The anti-melanoma roles and mechanisms of tricholoma isoflavone derivative CA028

Introduction
Melanoma is a tumor originating from malignant transformation of melanocytes, and its incidence has been increasing in recent years1. According to the Global Cancer Statistics in 2020, there were 324,635 new cases and 57,043 cancer-related deaths from melanoma, which accounted for ~1.7% of all cancer deaths globally2,3, and melanoma accounted for 73% of all skin cancer-related death4. Other than occurring in the skin, melanoma can also be found in non-skin areas such as eye, digestive system, genitourinary system and nasopharynx1. The main risk factors for melanoma include gender, age, UVR (ultraviolet radiation), number of gravidas, family history (family/personal history of melanoma), and areas of frequent sun exposure5. Melanoma has the highest frequency of mutations in all cancers, with a maximum of 100 mutations per Megabase position6. Melanoma can cause serious complications as it is highly metastasis, with the median survival of patients with metastatic melanoma ranging from 5 to 8 months3. In addition, melanoma is apparently resistant to chemotherapy, leading to poor prognosis. The 5-year survival rates for cutaneous melanoma are 97% (stage IA), 84% (stage IB), 68% (stage II), 55% (stage III), and 17% (stage IV)7. The prognosis is even worsened if the melanoma occurs in the mucosal region8. Melanoma patients have a poor prognosis, low responsiveness to existing targeted therapies, and resistance, which seriously hampers the treatment and control of the disease. Due to the inadequacy of existing melanoma treatments, there is an urgent need for new melanoma treatment options.
Cumulating reports indicated that ethnomedicines (e.g., turmeric, comfrey, amaranth, etc.) play an important antitumor roles9,10,11. With the development of modern pharmacology, screening and isolation of bioactive molecules from plants and herbs have been reported to be one of the promising approaches for the discovery of medicines12. A large number of isoflavones from Astragalus membranaceus have been identified and isolated. Among them, trichothecene isoflavones are the most abundant isoflavones. Many research groups have conducted long-term in-depth studies on the bioactivities and pharmacological effects of isoflavonoid phytoestrogens, including mullein isoflavones, spiny mangosteen flower flavonoids, and chickpea shootsin A. In which, mullein isoflavones have been demonstrated to exert anticancer effects in a wide range of tumor cells, including breast, colorectal, and osteosarcoma13,14,15, and the effects are better than those of spiny mangosteen flower flavonoids. In addition, tricholoma isoflavones have a wide potential for application in the development of anticancer drugs16. It was reported to inhibit the proliferation of breast cancer through targeting estrogen receptor β17. But the major problem is the relatively high IC50 of tricholoma. Therefore, our group modified the chemical structure of tricholoma isoflavones and identified the most effective one, tricholoma isoflavone derivative CA028 (C24H24O9) for treating cancers. Previous found that CA028 could target oral cancer and colorectal cancer through the control of different key hub genes18. However, the possible use of CA028 for treating melanoma and the underlying mechanisms is still unknown.
In the present study, we used the human melanoma cell lines A2058 and A375 as model to investigate the effectiveness of CA028 for treating melanoma. Our result showed that CA028 significant inhibited the proliferation of melanoma with the IC50 at 20.49 μM and 26.97 μM for A375 and A2058, respectively. In addition, the use of CA028 could inhibit the colony formation ability and migration ability. Also, the treatment of CA028 induced the apoptosis of melanoma. We then addressed the molecular mechanisms underlying the anti-melanoma effects of CA028 by applying relevant bioinformatics techniques such as comparative transcriptomic analysis and Ingenuity Pathway Analysis (IPA).
Materials and methods
Cell culture
A375 cell line and A2058 cell line were obtained from Wuhan Punosai Life Science and Technology Co., Ltd. Both cells belonged to melanoma cells of human origin, and the growth characteristics were adherent cells. The cell culture was performed according to the protocol provided by the source.
Cell viability assay
A375 and A2058 cells grown in logarithmic phase were prepared into 5 × 104 cells/mL cell suspension using complete medium DMEM and inoculated into 96-well plates at 100 μL per well. Inoculated 96-well plates were placed in the incubator, and after the cells were completely attached, the old medium was aspirated and Dulbecco’s Modified Eagle’s Medium (DMEM) complete culture medium containing different concentrations of CA028 was added at concentrations of 0 μM, 4 μM, 8 μM, 16 μM, 32 μM, and 64 μM for 48 h. After 48 h of incubation, the culture medium in the 96-well plate was sucked up, then 100 μL culture medium containing 10 μL CCK-8 solution was added to each well (prepared in advance to reduce errors), and the liquid containing CCK-8 needed to be prepared immediately before use. Care should be taken when adding samples to avoid producing bubbles to prevent affecting the subsequent microplate reader to detect the cell absorbance. After the end of adding samples, the 96-well plate was placed in the cell incubator and incubated in the dark for 60–90 min. The sample absorbance was then measured with a microplate reader at 450 nm, protected from light.
