Anti-inflammatory activity of collagen peptide in vitro and its effect on improving ulcerative colitis

Introduction
With the development of the global cod fishing and processing industry, the production of cod skin, a by-product of the cod processing industry, has also increased. If not effectively utilized, cod skin waste not only results in the loss of valuable resources but may also contribute to environmental pollution. Research has shown that cod skin is rich in protein, vitamin D, various essential minerals (such as calcium, iron, magnesium, and zinc), and unsaturated fatty acids (such as EPA and DHA), which are crucial for maintaining normal physiological functions, promoting bone health, protecting vision, supporting growth and development, and enhancing cardiovascular health1. Additionally, the collagen present in cod skin contributes to the health of the skin, bones, and joints2. Previous studies have demonstrated that cod skin can be utilized in various ways, including the extraction of collagen, gelatin, and collagen peptides for use as food additives and in the production of functional foods3.
Ulcerative colitis (UC) is a major form of Inflammatory Bowel Disease (IBD), resulting from a complex interplay among genetic, immune, microbial, and environmental factors. The disease primarily localizes in the colonic mucosa, causing congestion, swelling, and erosion of the mucosal lining. Clinically, UC presents with abdominal pain, distension, mucous, and bloody stools, often accompanied by weight loss, significantly increasing the risk of colon cancer and posing serious health risks4,5. Common treatments for UC include immunosuppressants, glucocorticoids, and biologics. Although these drugs can alleviate UC symptoms, they are often associated with adverse reactions such as rash and edema6. Consequently, functional foods aimed at modulating inflammation offer advantages over pharmacological treatments by avoiding the drawbacks of lengthy drug development and clinical trial cycles as well as potential side effects. Recent studies have demonstrated that collagen peptides from cod skin exhibit significant anti-tumor, anti-inflammatory, and immunomodulatory properties. WU et al. 7 prepared a gelatin-hydrolyzed oligopeptide from cod skin and found that it inhibited gastric cancer cell proliferation while inducing apoptosis by modulating the expression of pro-apoptotic proteins. NIU et al. 8 prepared collagen peptides from cod skin and found that it improved the symptoms of gastric ulcers and may be a viable treatment option. HAN et al. 9 demonstrated that collagen peptides from cod skin protected liver tissues from oxidative damage both in vivo and in vitro, with potential mechanisms involving enhanced antioxidant enzyme activity and reduced lipid peroxidation. GUO et al. 10 found that cod (Gadus) skin collagen peptide powder reduced inflammation, restored mucosal barrier function, and inhibited fibrosis in a mouse model of dextran sodium sulfate-induced colitis. Although most scholars have investigated the biological activities of collagen peptides, the precise mechanisms by which these collagen peptides alleviate ulcerative colitis remain unclear.
Therefore, to address the issue of extensive waste from cod skin and further investigate its potential for alleviating ulcerative colitis, we enzymatically synthesized collagen peptides from cod skin and evaluated their anti-inflammatory activity and mechanisms for mitigating ulcerative colitis both in vitro and in vivo. This study provides a scientific foundation for the development of functional foods utilizing collagen peptides as a raw material.
