds of the underlying mechanisms of cardiovascular diseases1. Atherosclerosis (AS) is a chronic inflammatory condition characterized by endothelial dysfunction, lipid accumulation, and immune cell involvement. A defining feature of AS is the development of yellowish fibro-fatty plaques in the intima layer of arterial walls. The development of AS is multifaceted processes involving endothelial cell inflammation, smooth muscle cell proliferation, phenotype conversion, lipid deposition, and foam cell formation by monocyte-derived macrophages. Current treatments for AS primarily consist of pharmacotherapy and intravascular interventions. However, long-term use of secondary prevention medications can result in side effects like muscle breakdown and liver damage2, adding to the patients’ healthcare burden. This underscores the critical need for safer and more effective therapeutic strategies.
Natural active compounds have recently attracted significant interest for their diverse physiological benefits, minimal side effects, and ability to act on multiple pathways and target. Research highlights the unique role of food-derived natural products in treating and preventing cardiovascular diseases. Compounds like puerarin3, Astragalus polysaccharides4, and ginsenosides5 have demonstrated potential in addressing AS. Recent findings reveal that various active compounds in Dendrobium exhibit anti-inflammatory and lipid-lowering properties, supporting their role in AS treatment and prevention. Xia et al. demonstrated that Dendrobium may treat AS by regulating the STAT3/FOXO3 signaling pathway and mitigating mitochondrial dysfunction and cellular aging, as shown through network pharmacology and in vitro studies6. Dong et al. reported that Dendrobium polysaccharides and flavonoids exhibit antioxidant, anti-platelet aggregation, and lipid-lowering activities, aiding in the prevention of atherosclerosis, hypertension and related cardiovascular diseases7. In HFD-induced ApoE-/- mice, Qi et al. observed that Dendrobium polysaccharides alleviate AS through lipid-lowering, antioxidant and anti-inflammatory effects, with a notable influence on gut microbiota8. Zhang et al. identified Moscatilin’s therapeutic potential in treating vascular calcification by targeting the IL13RA2/STAT3 and WNT3/β-catenin pathways, offering valuable insights for studying Dendrobium components in AS9. In addition, Dendrobium contains diverse phenolic compounds, such as bibenzyl and phenanthrene. Bibenzyl compounds exhibit strong antioxidant activity, with Dendrobium’s oxidative properties correlating to their bibenzyl content10. Some bibenzyl derivatives outperform vitamin C in free radical scavenging11. Phenanthrene derivatives like 4,7-dihydroxy-2,3-dimethoxy-9,10-dihydrophenanthrene12 and dendrochrysanene13 exhibit anti-inflammatory properties in vitro. At the same time, Flavonoids in Dendrobium, such as rutin, quercetin, and isorhamnetin, play a crucial role by reducing inflammation, oxidative stress, and lipid accumulation14. Despite these findings, the link between specific Dendrobium components and their anti-atherosclerotic effects remains underexplored, warranting further investigation into the impact of chemical composition on these therapeutic properties.
Dendrobium plants are perennial herbaceous epiphytic that thrives in cool, moist environments. Renowned in traditional Chinese medicinal, Dendrobium is known for its ability to nourish the stomach, promote fluid production, moisten the lungs, alleviate coughs, reduce internal heat, and enhance yin energy. Modern pharmacological research has identified active compounds in Dendrobium, including alkaloids, polysaccharides, flavonoids, phenols, and amino acids15. It exhibits significant physiological activities including enhancing immunity, lowering blood sugar, inhibiting cancer, relieving inflammation, and reducing oxidative stress, highlighting its medicinal potential. Currently, over 1000 Dendrobium species are found globally, including 76 species and two varieties native to China. Approximately 60 species are utilized in traditional medicine, highlighting the genus’s diversity and complex taxonomy. The 2020 edition of the pharmacopoeia recognizes cultivated species and closely related species like Dendrobium nobile Lindl. (D. nobile), Dendrobium huoshanense C. Z. Tang & S. J. Cheng (D. huoshanense), Dendrobium chrysotoxum Lindl. (D. chrysotoxum), and Dendrobium fimbriatum Hook (D. fimbriatum) as primary medicinal Dendrobium species. However, research has found substantial chemical variations in antioxidant activity and other effects among medicinal Dendrobium species, potentially influencing their clinical efficacy. Therefore, establishing a rapid and reliable system to identify and isolate medicinal Dendrobium compounds is critical for preserving germplasm resources and ensuring clinical effectiveness.
The identification of Dendrobium species primarily relies on traditional methods, spectroscopic and chromatographic techniques, and molecular approaches. Traditional methods rely on morphological traits for identification; however, the macroscopic characteristics of different Dendrobium species are very similar, complicating accurate differentiation, especially after deep processing. Spectroscopic and chromatographic techniques distinguish Dendrobium species based on their physicochemical characteristics of Dendrobium species, providing greater accuracy and objectivity than traditional methods. Molecular identification, which involves nucleic acid detection, is a more recent approach that is not limited by the plant’s growth conditions, developmental stage, or tissue type. However, these methods may not fully capture the biological effects seen in traditional Chinese medicine and can be challenging when isolating and identifying active compounds from different Dendrobium species16. Recently, liquid chromatography-mass spectrometry (LC-MS) has become widely used for qualitative analysis, targeted quantitative analysis, and quality control. In ultra-performance liquid chromatography (UPLC) Q-Exactive Orbitrap-MS, the quadrupole isolates precursor ions, while the Orbitrap analyzer provides high-resolution mass detection, allowing for the rapid identification of active compounds in complex herbal and biological samples. This technology greatly aids in the separation and identification of compounds among different Dendrobium species.