Plate colony formation assay
Take A375 and A2058 cells growing in logarithmic phase, prepare 250 cells/mL cell suspension with complete medium DMEM, inoculate in a 6-well plate, 2 mL in each well, and place in the incubator. After cell attachment, complete culture medium containing different concentrations of CA028 was administered at concentrations set as 0 μM, 8 μM, 16 μM, and 32 μM. After another day of culture, more than 50 cell colonies composed of single cells could be observed under the microscope, and the culture medium was discarded to terminate the culture. Each well was washed three times with 1 mL PBS, and the drug, dead cells and cell metabolites were washed off. One mL of 4% tissue/cell fixative was added to fix for 30 min, and then each well was washed three times with 1 mL PBS to completely remove the cell fixative. Then add 1 mL of 1% crystal violet solution to ensure that the staining is performed in the dark for 10 min. Rinse the unbound crystal violet staining solution in the 6-well plate of cells with water, move gently to avoid the detachment of cell clusters, place at room temperature for drying, and then take the crystal violet staining pictures of each well with a camera.
Cell scratch assay
A375 and A2058 cells that entered logarithmic phase growth were inoculated into 6-well plates at 1 × 105 and 1.5 × 105 cells per well (marked to ensure consistent location at each observation) and placed in an incubator until the cell density reached 90%. The old medium was discarded. Two parallel lines were drawn in the 6-well plate with a 10 μL tip and washed twice with PBS, followed by serum-free DMEM medium. The drug concentration of CA028 was set as the concentration of 0 μM, 8 μM, 16 μM and 32 μM, and placed in the incubator to continue the culture. Microscopically observed and photographed at 0, 24, and 48 h, and the healing of the scratched areas of the cells was recorded.
Transwell migration assay
A375 and A2058 cells entering the passage growth phase were starved and cultured for 12 h, digested to a single state of the cells, and 1 × 105 and 1.5 × 105 cells were added to each insert to inoculate a 24-well plate with 200 μL of drug-containing and serum-free medium in the insert and 600 μL of complete medium in the lower chamber. Ensure there are no air bubbles. CA028 drug concentrations were set at 0 μM, 8 μM, 16 μM, and 32 µM and kept in the incubator for another 24 h. Take out the culture pool, discard the culture medium in the upper chamber and wash twice in PBS, fix it with 4% cell fixative for 20 min, wash off the fixative with PBS, suck 200 μL of 1% crystal violet solution in the upper chamber, discard the crystal violet solution after 500 μL is stained in the lower chamber for 20 min, wash the unbound crystal violet staining solution in the 24-well plate of cells with PBS. Cells that did not penetrate the membrane on the upper chamber surface were removed in a gentle manner using a cotton swab and subsequently observed under a microscope and photographed for documentation.
Matrigel chamber invasion assay
Matrigel gel at 10 mg/mL was diluted appropriately. Serum-free medium was chosen as the dilution to maintain consistency of experimental conditions. We mixed Matrigel gel with serum-free medium at a ratio of 1:8, and the mixing should be uniform to facilitate subsequent gel preparation while avoiding the generation of bubbles (to prevent uneven gel preparation). Subsequently, 100 μL of diluted matrigel was pipetted gently into the upper chamber to form a thin film using a pipette. Place the 24-well plate in a constant temperature incubator at 37 °C for about 3 h. After gel solidification, use a tip to suck off the precipitated water, so as to make subsequent administration operation. The cell density was 2.5 × 104 cells/well, and the other experimental steps were the same as the conventional transwell cell migration assay except for the above steps. Subsequently, the cells were incubated under appropriate conditions for a period of time to observe the passage of the cells through the inserts.
Hoechst 33258 staining
Take A375 and A2058 cells growing in logarithmic phase, use complete medium DMEM to prepare cell suspension of 5 × 104 cells/mL and 7.5 × 104 cells/mL, inoculate them in a 6-well plate (2 mL for each well), and place them in an incubator. After overnight incubation in the incubator, the cells had successfully adhered. Subsequently, the old medium was removed and complete medium containing various concentrations of CA028, set at concentrations of 0 μM, 8 μM, 16 μM, and 32 μM, was added to the 6-well plates for concentration labeling for subsequent experiments. After 48 h of incubation, the incubation was terminated and the medium was removed. Following this, 0.5 mL of 4% tissue/cell fixative was added to each well and fixation continued for 30 min. Following fixation, each well was washed three times with 1 mL PBS to thoroughly remove the cell fixative. Then, 0.5 mL of Hoechst 33258 stain solution was added to a 6-well plate to ensure staining in the dark for 5 min. After staining was completed, the stain was removed by washing again with 1 mL of PBS, and then the sample was placed under a fluorescence microscope for observation and photographic recording.
Annexin V/propidium iodide apoptosis assay
Take A375 and A2058 cells growing in logarithmic phase, use complete medium DMEM to prepare cell suspension of 5 × 104 cells/mL and 7.5 × 104 cells/mL, inoculate them in a 6-well plate (2 mL for each well), and place them in an incubator. After incubation in the incubator, the cells had successfully adhered. Subsequently, the old medium was removed and treated with different concentrations of CA028 at concentrations set at 0 μM, 8 μM, 16 μM, and 32 μM. After the cells were cultured in CA028 drug environment for 48 h, 15 mL centrifuge tubes were prepared, the supernatant was collected, each group of cells was washed with 1 mL PBS, and PBS liquid was collected. Subsequently, cells were digested with EDTA-free trypsin, and digestion was stopped using twice the volume of trypsin complete medium. Cells were detached from the culture surface by gentle pipetting and centrifuged at 1000 × g for 5 min to remove the supernatant. 195 μL buffer was added to each tube of cell sample, and 5 μL AnnexinV-FITC and 10 μL PI mixture solution were added to each tube. All samples with added dye were allowed to react in an ice bath for 15 min in the dark. After cell staining, aspirate the fluid from the EP tube with a 1 mL pipette, filter through a 300 mesh filter into a flow tube, and load on the machine for flow cytometry.