Results
Characterization results of collagen peptides
The results of the characterization of collagen peptides are shown in Fig. 1. Figure 1A demonstrates that the infrared spectrum of collagen peptides exhibits the characteristic absorption peaks of type I collagen, specifically amides A, B, I, II, and III. The absorption peak of amide A is observed at 3342.64 cm−1, corresponding to N-H stretching vibrations, typically occurring in the range of 3400 cm−1 to 3440 cm−1. However, when the N-H group forms hydrogen bonds, the absorption peak is reduced by approximately 100 cm−[1 11, indicating the presence of hydrogen bonds in the collagen peptides. The absorption peak of amide B is observed at 2960.73 cm−1, primarily produced by the asymmetric stretching vibration of the CH2 group, characteristic of the peptide’s tertiary structure, suggesting that the tertiary structure remains intact. Amide I is responsible for the characteristic absorption band of the secondary structure of collagen, with the C = O stretching vibration producing the amide I band, typically found between 1690 and 1625 cm−1. The absorption peak of amide II is observed at 1544.98 cm−1, produced by N-H in-plane bending vibrations and C-N stretching vibrations12. Finally, the absorption peak of amide III is detected at 1247.94 cm−1, indicating that the collagen peptides retain their complete triple helix structure. Figure 1B presents the UV absorption spectrum of the collagen peptides, with an obvious absorption peak observed near 235 nm, consistent with the UV absorption characteristics of type I collagen. The UV absorption spectrum serves as an important basis for determining collagen type. For type I collagen, the strongest absorption peak is typically observed at a wavelength of around 235 nm, characteristic of its triple helix structure. Figure 1C displays the high-resolution mass spectrometry results for collagen peptides, revealing a series of mass-to-charge ratio peaks. These peaks represent different peptides, and their mass-to-charge ratio distributions can be used to determine the relative mass of the polypeptide fragments. The molecular weight of the collagen peptides ranges from 600 Da to 1000 Da, with the maximum peak occurring between 0.08 and 0.19 min, indicating that most compounds eluted early in the analysis. The most significant peaks were m/z 215.03, 290.08, and 435.06. The peak at m/z 215.03 corresponds to dipeptides or tripeptides containing one or more smaller amino acids. The peak at m/z 290.08 corresponds to a larger peptide containing proline, alanine, or serine, among others. The peak at m/z 435.06 represents longer peptide fragments. The difference between m/z 290.08 and m/z 215.03 is approximately 75 Da, corresponding to serine, while the difference between m/z 435.06 and m/z 290.08 is about 145 Da, corresponding to phenylalanine. Figure 1D illustrates the scanning electron microscopy results of collagen peptides. The texture pores of the collagen peptides are small and relatively uniform, indicating a complete structure. This porous network structure suggests that the collagen peptides exhibit excellent moisture retention and water absorption properties.
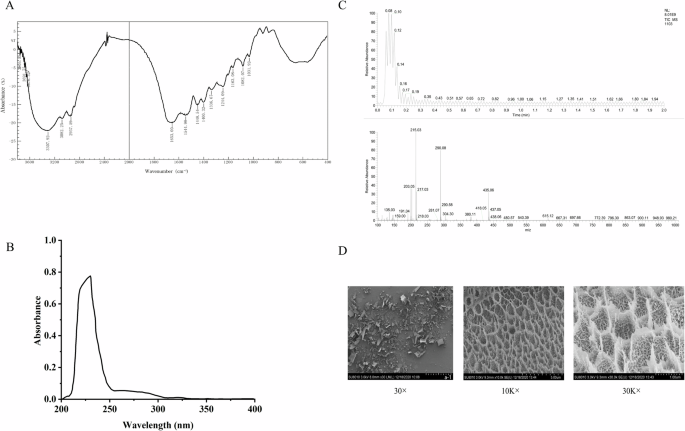
A FT-IR analysis of collagen peptides. B UV spectroscopy results of collagen peptides. C High-resolution mass spectrometry data of collagen peptides. D SEM images of collagen peptides.
In vitro anti-inflammatory activity of collagen peptides from cod skin
Collagen peptides suppress the production of inflammatory mediators in LPS-activated RAW264.7 cells without altering cell viability
In safety assessments, cell viability assays are among the commonly employed methods. By measuring the effect of collagen peptides on cell viability, we can make a preliminary assessment of their potential cytotoxicity and determine the therapeutic dosing of collagen peptides in vitro, providing important insights for further research. Figure 2A illustrates that cell viability was optimal when the collagen peptides concentration ranged between 25 and 100 μg/mL. Therefore, 25, 50, and 100 μg/mL were selected as the low, medium, and high therapeutic dosing concentrations in this study. When nitric oxide (NO) is overproduced in vivo, it can react with superoxide anions to form potent oxidizing agents, such as peroxynitrite ions, which can damage tissues and cells. Additionally, excessive NO can inhibit energy synthesis and interfere with DNA replication, posing significant health risks13. Figure 2B demonstrates that NO levels in RAW264.7 cells significantly increased after Lipopolysaccharides (LPS) stimulation (P < 0.01), but significantly decreased following collagen peptides treatment (P < 0.01), indicating that collagen peptides suppress the overproduction of NO, thereby mitigating inflammation. Excessive production of pro-inflammatory cytokines can induce an overactive inflammatory response, leading to tissue and cell damage. Figure 2C, D, and E illustrates that, compared to the control group, the levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were significantly elevated in the LPS group (P < 0.01). Conversely, different concentrations of collagen peptides significantly reduced the secretion levels of IL-1β, IL-6, and TNF-α in a dose-dependent manner (P < 0.01). These findings indicate that collagen peptides inhibit the secretion of pro-inflammatory cytokines induced by LPS in RAW264.7 cells, thereby attenuating inflammatory responses.