In this study, we employed UPLC-Q-Exactive Orbitrap-MS to analyze the chemical compounds of different Dendrobium species. We integrated network pharmacology and molecular docking to explore the differential active compounds (DACs) and shared active compounds (SACs) in different Dendrobium species, and assess how these compounds influence the variations in anti-atherosclerotic (anti-AS) effects across species. Our findings indicate that the chemical profile differences among Dendrobium species correlate with their distinct anti-AS effects, providing a basis for further research and development of Dendrobium’s medicinal and nutritional values.
This study utilized UPLC-Q-Exactive Orbitrap-MS to examine the chemical components of different Dendrobium varieties. We also investigated the differential and shared active components against AS by combining network pharmacology and molecular docking, and analyzed how these components contribute to the anti-AS mechanisms in different varieties. Our results suggest that the chemical composition variations among Dendrobium cultivars influence their distinct anti-AS effects. This will provide a material foundation for further research, development, and utilization of Dendrobium’s medicinal and nutritional potential.
Results
MS analysis of different Dendrobium species
Identification of compounds
The total ion chromatogram from the UPLC-Q-Exactive Orbitrap-MS analysis is presented in Fig. 1. A total of 77 active compounds were identified after screening, as detailed in Table S1. These compounds include 18 flavonoids, 15 organic acids, 12 amino acids, 7 alkaloids, 4 phenolic compounds, 3 fatty acids, 3 ketones, 2 coumarins, 2 naphthoquinones, 2 amides, 2 alcohols, 2 aldehydes, 1 heterocyclic compound, 1 amine, 1 pyridine, 1 ester, and 1 peptide.
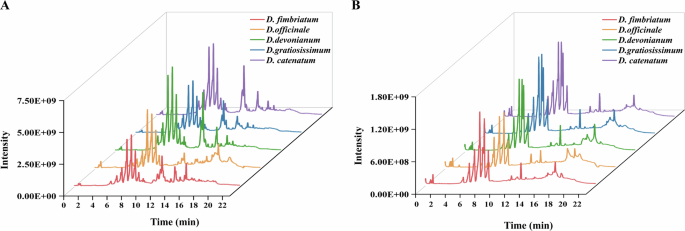
A positive ion mode. B negative ion mode.
Mathematical analysis and differential comparison
The PCA results (PC1: 25.3%, PC2: 13.7%), shown in Figure S1, reveal clear clustering of Dendrobium samples from the five species. Specifically, D. fimbriatum is situated in the first and fourth quadrants, while D. catenatum is placed in the second quadrant, with a wider and more dispersed distribution, which significantly distinguishes it from the other species. D. devonianum is positioned in the third and fourth quadrants, showing distinct characteristics compared to the other species. D.officinale and D.gratiosissimum overlap considerably, suggesting less differentiation between these two species.
The OPLS-DA model fit indices in analysis (Fig. 2A) were R2x = 0.761 (goodness of fit for X variables), R2y = 0.855 (goodness of fit for Y variables), and Q2 = 0.801 (model predictability). R2 and Q2 values above 0.5 indicate a well-fitting model. Permutation testing (200 permutations) showed no overfitting (Fig. 2B), confirming the model’s robustness in differentiating Dendrobium species with statistical significance. Analysis revealed that among the 77 identified compounds, 12 were designated as DACs (VIP ≥ 1, Fig. 2D) and 65 as SACs (VIP < 1). Table S1 highlights tropine, hesperetin, formononetin, n-trans-feruloyltyramine, and β-lapachone as high-content DACs specific to particular species. Specifically, tropine and hesperetin were abundant in D. catenatum, while formononetin and β-lapachone were unique to D. fimbriatum. N-trans-feruloyltyramine was exclusive to D. devonianum. Additionally, low-content compounds (peak area percentage<1%), including equol, trigonelline, and 2-amino-1,3,4-octadecanetriol, were detected, with trigonelline and 2-amino-1,3,4-octadecanetriol found only in D. catenatum. Among the 65 SACs, 26, such as 6-hydroxy-5-methoxyxanthone, 3-coumaric acid, 2-hydroxycinnamic acid, and sebacic acid, were more prevalent in specific species, particularly D. fimbriatum.
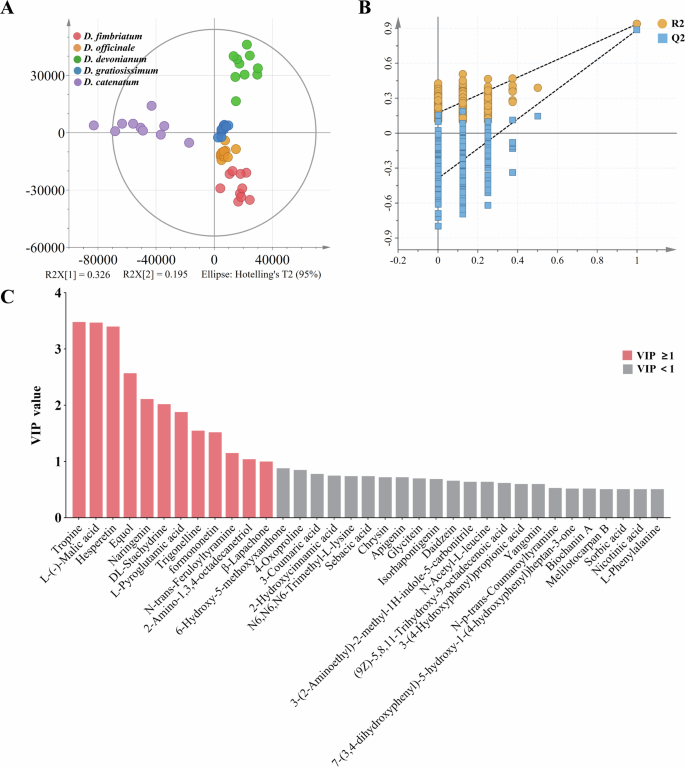
A OPLS-DA score plot. B OPLS-DA model cross-validation results. C VIP plot.