Total RNA extraction and transcriptome sequencing
After the treatment, total RNA of A375 cells (n = 4) were harvested by using TRIzol reagent according to the manufacturer’s instruction. 1 μg of RNA with RNA integrity value was greater than 7.0 was subjected for the transcriptome library construction. Sequencing was provided by Lianchuan Biological Company. Briefly, the mRNA was purified by using Dynabeads Oligo (dT) beads, followed by fragmentation in 94 °C using a magnesium ion interrupt kit (NEBNextR Magnesium RNA Fragmentation Module). The fragmented RNA was used for cDNA synthesis by using reverse transcriptase (Invitrogen SuperScriptTM II Reverse Transcriptase), followed by double strand synthesis using Escherichia Coli DNA polymerase I and RNase H. The library within 300 bp ± 50 bp was purified using magnetic beads and was subjected to illumina NovaseqTM 6000 for 150 bases pair end sequencing.
CDNA synthesis and quantitative PCR analysis
CDNA was synthesized by using MonScriptTM5x RTIII All-in-one Mix (Mona Biotechnology Wuhan Co., Ltd). QPCR was performed using MonAmp SYBR Green PCR Mix (Mona Biotechnology Wuhan Co., Ltd). Primers were shown in Supplementary Table 1. Results were quantified with 2-ΔΔCt by normalization to GAPDH.
Immunohistochemistry staining
A375 cells were seeded into 6-well plates with dedicated slides. After attachment, cells were treated with different concentrations of CA028 (0 μM, 8 μM, 16 μM, and 32 μM) for 48 h. Cells were washed twice with PBS and fixed with 4% paraformaldehyde; then permeabilized with 0.3% Triton-100 (prepared with PBS) at room temperature for 15 min. The cells were incubated with 5% BSA (containing goat serum) at room temperature for 30 min. Then P21 and SUFU antibodies (1:500) were added, and incubated overnight at 4 °C. Cells were washed three times with TBS for 10 min, followed by incubation with secondary antibody for 1 h and washing with TBS. Finally, chromogenic substrate (DAB) was added to form a color reaction product that could be observed microscopically, and the slides were mounted after being dried.
Establishment of xenograft mice model
Twelve female BALB/c nude mice (~5 weeks old) were purchased from Guangdong Zhiyuan Biomedical Technology Co. All animal experiments in this study were approved by the Animal Care and Use Committee of Guilin Medical College. After 1 week of acclimatisation, 4 × 106 of A2058 cells were resuspended in phosphate buffered saline (PBS) and were inoculated on the right dorsum of nude mice. After 12 days the mice were randomly divided into two groups, the control group and the CA028 group. Then, the mice were injected intraperitoneally with 100 μL of vegetable oil (control group) or 90 mg/kg CA028 (CA028 group), once a day for 14 days. Mouse body weight and tumor volume were monitored every 2 days. Tumor volume was calculated using the following formula: (tumor length) × (tumor width)2 × 0.5. Nude mice were euthanized by the cervical dislocation after 14 days of treatment. Tumors were then isolated, weighed and photographed.
Statistical analysis
All experiments in this part were repeated more than three times, and data were described in the form of mean ± standard deviation. In analyzing data differences between groups, one-way analysis of variance was used for overall comparisons and Tukey test was used for comparisons between groups. * represents a P value < 0.05, where P < 0.05 indicates a statistically significant difference.
Results
CA028 inhibited the proliferation and carcinogenicity of melanoma
A375 and A2058 cells were treated with different concentrations of CA028 for 48 h, and the activity of the cells was detected using CCK-8 assay. Our result showed that the treatment of CA028 significantly inhibited the proliferation of melanoma cells A375 and A2058 at IC50 20.49 μM and 26.97 μM, respectively (Fig. 1A), which were lower than that of parental compound calycosin (A375: 116.8 μM and A2058: 138.8 μM) Moreover, treatment of human normal skin cells with CA028 had no significant effect on HaCaT activity (Supplementary Fig. 1). On the other hands, the CA028 treatment reduced the colony formation ability of A375 and A2058 cells (Fig. 1B). In the Annexin V/PI analysis, the CA028 treatment significantly induced the proportion of apoptotic cells in melanoma (Fig. 1C). A similar finding was observed in Hoechst33258 staining that the treatment of CA028 induced the % of apoptotic melanoma cells (Fig. 1D). Taken together, our results demonstrated the inhibitory effect of CA028 in melanoma cells.