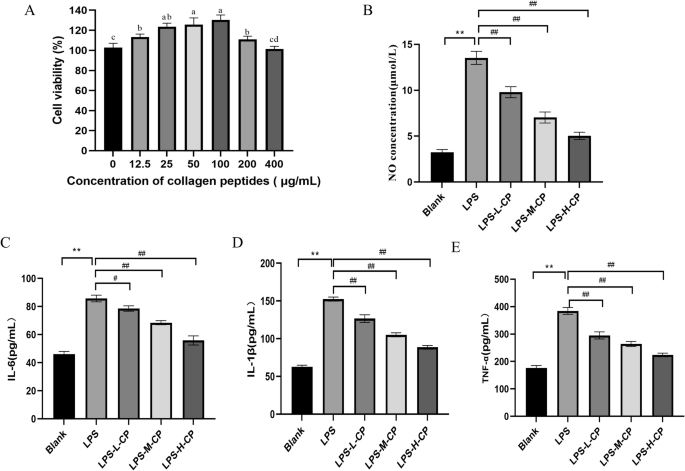
A Cell viability. B NO secretion levels. C IL-6 content. D IL-1β content. E TNF-α content. Values are expressed as the mean ± SD (n = 3). Different lowercase letters indicate significant differences (p < 0.05). *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the LPS group.
Collagen peptides improve the oxidative stress response of RAW264.7 cells activated by LPS
There is a reciprocal relationship between oxidative stress and the inflammatory response. Oxidative stress can activate the inflammatory response, while inflammation can amplify the intensity and duration of oxidative stress, creating a vicious cycle14. Figure 3A illustrates that, compared with the control group, the average fluorescence intensity of reactive oxygen species (ROS) in RAW264.7 cells stimulated by LPS was 37.45%. After treatment with varying concentrations of collagen peptides, ROS levels in LPS-stimulated RAW264.7 cells were significantly reduced (P < 0.01). Figure 3B, C, and D displays the alterations in the levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) within the cells. The results indicate that LPS stimulation significantly decreased the levels of GSH and SOD (P < 0.01) while markedly increasing MDA production (P < 0.01). Collagen peptides treatment significantly mitigated these alterations and attenuated oxidative damage in a dose-dependent manner.
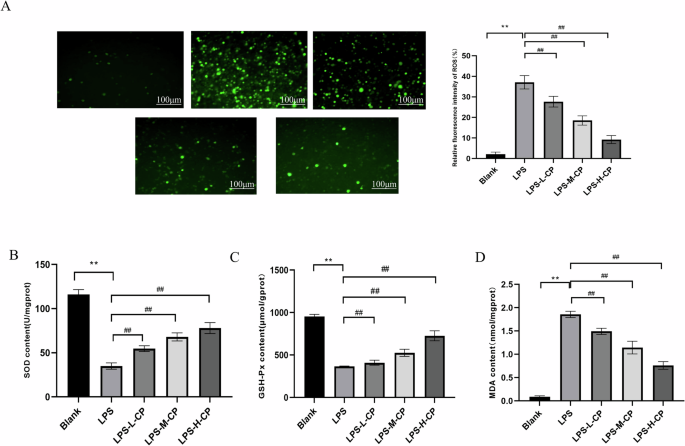
A ROS levels. B SOD activity. C GSH-Px content. D MDA content. Values are expressed as the mean ± SD (n = 3). Statistical comparisons: *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the LPS group.