Network pharmacology study on anti-AS activity
Core target for Dendrobium in AS treatment
The analysis identified 838 related to the 77 active compounds from Dendrobium and 1884 AS-related targets (Fig. 3A). The Venn diagram (Fig. 3B) revealed 300 potential Dendrobium targets for AS treatment. Network analysis using Cytoscape 3.10.1 identified 70 core targets, represented by a graph of 70 nodes (core targets) and 380 edges (protein-protein interactions, PPIs) (Fig. 3C). Node size and color intensity correspond to target significance, positively correlating with degree values.
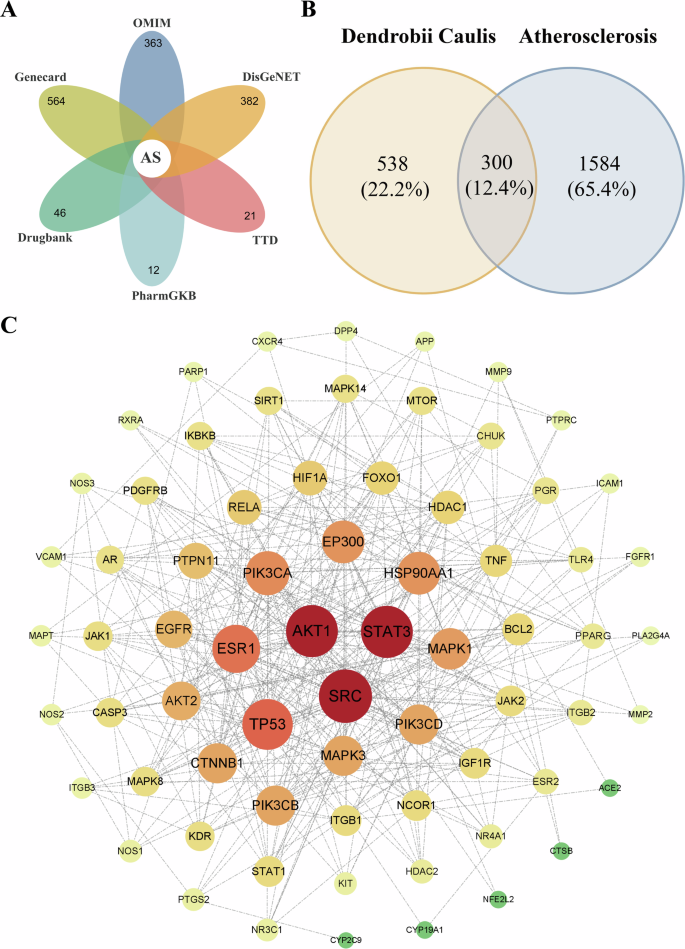
A Venn diagram of AS disease targets. B Venn diagram of intersections between Dendrobium compounds and anti-AS targets, where the orange circle represents the targets of Dendrobium active compounds (totaling 838); the blue circle represents AS disease targets (totaling 1884); the overlapping area of the two circles represents the potential targets for Dendrobium in treating AS (totaling 300). C PPI network, where circular nodes represent protein genes and lines between circles indicate interactions between targets. Larger and darker circles represent higher degree values and stronger significance.
The analysis indicates that SRC, AKT1, and STAT3 are in the anti-atherosclerotic process of Dendrobium, establishing them as key targets for the AS treatment. SRC, a well-characterized non-transmembrane protein activated by various extracellular signals, is involved in cancer biology and macrophage regulation. It plays a crucial role in foam cell formation via CD36, expression of pro-inflammatory cytokines, lesion development, and plaque instability in AS models. Research demonstrates that SRC disrupts adhesive connections, facilitates monocyte transport, and promotes smooth muscle proliferation and migration. It also regulates macrophage lipid uptake and endothelial cell inflammation, all contributing to AS progression17. AKT1, a key effector protein in the PI3K/Akt signaling pathway, regulates endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) generation, offering protective effects against AS plaques development. Various AKT1 subtypes influence AS progression by regulating inflammation and apoptosis. This includes activating the PI3K/Akt signaling pathway, increasing VEGF expression, and enhancing angiogenesis18. STAT3, part of the STAT protein family, is central to cell growth, apoptosis, and oncogenesis. Inflammation-related STAT3 pathways are strongly associated with AS. They regulate endothelial dysfunction, lipid metabolism, macrophage polarization, inflammation, and smooth muscle proliferation and migration19,20. STAT3 is implicated in unstable plaques development. Its inhibition may suppress endothelial cell proliferation and migration, limit vascular genesis, and slow the progression of unstable plaque progressio21.
Identification of core targets for DACs in the anti-AS effects of different Dendrobium species
Analysis of 12 DACs across different Dendrobium species identified 70 core targets linked to anti-AS activity, encompassing 59 active compounds. Among these, 9 SACs were revealed, with contents in the different Dendrobium depicted in Fig. 4. A Venn diagram illustrating DACs with anti-AS properties across the analyzed Dendrobium species, as shown in Fig. 5A. Target prediction for the 9 DACs identified 142 shared targets related to AS, as depicted in a Venn diagram (Fig. 5B). These common targets represent the potential mechanisms of the DACs in mediating anti-AS effects across various Dendrobium species. The protein interaction network (Fig. 5C) includes 101 nodes and 245 edges, with node size and color intensity indicating the importance of targets based on their degree values. Topology analysis results showed that 34 core targets for the anti-AS effects of DACs in different Dendrobium species.