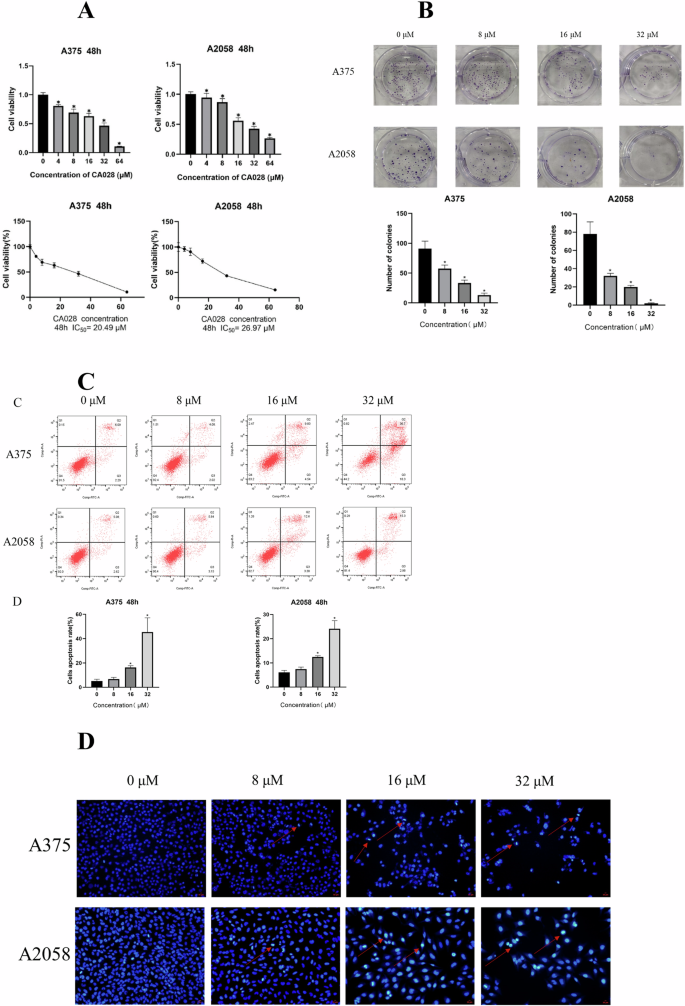
A CCK8 proliferation assay showed the inhibition of proliferation of melanoma cells A375 and A2058 caused by CA028 treatment. B Colony formation showed the reduced colony formation ability of melanoma cells A375 and A2058 caused by CA028 treatment. C Annexin V/PI staining followed by flow cytometry analysis showed the induction of melanoma cell apoptosis caused by CA028 treatment. D Hoechst33258 staining showed that the treatment of CA028 induced the % of apoptotic melanoma cells.
CA028 inhibited migration and invasion ability of melanoma cells
Then we study the effect of CA028 on the metastatic ability of melanoma cells. The result of wound healing assay showed that the treatment of CA028 could significantly shorten the closure time of wound, suggesting the inhibition of the migration ability of melanoma cells (Fig. 2A). A similar finding was observed in migration assay that the CA028 treatment could reduce the number of migrated cells (Fig. 2B). Moreover, the CA028 treatment could inhibit the invasiveness of melanoma cells, which was reflected by invasion assay (Fig. 2C).
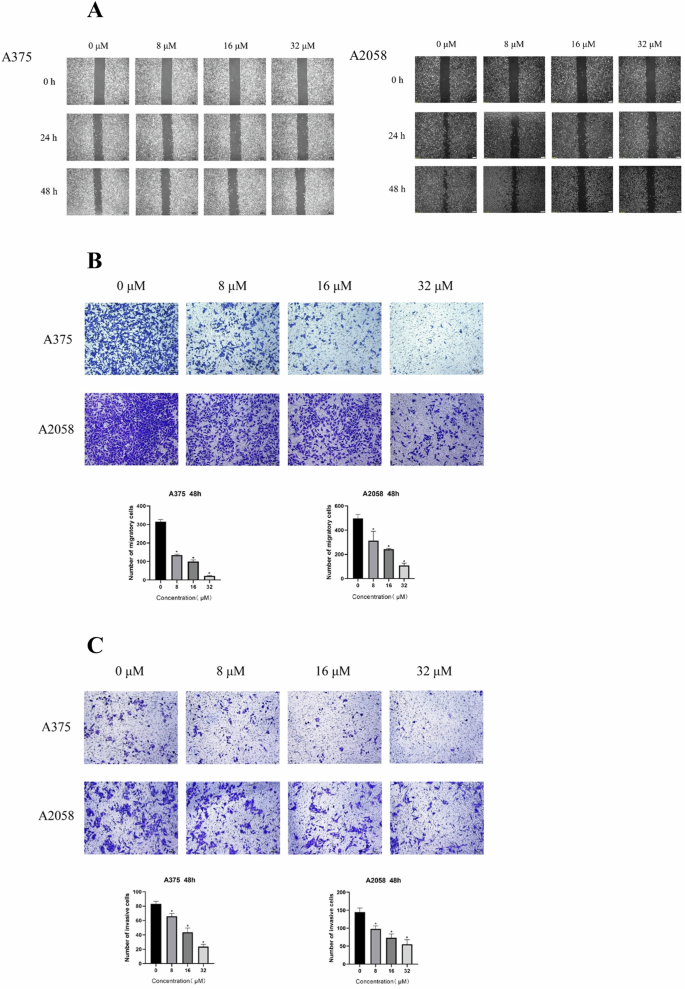
A Wound haling assay showed the reduced migration ability of melanoma cell caused by CA028 treatment. B Migration assay showed the reduced migration ability of melanoma cell caused by CA028 treatment. C Invasion assay showed the reduced invasiveness of of melanoma cell caused by CA028 treatment.