Collagen peptides suppress the mRNA levels of inflammatory genes stimulated by LPS
Under normal physiological conditions, inducible nitric oxide sythase (iNOS) expression is minimal; however, during an inflammatory response, iNOS expression increases significantly, catalyzing the production of large amounts of NO, which subsequently participates in regulating inflammation. Therefore, detecting the mRNA expression levels of iNOS can assess the intensity of the cellular inflammatory response. cyclooxygenase 2 (COX-2) is another key enzyme involved in inflammation, catalyzing the conversion of arachidonic acid into prostaglandins and other inflammatory mediators. COX-2 expression is also upregulated in response to inflammatory stimuli15. Measuring the mRNA expression levels of COX-2 can further indicate the inflammatory status of the cells. As shown in Fig. 4, the mRNA expression levels of iNOS and COX-2 in RAW264.7 cells were significantly upregulated by LPS (P < 0.01), consistent with the observed increase in NO secretion. Conversely, collagen peptides significantly reduced their expression levels (P < 0.01).
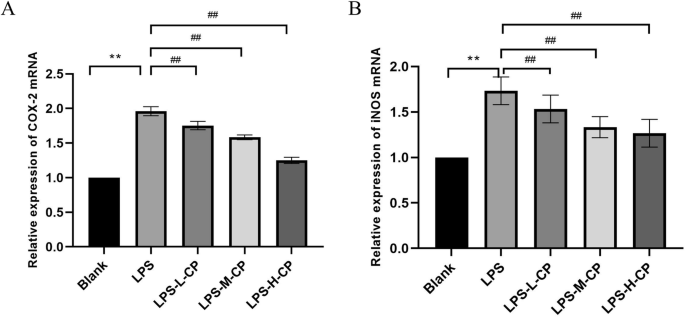
A COX-2 mRNA expression. B iNOS mRNA expression. Values are expressed as the mean ± SD (n = 3). Statistical comparisons: *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the LPS group.
Effect and mechanism of collagen peptides from cod skin on colitis in mice
Effects of collagen peptides on body weight, colon length, and DAI score of mice
As shown in Fig. 5A and B, DSS treatment significantly reduced both the body weight change rate and colon length in mice compared to the blank group (P < 0.01). In comparison to the model group, collagen peptides administration significantly increased both the body weight change rate and colon length (P < 0.05). As shown in Fig. 5C, the disease activity index (DAI) score of the model group was significantly higher than that of the normal group (P < 0.01), confirming the successful construction of the ulcerative colitis model. In comparison to the model group, collagen peptides treatment significantly reduced the DAI score (P < 0.05).
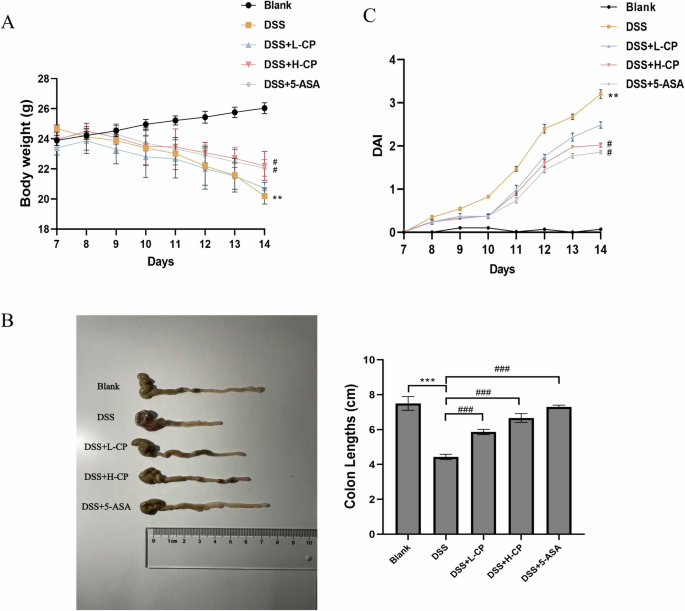
A Body weight changes. B Colon length. C DAI score. Values are expressed as the mean ± SD (n = 10). Statistical comparisons: *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the DSS group.