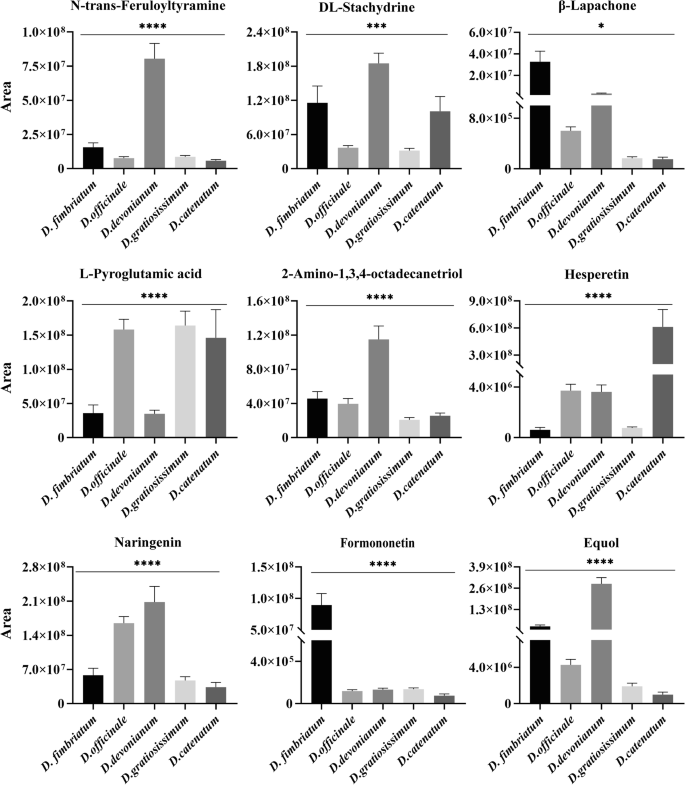
Visualized the content comparison of nine compounds, including N-trans-Feruloyltyramine, DL-Stachydrine, β-Lapachone, L-Pyroglutamic acid, 2-Amino-1,3,4-octadecanetriol, Hesperetin, Naringenin, Formononetin, and Equol, in five different varieties of Dendrobium.
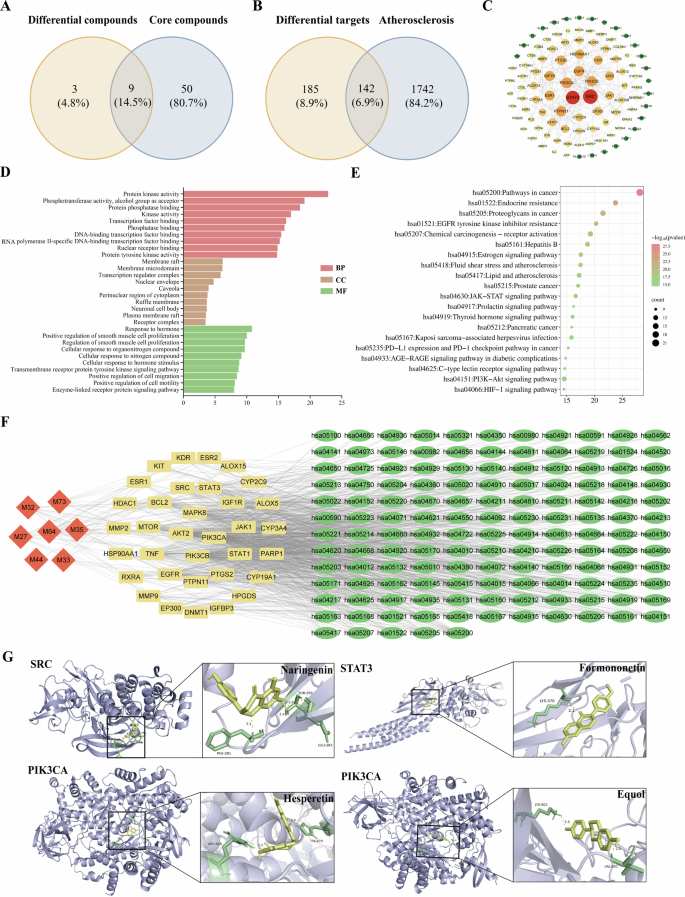
A Venn diagram of DACs of different Dendrobium species and core anti-AS compounds, where the orange circle represents DACs of different Dendrobium species (VIP ≥ 1), totaling 12; the blue circle represents core anti-AS compounds of Dendrobium (compounds corresponding to core targets), totaling 59; the overlapping area of the two circles represents the DACs of different Dendrobium species against AS, totaling 9. B Venn diagram of the intersection between the target points of DACs of different Dendrobium species against AS and anti-AS targets, where the orange circle represents the targets of DACs of different Dendrobium species against AS, totaling 327; the blue circle represents AS disease targets, totaling 1884; the overlapping area of the two circles represents the potential targets of DACs of different Dendrobium species against AS, totaling 142. C PPI network of the targets of DACs of different Dendrobium species against AS. D GO functional enrichment analysis (top 10) of DACs of different Dendrobium species against AS. E KEGG signaling pathway enrichment analysis (top 20) of DACs of different Dendrobium species against AS, where the x-axis represents enrichment scores; larger bubbles indicate more genes enriched in the pathway, and redder bubbles signify higher enrichment significance. F “compound-target-pathway” interaction network of DACs of different Dendrobium species against AS. In the diagram, diamonds represent various active compounds of Dendrobium, rectangles represent key targets for Dendrobium in treating AS, and ellipses represent KEGG pathways; lines indicate connections between active compounds, key targets, and pathways. Larger and darker shapes denote higher degree values and greater significance. G Molecular docking results of DACs of different Dendrobium species with core anti-AS targets.
SRC, STAT3, and PIK3CA were identified as the top targets proteins based on degree value, interacting with 23, 21, and 15 other proteins, respectively. These proteins play critical roles in the anti-atherosclerosis effects of DACs across various Dendrobium species. Particularly, PIK3CA is a key effector in the PI3K/Akt signaling pathway. The PIK3CA gene encodes a critical catalytic subunit that regulates PI3K activity. Mutations in PIK3CA can result in prolonged PI3K activation, disrupting cellular signaling and compromising pathway integrity.