CA028 controlled the genes related to cell proliferation, cell apoptosis, and migration ability of melanoma cells
To further understand the potential mechanism of CA028 against melanoma, we screened for differential gene expression in A375 cells after CA028 treatment by transcriptome sequencing. When comparing gene profiles between control and CA028-treated groups, 4250 differentially expressed genes (DEGs) were identified, including 4,050 upregulated and 200 downregulated genes (Fig. 3A). The DEGs were submitted to DAVID tool for GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. The result of GO analysis found the involvement of DEGs in the biological processes related to DNA damage response and associated cell cycle arrest such as cell cycle phase transition and regulation of cell cycle process (Fig. 3B). Also, the DNA damage response would lead to programmed cell death (Fig. 3C), especially cell apoptosis through the regulation of intrinsic apoptotic signaling pathway by p53 class mediators, including CD74, CDKN1A, SHISA5, AEN, MIF, PML, BBC3, BCL2L12, BAG6, MUC1, DDIT4, BCL3, TMEM109, CDIP1, NUPR1, ZNF385A, TP73, PHLDA3 (Fig. 3D). In addition, the treatment of CA028 deregulated a cluster of genes involved in cell growth and cell proliferation (Fig. 3E). Furthermore, the CA028 targeted the genes that controlled the wound healing and cell migration ability (Fig. 3F). All these processes further supported the inhibitory proliferative and invasive roles of CA028 on melanoma cells.
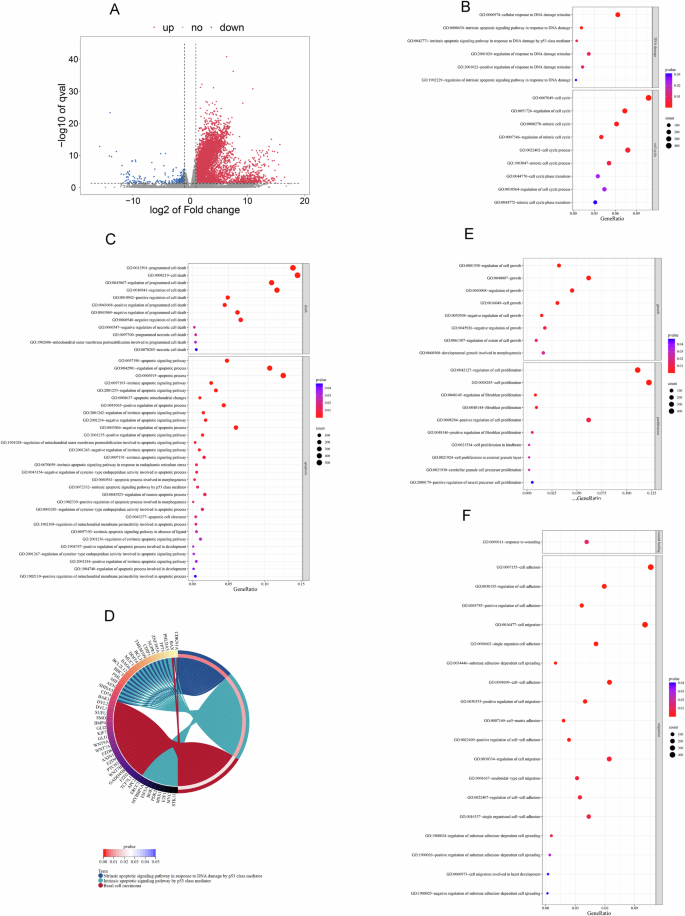
A Comparative transcriptome sequencing demonstrated the differential gene expression in melanoma cell A375 under CA028 treatment. Blue dots represented downregulated genes; red dots represented upregulated genes. Cutoff: 1<log2 fold change < –1 and log10 q-value > 1.3. Gene ontology (GO) enrichment analysis highlighted the roles of CA028’ target genes in (B) DNA damage and cell cycle and C cell death and apoptosis. D Cricos plot showed the involvement of genes in the selected GO terms. GO enrichment analysis highlighted the roles of CA028’ target genes in (E) cell growth and proliferation and F wound healing and cell migration. The size of bubble represented the number of genes involved in the biological processes. The color of bubble represented the significance of the biological processes.
CA028 targeted the genes involved in the cell signaling related to carcinogenicity and metabolism of melanoma
Then we further conducted the KEGG pathway enrichment analysis using the CA028-mediated DEGs. Our result highlighted important signaling pathways related to the carcinogenicity of many cancer types, especially basal cell carcinoma (Fig. 4A), including MAPK signaling, Rap1 signaling, TNF signaling, Relaxin signaling, Phospholipase D signaling, Hippo signaling, Notch signaling, VEGF signaling, GnRH signaling, Insulin signaling, Neurotrophin signaling, ErbB signaling, IL-17 signaling, Toll-like receptor signaling, and NF-kappa B signaling (Fig. 4B). Also, CA028’ targets were involved in apoptosis and different metabolism pathways and biosynthesis such as fructose and mannose metabolism, insulin resistance, and biosynthesis of amino acids (Fig. 4C). IPA was further used to construct the gene network involved in the anti-melanoma roles of CA028. The result of canonical pathway analysis showed highlighted the alteration of CA028 on the DNA damage and cell apoptosis signaling through targeting p53 signaling and death receptor signaling (Fig. 4D & Table 1). More importantly, CA028 targeted melanocyte development and pigmentation signaling and melanoma signaling in melanoma cells. The gene network was unpinned to visualize the anti-melanoma effects of CA028 (Fig. 4E).