Effect of collagen peptides on colonic pathology in mice
As an essential part of the mouse intestine, the colon plays a crucial role in absorbing substances to provide nutrients to the body. As shown in Fig. 6, the colon mucosa of the normal group appears smooth and intact, with well-defined crypts and goblet cells, abundant and neatly arranged intestinal glands, uniform submucosal space, and no signs of inflammatory cell infiltration. In contrast, the model group exhibited severe colon edema, necrosis of the mucosal layer, disappearance of crypt structures, and diffuse inflammatory cell infiltration. In comparison to the model group, collagen peptides treatment significantly reduced inflammatory cell infiltration and ameliorated the pathological changes in colon tissues induced by dextran sulfate (DSS).
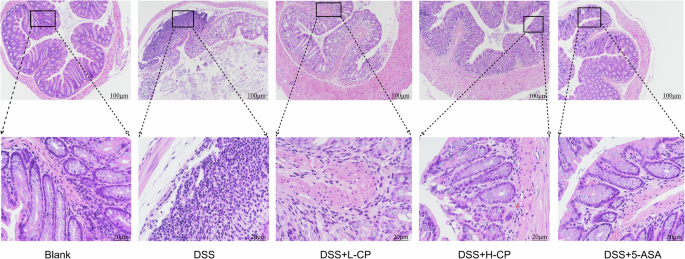
Effect of collagen peptide on DSS-induced colonic histopathological changes in UC mice.
Effects of collagen peptides on cytokines, NO secretion, and MPO activity in mice
When assessing the degree of inflammation, changes in inflammatory cytokine levels are critical. The balance between proinflammatory and anti-inflammatory cytokines plays a pivotal role in the regulation of inflammation. As shown in Fig. 7A and B, IL-6 and TNF-α levels in the model group were significantly higher than in the blank group (p < 0.001). Compared with the DSS group, IL-6, and TNF-α levels were significantly reduced in the collagen peptides-treated group (p < 0.001). Figure 7C shows the changes in the levels of the anti-inflammatory cytokine IL-10. Compared with the blank group, IL-10 levels were significantly decreased in the DSS model group (p < 0.001), whereas collagen peptides treatment significantly increased IL-10 levels (p < 0.001). These findings suggest that collagen peptides may help prevent and manage ulcerative colitis by regulating the balance between proinflammatory and anti-inflammatory factors. Myeloperoxidase (MPO) activity and NO levels are closely associated with inflammatory diseases. Figure 7D and E demonstrates that MPO activity and NO levels were significantly higher in the DSS model group than in the blank group (p < 0.001). In contrast, collagen peptides treatment significantly reduced MPO activity and NO levels in a dose-dependent manner (p < 0.001).
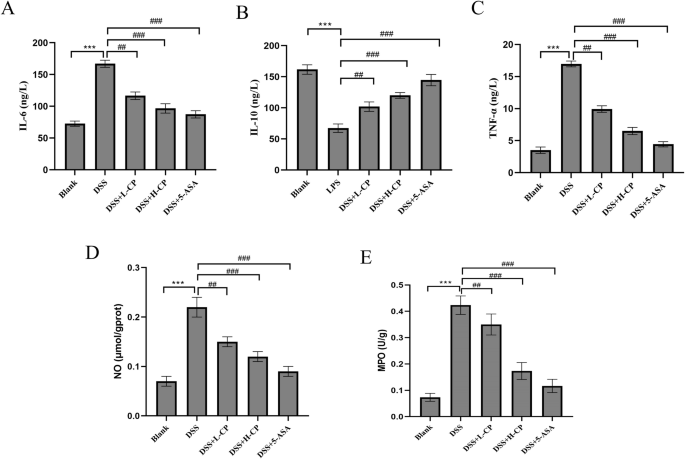
A IL-6 content. B IL-10 content. C TNF-α content. D NO secretion. E MPO activity. Values are expressed as the mean ± SD (n = 10). Statistical comparisons: *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the DSS group.