Identification of core targets for SACs in the anti-AS effects of different Dendrobium species
Among 65 DACs identified across Dendrobium species, 70 core targets were linked to anti-AS activity. Analysis revealed 50 SACs specific to different species, as shown in Fig. S2A. Target prediction for the 50 SACs identified 283 common targets for AS, visualized in a Venn diagram (Fig. S2B). These shared targets represent the potential mechanisms underlying SACs’ anti-AS effects across Dendrobium species. The protein interaction network (Figure S2C) consists of 212 nodes and 783 edges, with node size and color intensity indicating the relative significance of targets. Topology analysis identified 65 core targets associated with SACs’ anti-AS effects in different Dendrobium species.
SRC, TP53, and AKT1 emerged as the top-ranked targets, interacting with 40, 36, and 32 other proteins, respectively, underscoring their significance in SACs’ anti-AS mechanisms. TP53, a key apoptosis-related gene, plays a crucial role in anti-AS activity. Research suggests TP53 influences anti-AS activity via autophagy. Under normal conditions, it inhibits autophagosome formation, while stress conditions activate autophagy through the mTOR pathway22. Additionally, TP53 also plays a critical role in ferroptosis23, driving pathological changes in the redox state and metabolic processes, which can worsen AS progression24.
GO functional enrichment and KEGG pathway analysis
GO analysis identified 837 terms associated with DACs from different Dendrobium species, including 742 Biological Process (BP) terms linked to hormone response, smooth muscle cell proliferation, and transmembrane receptor protein tyrosine kinase signaling. Additionally, 38 Cellular Compound (CC) terms were identified, primarily enriched in structures such as membrane raft, membrane microdomain, and caveola. The analysis also revealed 57 Molecular Function (MF) terms, mainly involving nitric-oxide synthase regulator activity, iron ion binding, and insulin receptor substrate binding. For SACs from different Dendrobium species, 1767 GO terms were identified, including 1540 BP terms related to enzyme-linked receptor signaling, apoptotic signaling pathway regulation, and transmembrane tyrosine kinase signaling pathway. The analysis revealed 97 CC terms enriched in membrane raft, membrane microdomain, plasma membrane raft, and caveola. Among the 130 MF terms, key functions included phosphotransferase activity, alcohol group as acceptor, estrogen response element binding, and ubiquitin-like protein ligase binding. The top 10 BP, CC, and MF terms, ranked by significance (P-values), are displayed in Fig. 5D and S2D.
KEGG pathway analysis identified 137 entries pathway for DACs in various Dendrobium species, with key entries including lipid and AS regulation (12 target proteins), PI3K-Akt signaling pathway (12 target proteins), fluid shear stress and AS (11 target proteins), JAK-STAT signaling pathway (11 target proteins), and AGE-RAGE signaling pathway in diabetic complications (9 target proteins). SACs showed enrichment in, with primary entries including lipid and AS regulation (30 target proteins), PI3K-Akt signaling pathway (29 target proteins), fluid shear stress and AS (25 target proteins), AGE-RAGE signaling pathway in diabetic complications (20 target proteins), and HIF-1 signaling pathway (18 target proteins). The top 20 pathways were visualized using KEGG enrichment bubble plots, as shown in Fig. 5E and S2E.
AS progresses in multiple stages, with altered lipid metabolism being a key characteristic and risk factor. Enrichment in lipid and AS signaling pathways also highlights the role of core targets in regulating lipid metabolism and influencing AS progression25. Blood flow shear stress significantly affects vascular endothelial cells, with mechanosensitive cation channels and epigenetic pathways mediating endothelial function and influencing AS progression. This finding validates the relevance of fluid shear stress and AS-related pathways. Recent studies emphasize the link between diabetes and AS, with poor glycemic control accelerating AS and cardiovascular complications26. The AGE-RAGE pathway significantly contributes to diabetic stress, increasing intracellular calcium levels, oxidative stress, endothelial cell proliferation, and coronary artery AS27. Inhibiting the AGE-RAGE pathway mitigates vascular smooth muscle cell migration, foam cell formation, and coronary AS. Active compounds in Dendrobium target p-PI3K/PI3K and p-Akt/Akt in the PI3K/Akt signaling pathway, reducing their phosphorylation levels and thereby inhibiting the pathway. This inhibition produces anti-inflammatory17, antioxidant28, and lipid metabolism-regulating29 effects, contributing to AS treatment. For example, flavonoids in Dendrobium18 inhibit the PI3K/Akt pathway, regulate ribosomal translation, and s suppress inflammatory mediators like TNF-α, IL-6. These effects help regulate macrophage polarization and foam cell formation, showcasing their anti-inflammatory properties30. Recent studies highlights the role of the PI3K/Akt pathway in endothelial cell lipid metabolism. Its inhibition reduces lipid deposition in vascular smooth muscle cells (VSMCs), increase lipid efflux, improves lipid metabolism disorders, and lowers plaque formation risk31. These findings demonstrate that Dendrobium can combat AS through various targets and signaling pathways. The pathways related to DACs and SACs across various Dendrobium species show significant overlap.