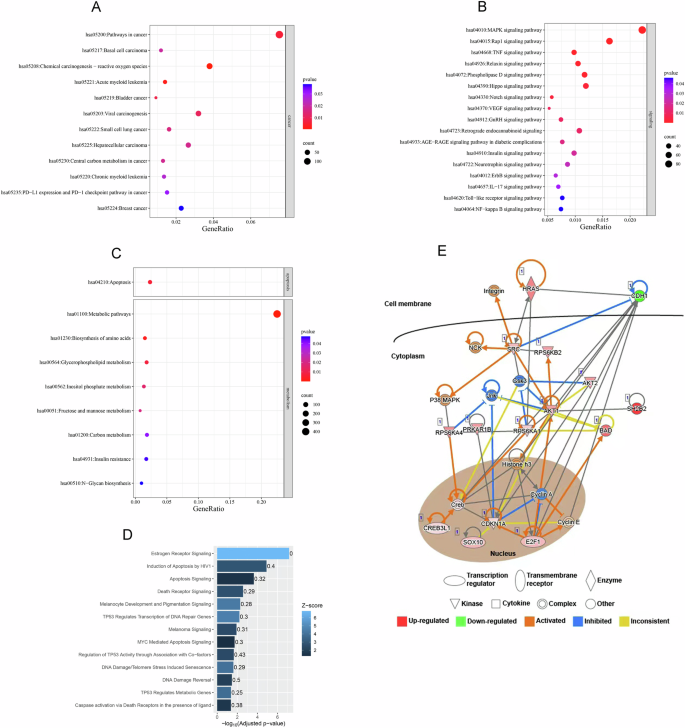
KEGG pathway enrichment analysis showed the involvement of CA028’s targets in A carcinogenicity, B cancer signaling, and C apoptosis and metabolisms. The size of bubble represented the number of genes involved in the pathways. The color of bubble represented the significance of the pathways. D Ingenuity Pathway Analysis highlighted the importance of CA028’s targets in cell death and DNA damage response. E Gene network showed the anti-melanoma roles of CA028.
Validating the findings of comparative transcriptomic analysis
In order to validate the findings from comparative transcriptomic analysis, we conducted qPCR analysis on the genes involved in anti-proliferation (BAK1 and P21) and anti-migration and -invasion (SUFU and BMP4). Our result showed that the treatment of CA028 could significantly induce the expression of this gene cluster (Fig. 5A). Furthermore, immunohistochemical (IHC) staining demonstrated the induction of protein expression of SUFU and P21 in melanoma caused by CA028 treatment. Taken together, both qPCR and IHC analyses supported the findings from transcriptome sequencing.
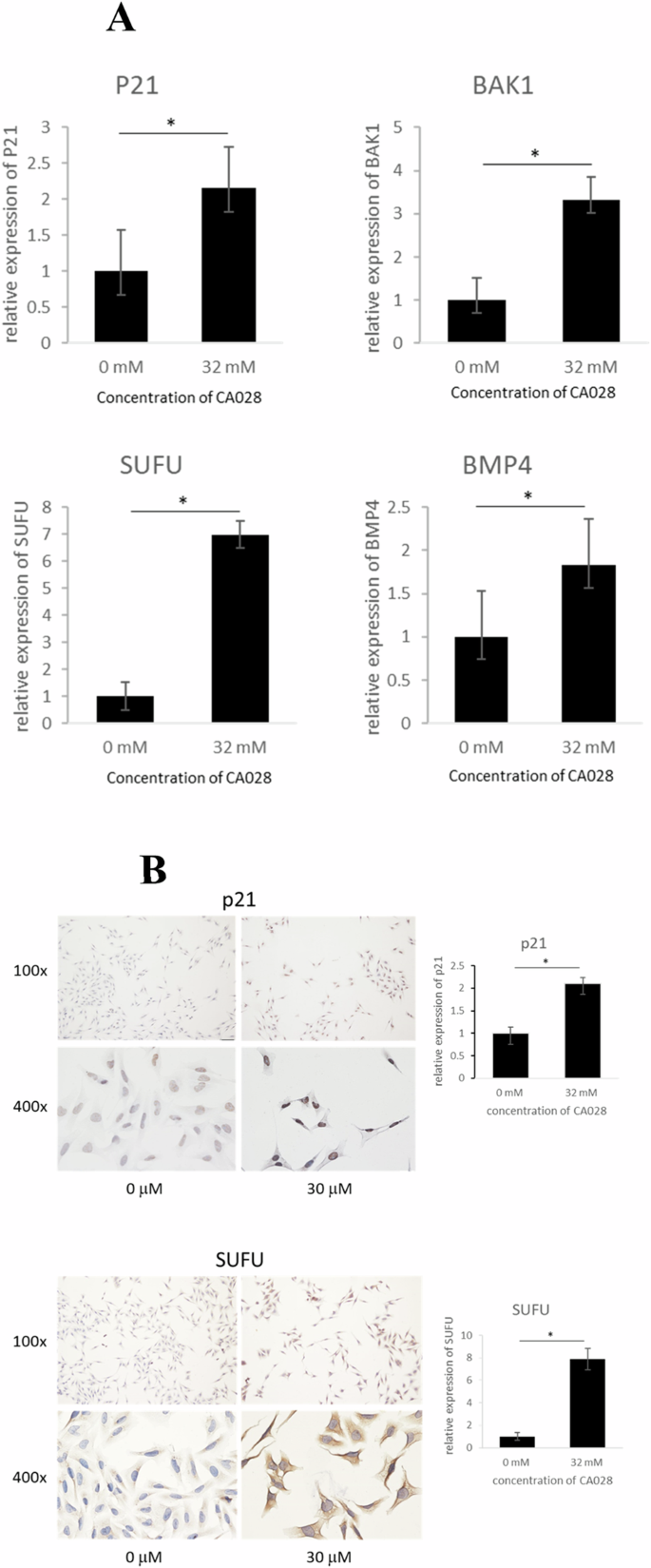
A qPCR analysis was used to determine the expression of anti-proliferation genes (BAK1 and P21) and anti-migration and -invasion genes (SUFU and BMP4). B immunohistochemical staining was used to determine the change of protein expression of SUFU and P21 in melanoma after CA028 treatment.