Effect of collagen peptides on the protein expression of ZO-1, Occludin and Claudin-1 in colon tissue of mice
The expression of tight junction proteins zonula occludens 1 (ZO-1), Occludin, and Claudin-1 was assessed via immunohistochemical analysis. As shown in Fig. 8, the expression levels of Occludin, Claudin-1, and ZO-1 in the colon tissue were significantly decreased following DSS induction (p < 0.001), indicating impaired intestinal barrier function. After intervention and treatment with collagen peptides, the expression levels of Occludin, Claudin-1, and ZO-1 were significantly increased (p < 0.001), suggesting that collagen peptides helped maintain intestinal barrier integrity, thereby preventing further damage associated with ulcerative colitis.
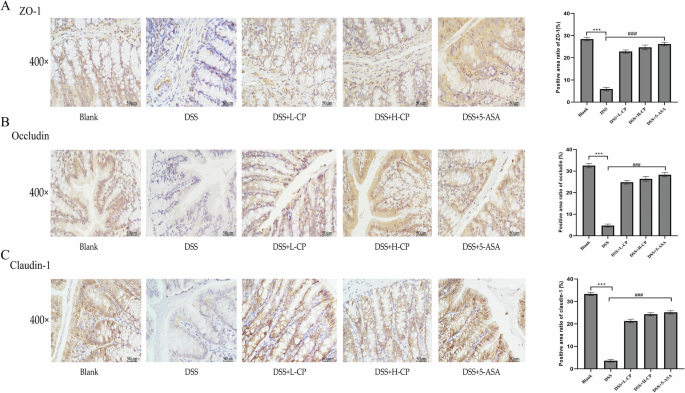
A ZO-1 expression. B Occludin expression. C Claudin-1 expression. Values are expressed as the mean ± SD (n = 10). Statistical comparisons: *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the DSS group.
The regulatory effect of collagen peptides on NF-κB /MAPK signaling pathways in colon tissue
The expression of the nuclear factor kappa-B/mitogen-activated protein kinase (NF-κB/MAPK) signaling pathway in colon tissue was further examined. As shown in Fig. 9A-B, the NF-κB/MAPK signaling pathway was activated in the colon of the DSS group, with increased phosphorylation levels of IκBα, NF-κB p65, and P38 MAPK. Collagen peptides inhibited the phosphorylation of IκBα, NF-κB p65, and P38 MAPK proteins in the colon of mice. The ratios of p-IκBα/IκBα, p-p65/p65, and p-p38 MAPK/P38 MAPK were decreased.
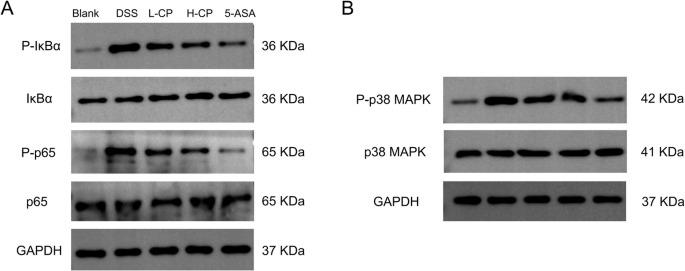
A Protein expression of IκBα, P-IκBα, p65, and P-p65 was assessed by Western blot, with GAPDH serving as a loading control. B Protein expression of P38 MAPK and P-P38 MAPK was assessed by Western blot, with GAPDH serving as a loading control.