Construction of the “compound-target-pathway” network
The “compound-target-pathway” network for Dendrobium is shown in Fig. 5F and S2F. Among DACs, seven key anti-AS compounds were identified, including naringenin, hesperetin, equol, n-trans-feruloyltyramine, and formononetin (Table 1). SACs analysis revealed 48 important anti-AS compounds, such as butin, yangonin, diosmetin, chrysin, licochalcone B, eriodictyol, senecionine, isorhapontigenin, and apigenin (Table S2). Notably, many of these compounds are flavonoids, such as naringenin32, hesperetin33, butin34, diosmetin35, chrysin36, and eriodictyol37, consistent with previous studies on Dendrobium phytochemistry. Studies show these flavonoids exhibit pharmacological effects like anti-inflammatory, antioxidant, and anti-apoptotic properties. Naringenin’s anti-AS effects may involve promoting foam cell autophagy through increased AMPK phosphorylation levels, which inhibits foam cell formation and slows AS progression. Naringenin also stabilizes plaques by activating STAT6, boosting TIMP-3 expression, and preventing the degradation of collagen and elastin fibers. Hesperetin improves inflammation and dyslipidemia, reduces macrophage infiltration in aortic sinus plaques, and exerts anti-AS effects by activating the Nrf2/ARE pathway to maintain oxidative balance in endothelial cells. Chrysin reportedly lowers serum levels of pro-inflammatory cytokines (IL-1β, TNF-α), and triglycerides in rats, exhibiting acute lipid-lowering effects38. Animal experiments reveal that chrysin prevents cholesterol-induced AS by reducing oxidative stress and enhancing gut microbiota colonization39. Eriodictyol exerts anti-inflammatory effects by inhibiting the MAPK pathway, reducing NO synthesis, VEGF expression, and endothelial adhesion molecule levels, thereby preventing AS development40. These findings highlight the characteristic multi-compound and multi-target therapeutic approach of different Dendrobium species in combating AS using DACs/SACs.
Molecular docking
Lower binding energies typically reflect greater stability of ligand-receptor complexes, with a binding energy < −5 kJ·mol−1 generally suggesting significant interaction. Among DACs from different Dendrobium species, all except 2-amino-1,3,4-octadecanetriol (binding energy > −5 kJ·mol⁻¹ with STAT3) demonstrated effective binding to key targets, including naringenin, hesperetin, and six other active compounds. Binding energies ranged from −6.4 to −8.9 kJ·mol−1, with all compounds showing stronger binding to PIK3CA than to SRC or STAT3 (Table S3). Compared to reference ligands (SRC-JA6 (C18H23FN2O2S): −7.5 kJ·mol−1, STAT3-KQV (C40H42F2N7O9P): −9.1 kJ·mol−1, PIK3CA-Taselisib (C24H28N8O2): −10 kJ·mol−1), most DACs exhibited binding energies close to or lower than the controls, except for 2-amino-1,3,4-octadecanetriol. Notable pairings included naringenin with SRC (−8.1 kJ·mol−1), formononetin with STAT3 (−7.4 kJ·mol−1), hesperetin with PIK3CA (−8.7 kJ·mol−1), and equol with PIK3CA (−8.9 kJ·mol−1) (Fig. 5G). Hydrogen bonding was observed: naringenin-SRC (THR-299, GLU-283, PHE-281, PDB: 8JN8), formononetin-STAT3 (LYS-370, PDB: 6NJS), hesperetin-PIK3CA (ARG-818, HIS-670, PDB: 8EXL), and equol-PIK3CA (LYS-802, VAL-851).
These findings suggest DACs from Dendrobium exhibit good binding activity for key AS targets SRC, STAT3, and PIK3CA. SACs docking results revealed stronger interactions with SRC and AKT1 compared to TP53. All SACs except cis-3-hexenyl tiglate with SRC (binding energy > −5 kJ·mol⁻¹) showed binding energies ranging from −5 to −10.3 kJ·mol⁻¹ (Table S4). Compared to reference ligands (SRC-JA6 (C18H23FN2O2S): −7.5 kJ·mol−1, TP53-R4F (C13H13NO2S): −5.2 kJ·mol−1, AKT1-UC8 (C35H36N6O3): −12.9 kJ·mol−1), most SACs exhibited comparable or better binding energies. Top combinations included apigenin-SRC (−8.4 kJ·mol−1), melilotocarpan B-SRC (−8.5 kJ·mol−1), senecionine-SRC (−8.6 kJ·mol−1), quercetin-TP53 (−7 kJ·mol−1), butin-TP53 (−7.4 kJ·mol−1), eriodictyol-TP53 (−7.8 kJ·mol−1), glycitein-AKT1 (−10.3 kJ·mol−1), quercetin-AKT1 (−10.2 kJ·mol−1), and α-lapachone-AKT1 (−10 kJ·mol-1) (Fig. S2G). Hydrogen bonds formed at active sites included apigenin-SRC (GLU-181, ARG-178, GLN-531, PDB: 8JN8), melilotocarpan B-SRC (LEU-66), senecionine-SRC (ASN-394, CYS-280, PHE-281), butin-TP53 (SER-96, SER-99, ARG-213, PDB: 8E7A), eriodictyol-TP53 (ARG-213, ARG-267, SER-99, THR-256), quercetin-TP53 (THR-231, HIS-233, GLY-199, PRO-222), α-lapachone-AKT1 (SER-205, ASN-204, PDB: 7NH5), and glycitein-AKT1 (ASN-53, SER-205, ASN-204, THR-211). These results indicate SACs from Dendrobium exhibit good binding activity with SRC and TP53, with most compounds also showing good binding activity with the TP53.
In conclusion, molecular docking results demonstrate that DACs/SACs from different Dendrobium species interact effectively with key anti-AS targets. These interactions may contribute to anti-AS effects by modulating inflammation, oxidative stress, apoptosis, and lipid metabolism, supporting the accuracy and reliability of the study’s predictions.
Discussion
Dendrobium demonstrates potential benefits in combating AS. However, variations in the chemical composition of Dendrobium species have made it challenging to identify the key compounds responsible for these interspecies differences in therapeutic efficacy. This study focused on five representative Dendrobium cultivars—D. fimbriatum, D. officinale, D. devonianum, D. gratiosissimum, and D. catenatum—to explore whether variations in their chemical profiles correlate with differences in their anti-AS effects. D. fimbriatum and D. officinale are included in the “Chinese Pharmacopoeia,” while D. devonianum and D. gratiosissimum are commonly sold as mixed varieties of D. officinale. D. catenatum, prized for its horticultural value, was also selected, making these five cultivars ideal for studying interspecies differences.