CA028 reduced the growth of xenograft in nude mice model
By using nude mice model, we further assessed the inhibitory effect of CA028 on the in vivo proliferation of melanoma. Our result showed that the treatment of CA028 could significantly reduce both of tumor size of melanoma in nude mice (Fig. 6A). In addition, the use of CA028 attenuated the reduced body weight nude mice (Fig. 6B). Taken together, the results suggested that the use of CA028 could reduce the melanoma.
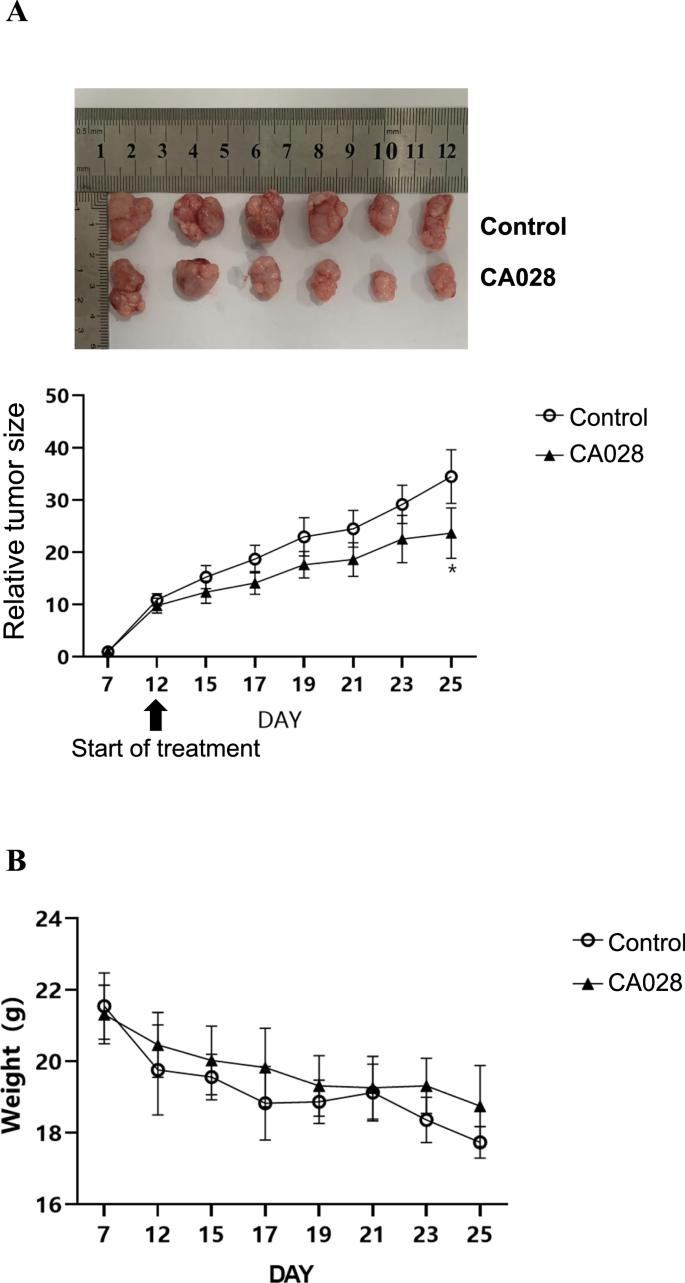
A The use of CA028 significantly reduced the tumor growth of melanoma raised by A2058 in nude mice as compared with control group. B The use of CA028 attenuated the reduced body weight in nude mice inoculated with A2058 melanoma cells.
Discussion
Isoflavones, a flavonoid, are one of the plant secondary metabolites with the chemical formula C15H10O2 and belong to aromatic compounds containing oxygenated heterocycles. It is structurally similar to the endogenous estrogen 17-beta estradiol (E2). Isoflavones are widely present in soy products such as soybeans. Isoflavones have been reported to be effective in reducing the risk of cancer. If soy isoflavones are supplemented in the diet, they can reduce the risk of death by 29% and the risk of cancer recurrence by 32% in breast cancer patients19. Melanoma is a highly aggressive malignancy, and surgical resection is the most important method for the treatment of melanoma in clinical practice, however for metastatic melanoma, surgical treatment is not enough and drug chemotherapy is also required. Dacarbazine was the first chemotherapy drug approved by the FDA for the treatment of melanoma, and the median survival with dacarbazine was 5–11 months, with a 1-year survival rate of only 27%20,21,22,23. Although some progress has been made in chemotherapeutic agents, the side effects of chemotherapeutic agents remain inevitable. With the continuous improvement of medical level, the application of targeted therapy and immunotherapy has also increased. Current effective treatments for metastatic melanoma are immune checkpoint inhibitors24. Immune checkpoints can be manipulated by PD124, PD-L1/220, and CTLA-425 in treating melanoma. Despite these breakthroughs in cancer treatment, a large proportion of patients still do not respond to these drugs, and some patients who respond develop secondary resistance, and the treatment is expensive and can be serious once side effects occur.