Effects of collagen peptides on intestinal microbiota in mice
Microbes are crucial in the development of colitis, and regulating intestinal flora to improve colitis is a focus of current research. Therefore, the present study examined changes in gut microbiota. Figure 10A shows that as sequencing volume increased, rarefaction curves and operational taxonomic units (OUT) ranks of the different groups first increased and then stabilized, indicating that the sequencing volume is reasonable and the depth is sufficient to reflect sample diversity and ensure result accuracy. Figure 10B shows that compared with the blank group, the Chao1 index, Simpson index, Shannon index, and Faith’s PD index were significantly lower in the DSS group (P < 0.01), indicating that DSS-induced UC led to a decrease in the overall abundance of intestinal flora. After treatment with collagen peptides, the overall abundance of intestinal flora significantly increased (P < 0.01), indicating that collagen peptides restored the reduced abundance of intestinal flora in UC mice. As shown in Fig. 10C, principal coordinate analysis revealed significant differences in microbial community structure between the groups. After collagen peptides treatment, the microbial community structure in DSS-induced mice shifted closer to that of the Control group, indicating that the structure of intestinal flora resembled that of the healthy group after treatment. Figure 11A shows that Firmicutes, Bacteroidetes, and Proteobacteria were the dominant phyla of intestinal flora. The relative abundance ratio (F/B) of Firmicutes to Bacteroidetes in the colon of mice increased after DSS induction. An imbalance in the F/B ratio is closely related to conditions such as obesity and intestinal inflammation, and an increase in this ratio is a typical characteristic of intestinal flora imbalance. The figure shows that the collagen peptides group effectively reversed the increase in F/B in DSS-induced colitis mice. At the phylum level, compared with the blank group, the abundance of Proteobacteria in the DSS group was significantly increased. This increase is considered a diagnostic marker of UC, while collagen peptides treatment significantly reduced the abundance of Proteobacteria, thereby contributing to the restoration of intestinal flora health. Figure 11B shows that, at the genus level, the beneficial microorganisms Lachnospiraceae NK4A136 group were significantly reduced in the DSS group, while their abundance significantly increased in the collagen peptides treatment group. Pseudomonas abundance increased after DSS treatment, but collagen peptides treatment significantly reduced the relative abundance of Pseudomonas. Combined with previous studies, collagen peptides were shown to improve gut microbiota imbalance in mice with colitis by increasing the relative abundance of probiotics, reducing harmful bacteria, and repairing the damaged intestinal mucosa and intestinal barrier.
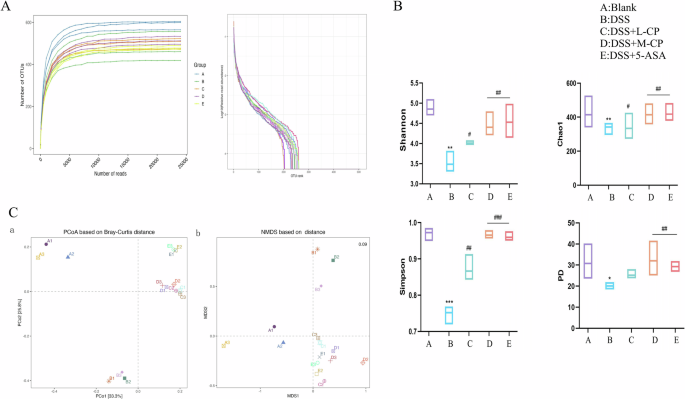
A, B α-Diversity. C β-Diversity. Data are expressed as the mean ± SD (n = 3). Statistical comparisons: *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and###p < 0.001 versus the DSS group.