In the study, UPLC-Q-Exactive Orbitrap-MS technology was used to analyze and compare the chemical compositions of different Dendrobium species. Key active compounds, including DACs and SACs, were identified and further investigated using network pharmacology and molecular docking to understand their interactions with core therapeutic targets. Analysis showed that among DACs in different Dendrobium species exhibit anti-AS effects through key compounds. For example, D. fimbriatum contains equol, formononetin, and β-lapachone; D. devonianum includes equol and n-trans-feruloyltyramine; and D. catenatum features hesperetin and naringenin. These compounds likely target core receptors such as SRC, STAT3, and PIK3CA. SACs, including butin, apigenin, glycitein, biochanin A, and melilotocarpan B were more abundant in D. fimbriatum. These compounds may exert their effects by interacting with core receptors like SRC, TP53, and AKT1. Despite being present in smaller amounts, compounds like quercetin, α-lapachone, and eriodictyol may still influence core receptors to exert anti-AS effects. Previous studies indicate that the identified compounds in Dendrobium possess notable anti-inflammatory and antioxidant properties. These include flavonoids such as hesperetin, formononetin, biochanin A, butin, apigenin, equol, and chrysin32,33,34,35,36,37,38,39, along with phenolic compounds like myristicin and apocynin41,42. Certain compounds, such as norharman and 3-coumaric acid, exhibit unique bioactivities, including restoring lipid metabolism balance and reducing blood cholesterol levels43. Amino acids like pyroglutamine, N-acetyl-L-leucine, and L-phenylalanine contribute to immune modulation by enhancing immune cell activity. Compounds such as DL-stachydrine and nicotinic acid have anticoagulant properties, preventing thrombosis and improving microcirculation44. These findings suggest that Dendrobium compounds work synergistically through multiple pathways and targets to combat AS, with the variation in compound composition accounting for differences in anti-AS efficacy among species. Pathway enrichment analysis indicated that both DACs and SACs from different Dendrobium species influence critical pathways, including PI3K/Akt, JAK-STAT, lipid metabolism, fluid shear stress, and AGE-RAGE signaling related to diabetes. These pathways enable multi-target, multi-pathway, and multi-route regulation, contributing to inflammation control, oxidative damage reduction, apoptosis modulation, and lipid metabolism regulation, which collectively support therapeutic and preventive effects against AS.
The study utilized advanced methodologies, including UPLC-Q-Exactive Orbitrap-MS, literature review, public database mining, network pharmacology, and molecular docking, to provide the mechanisms of Dendrobium in AS prevention and treatment. Despite these findings, the study has limitations, necessitating additional in vivo and in vitro experiments to clarify the specific mechanisms and biological effects of Dendrobium active compounds on identified targets and pathways. In vivo studies involved feeding ApoE-/- mice a high-fat diet to induce an AS model, allowing observation of the anti-AS effects of five Dendrobium species. Tissue plaque changes were analyzed using histological staining methods (HE, Masson, and oil red O staining) alongside lipid and inflammation level measurements to evaluate the varying anti-AS effects among Dendrobium species. Western blot analysis was used to measure the expression levels of SRC, STAT3, PIK3CA, TP53, and AKT1, elucidating differences in the mechanisms of action. In vitro experiments involved ox-LDL-induced THP-1 and HUVEC cells treated with drug-containing serum from the five Dendrobium species. Additional tests included individual compounds such as equol, formononetin, β-lapachone, n-trans-feruloyltyramine, hesperetin, naringenin, butin, apigenin, glycitein, biochanin A, and melilotocarpan B. Anti-AS effects and mechanisms were assessed through cell viability (CCK-8 assay), lipid accumulation (oil red O staining), inflammation levels (ELISA), chemokine expression (immunofluorescence), and protein expression of key pathways (Western blotting). Molecular docking results were validated using enzyme inhibition assays for equol, formononetin, β-lapachone, n-trans-feruloyltyramine, hesperetin, and naringenin with SRC, STAT3, PIK3CA, as well as butin, apigenin, glycitein, biochanin A, and melilotocarpan B with SRC, TP53, and AKT1.
Methods
Sample preparation
This study selected five Dendrobium species: Dendrobium fimbriatum Hook (D. fimbriatum), Dendrobium officinale Kimura & Migo (D. officinale), Dendrobium devonianum Paxt. (D. devonianum), Dendrobium gratiosissimum Rchb. f. (D. gratiosissimum), and Dendrobium catenatum Lindl. (D. catenatum). All Dendrobium samples were 3 years old and collected within the past 3 months. Ten samples (n = 10) for each Dendrobium species were selected. An appropriate amount was weighed, ground, and 0.2 g was placed into a conical flask. To each flask, 25 mL of 70% methanol was added with precision, the mass was recorded, and the mixture was sonicated for 30 min. After cooling, the mass was recorded again. Any loss in mass was corrected by adding 70% methanol. The solution was shaken, filtered, and the filtrate was collected. The filtrate was then passed through a 0.22 μm microporous membrane to obtain the final solution.
UPLC-Q-Exactive Orbitrap-MS
Chromatographic separation was performed using a Waters Acquity BEH C18 column (2.1 × 100 mm, 1.7 μm) with a gradient elution of acetonitrile (A) and 0.1% formic acid aqueous solution (B) as follows: 0–20 min, 5%–100% A; 20–22 min, 100% A; 22–23 min, 5% A. The column temperature was maintained at 30 °C, with a flow rate of 0.4 mL·min−1 and an injection volume of 2 μL.