In the recent years, cumulating reports suggested the possible use of flavonoid for treating melanoma. For example, the use of genistein, a flavonoid, inhibited the growth and invasiveness of B16-B16 melanoma cells through reducing cytoskeleton-associated proteins and oncoprotein c-Myc, and increasing p53 level26,27. Isoflavones promote ROS generation and trigger oxidative stress in mitochondria, leading to cytochrome c release that are decisive factors in induction of apoptosis in melanoma cells28. In the present study, we aimed to investigate the possible use of a modified tricholoma isoflavone, CA028 for treating melanoma. CA028 is a derivative of calycosin with molar mass 284.26 g/mol. The chemical structure of calycosin is 7-hydroxy-3-(3-hydroxy-4-methoxyphenyl) chromen-4-one (C16H12O5) (Supplementary Fig. 2). Calycosin was reported to target many signaling pathways such as TGFBR1 signaling, PI3K/AKT signaling, HMGB1/TLR4/NF-kappaB signaling, MAPK/STAT3/NF-kappaB and NF-kappaB signaling29,30,31,32,33. CA028 is synthesized by replacing the two H atoms of calycosin with CH2COOCH2CH3. By using in vitro melanoma cell line models A375 and A2058, we demonstrated the treatment of CA028 could induce apoptosis of melanoma cells; and inhibit the proliferation, migration, and invasion abilities; and it further supported the anti-cancer properties of CA02818. The result of comparative transcriptomic analysis followed by bioinformatic analysis highlighted the anti-melanoma role of CA028 through targeting p53 pathways. P53, one of the most reported tumor suppressors, is frequently inactivated in melanoma by diverse mechanisms34, in which, near 90% of human melanoma harbor inactivated wild-type p535. More importantly, it has been demonstrated that the p53 pathway is helpful for acquired resistance to targeted therapy in melanoma34. So, targeting p53 pathway could be a promising approach for treating melanoma. For example, p53-activating agent SLMP53-2 was reported to inhibit the growth and induce cell cycle arrest and apoptosis of human melanoma cells36. Other than p53 pathways, our result also demonstrated that the CA028 could target many genes involved in the key pathways of melanoma carcinogenesis, including MAPK pathway. Highly metastatic melanoma was found to harbor frequent mutations on the genes involved in the MAPK pathway, especially protein kinase BRAF (particularly the V600E mutation, 40–50%), which leads to a substantial increase in the kinase activity of the BRAF protein37. Another common mutation in melanoma is NRAS (15–20% of cutaneous melanomas), which leads to activation of MAPK pathway43, eventually promotes melanomagenesis and tumor cell metastasis of melanoma38.
In addition, the KEGG pathway analysis further suggested the signaling pathways involved in the anti-melanoma roles of CA028 through targeting and inducing a gene cluster, including induction of BMP4, CDKN1A, WNT7B, SUFU, and BAK1, to regulate tumorigenicity and metastasis of basal cell carcinoma. Bone morphogenetic protein 4 (BMP4), belongs to the bone morphogenetic protein (BMP) family, is an important class of cell signaling proteins involved in a variety of biological processes, including embryonic development, cell proliferation and differentiation, skeletal development, and tissue repair39. BMP4 was reported to inhibit melanogenesis, partially by downregulation tyrosinase activity40. Some studies have shown that BMP4 has the potential to inhibit tumor growth. BMP4 acts as an autocrine mediator and is able to regulate a range of metastasis-associated genes, including Smad7, which in turn is involved in the activation of the canonical BMP-Smad signaling pathway. Restoration of BMP4 expression or therapeutic administration of BMP4 protein reduces the number of circulating tumor cells, leading to reduced metastasis and increased patient’s survival rate41. Cyclin-dependent kinase inhibitor 1A (CDKN1A), a cell cycle regulatory protein, is also known as p21 or p21CIP1. The regulation of CDKN1A is closely related to cellular stress, DNA damage, and cellular aging. It has the function of inhibiting cell proliferation and cell cycle progression of cancer cells42. CDKN1A was reported to be suppressed in melanoma43, and the low expression of CDKN1A resulted in deregulation of cell cycle; and excessive cell proliferation and metastasis, leading to development of melanoma44. Wingless-type MMTV integration site family, member 7B (WNT7B), a secreted protein, is a key member of the Wnt signaling pathway, which plays an important regulatory role in biological processes such as embryonic development, tissue formation and cell growth. A histopathological study of malignant melanoma demonstrated that WNT7B is expressed at relatively low levels in melanoma. However, its functional role in melanoma is still not reported45. But, low expression of WNT7B may be associated with the progression of melanoma by enhancing proliferation, migration, and invasion of melanoma cells. Suppressor of fused (SUFU) is an inhibitor of hedgehog signaling pathway through the regulation of transcription factors GLI. The low expression level of SUFU in melanoma tissues suggests the hyperactivation of hedgehog signaling pathway, which is associated with pathological features including progression, metastasis, and drug resistance in melanoma. An in vitro study using melanoblasts showed that knockdown of Sufu significantly increase cell migration by increasing the phosphorylation of ERK and reducing the level of GLI346. Bcl-2 antagonist/killer 1 (BAK), belongs to the Bcl-2 family, is a key apoptosis regulatory protein that activates cell apoptotic pathways by interacting with other Bcl-2 family members such as BAX47. Deletion or dysfunction of the BAK1 gene in tumor cells make them evading from the apoptotic mechanism, thereby promoting their growth and survival48.
In conclusion, this study identifies many possible targets of CA028 and bioinformatic analysis further unfolds their biological functions and signaling pathways involved in the anti-melanoma roles of CA028, suggesting the possible use of CA028 for treating melanoma. All these target genes could serve as promising therapeutic targets for further study.

Responses