Mass spectrometry was conducted using a heated electrospray ionization (HESI) source in negative ion detection mode, with a sheath gas pressure of 206.84 kPa, an auxiliary gas flow rate of 8 L·min−1, a spray voltage of 2.00 kV, and an ion transfer tube temperature of 320 °C. The scanning mode used was Full MS/dd-MS2, with a Full MS resolution of 70,000 and a dd-MS2 resolution of 17,500, scanning from m/z 100 to 1000.
Network pharmacology
Prediction of targets for Dendrobium chemical compounds
Targets related to effective active compounds of Dendrobium were predicted using the Swiss Target Prediction database (http://swisstargetprediction.ch/). The obtained target proteins were standardized and named according to the UniProt database (https://www.uniprot.org) for normalization.
Screening for AS disease targets
AS-related targets were collected by searching for “atherosclerosis” across six databases: OMIM (https://www.omim.org/), GeneCards (https://www.genecards.org/), DrugBank (https://go.drugbank.com/), PharmGKB (https://www.pharmgkb.org/), TTD (https://db.idrblab.net/ttd/), and DisGeNET (https://www.disgenet.org/). The obtained targets were standardized using the UniProt database, integrated from all databases, and duplicates were removed to obtain a comprehensive list of AS-related targets.
Selection of core targets and construction of the protein-protein interaction (PPI) network
Venny 2.1.0 was used to identify shared targets by intersecting the predicted targets of Dendrobium’s active compounds with AS-related genes. A Venn diagram was created to visualize potential core targets responsible for Dendrobium’s anti-AS effects.
The identified targets were entered into the STRING database (https://string-db.org/) for protein-protein interaction (PPI) network analysis. Only interactions involving Homo sapiens proteins were considered, with a confidence threshold of > 0.9, and isolated nodes were hidden. A PPI network was constructed using Cytoscape 3.10.1, based on three important topological parameters: degree, betweenness centrality, and closeness centrality. Targets with values above the median for these parameters were selected as core targets for Dendrobium’s anti-AS effects, representing the material basis for its therapeutic effects against AS. The same method was used to screen for DACs among various Dendrobium species and identify core targets of SACs against AS.
Screening DACs/SACs for anti-AS effects in different Dendrobium species
The DACs/SACs identified by SIMCA 14.1 software from different Dendrobium species were intersected with the material basis of Dendrobium’s anti-AS effects using Venny 2.1.0. This process revealed DACs/SACs contributing to anti-AS effects among different Dendrobium species, which were visualized in a Venn diagram.
GO functional enrichment analysis and KEGG pathway enrichment analysis
GO functional enrichment and KEGG pathway enrichment analyzes for the core targets of DACs/SACs in Dendrobium’s anti-AS effects were performed using the Metascape database (https://metascape.org/). GO functional enrichment analysis categorized gene products into biological process (BP), cellular compound (CC), and molecular function (MF). KEGG pathway enrichment analysis further clarified the biological functions of the target proteins. Statistical significance for both GO and KEGG analysis analyzes was set at P < 0.05. The results of the enrichment analysis were visualized using online bioinformatics tools.
Construction of “compound-target-pathway” networks
A “compound-target-pathway” analysis network was constructed using Cytoscape 3.10.1 software, based on the DACs/SACs, their core targets, and associated pathways identified for Dendrobium’s anti-AS effects in different species.
Molecular docking
Based on the results of network pharmacology, DACs/SACs associated with anti-AS effects in different Dendrobium species were selected. The 2D structures of potential active compounds were obtained from the PubChem online database (https://pubchem.ncbi.nlm.nih.gov), and converted to 3D structures using Chem3D 20.0 software. Crystal complexes of the top three core targets were selected from the RSCB PDB database (https://www.rcsb.org/), using criteria such as “Homo sapiens”, “X-ray crystallography”, and “resolution < 3 Å”. Selection was based on degree values.
Pymol 2.2.0 and AutoDock Tool 1.5.7 software were used to process the crystal structures by removing ligands, performing desolvation, hydrogenation, and adding charges. The processed structures were saved in pdbqt format. In AutoDock Tool 1.5.7, the protein and ligand were loaded to define the positions and sizes of the binding site grid boxes. The mesh parameters of each protein were defined as follows: SRC (center_x = - 29.374, center_y = 83.674, center_z = 121.278, size_x = 68.25, size_y = 68.25, size_z = 68.25), STAT3 (center_x = -3.614, center_y = 19.752, center_z = 26.103, size_x = 74.0, size_y = 116.55, size_z = 90.65), PIK3CA (center_x = 2.782, center_y = 6.69, center_z = 18.485, size_x = 80.58, size_y = 86.33, size_z = 90.65), TP53 (center_x = -25.032, center_y = 20.472, center_z = -9.612, size_x = 44.03, size_y = 49.31, size_z = 42.27), AKT1 (center_x = 20.432, center_y = -8.603, center_z = -16.934, size_x = 45.47, size_y = 64.41, size_z = 56.83). The top 10 ligand conformations were generated by setting the exhaustiveness to 32. Molecular docking was conducted using AutoDock Vina 1.1.2 to evaluate the affinity between the compounds and target proteins. Pymol 2.2.0 software was used for visualizing and analyzing the molecular docking results.
Data processing
The chemical compounds in different Dendrobium species were identified using MS data, supported by the mzCloud and mzVault databases, along with previously reported literature, combined with MS2 data. Active compounds were validated using the SwissADME website (http://www.swissadme.ch/) ensuring they met criteria such as high gastrointestinal absorption and drug likeness, with two or more “yes” responses.
Unsupervised principal component analysis (PCA) was performed using Origin 9.8.0.200 to assess overall metabolic differences between sample groups and variations within each group. Supervised orthogonal partial least squares discriminant analysis (OPLS-DA) was performed using SIMCA 14.1 software to identify metabolites that significantly contributed to inter-group differences, with variable importance in projection (VIP) calculated. Compounds with a VIP ≥ 1 were selected as DACs/SACs among different Dendrobium species.
Responses