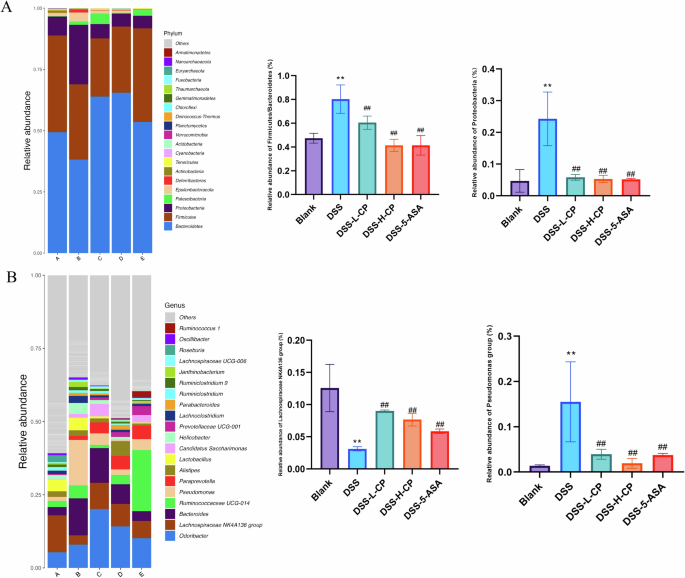
A Phylum-level composition. B Genus-level composition. Data are expressed as the mean ± SD (n = 3). Statistical comparisons: *p < 0.05, **p < 0.01, and ***p < 0.001 versus the blank group; #p < 0.05, ##p < 0.01, and ###p < 0.001 versus the DSS group.
Discussion
Current studies have demonstrated that collagen peptides exhibit anti-inflammatory, antioxidant, and immunomodulatory functions. LUO et al. 16 discovered that a novel collagen peptide derived from Atlantic salmon (Salmo salar) bone can delay osteoarthritis development by inhibiting cartilage matrix degradation and exerting anti-inflammatory effects. LIU et al. 17 reported that collagen peptides derived from the enzymatic hydrolysate of Salmo salar skin exhibit anti-inflammatory activity in the LPS-induced RAW264.7 inflammatory model. LIN et al. 18 demonstrated that marine bioactive peptides can protect rats from early alcoholic liver injury through their antioxidant activity and by improving lipid metabolism. REN et al. 19 indicated that tilapia skin collagen peptides exhibit antioxidant and protective effects on red blood cells. Our previous research also demonstrated that collagen peptides from cod skin can delay the senescence of 2BS cells20. Although extensive research has explored various bioactive functions of collagen peptides from different sources, the mechanism of action of these peptides in ulcerative colitis remains unclear. Therefore, this study aims to investigate the effects and potential mechanisms of collagen peptides in the context of ulcerative colitis. Inflammation is an adaptive response of the immune system, triggered by pathogens, damaged cells, exogenous stimuli, and various other factors. It plays a central role in the host defense system and is crucial for maintaining homeostasis. However, excessive inflammation can have detrimental effects on the body, leading to tissue damage and various diseases21. LPS, a potent inducer of cytotoxic inflammation, interacts with Toll-like receptor 4 (TLR4) on macrophages to initiate the production of inflammatory mediators. Therefore, we employed LPS to establish a RAW264.7 inflammatory cell model for investigating the anti-inflammatory effects of collagen peptides in vitro. NO is a crucial signaling molecule in the inflammatory response. TNF-α, an initiating factor in the inflammatory response, can induce the production of reactive oxygen species by adherent neutrophils and promote the expression of other pro-inflammatory cytokines. Excessive release of inflammatory mediators can contribute to the development of inflammation-related diseases; thus, regulating the levels of pro-inflammatory cytokines is a crucial and effective strategy for mitigating inflammation22. The results of this study indicate that collagen peptides can reduce the secretion of NO and the inflammatory cytokines TNF-α, IL-1β, and IL-6, suggesting that the anti-inflammatory effect of collagen peptides is associated with the inhibition of inflammatory mediator expression. As a precursor to NO, the expression of iNOS mRNA in activated cells can drive excessive NO production, which is linked to various inflammatory immune disorders. Concurrently, when cells are stimulated by inflammatory and other stimuli, the mRNA expression of COX-2, which is typically low in normal cells, increases, exacerbating the inflammatory response and tissue damage. Importantly, NF-κB plays a crucial role in regulating inflammatory mediators, and its translocation is involved in the expression of iNOS and COX-223. Our results demonstrated that collagen peptides exert anti-inflammatory effects by inhibiting the expression of iNOS and COX-2. ROS, as chemically active oxygen-containing molecules produced during oxygen metabolism in biological systems, play a crucial role in the inflammatory response by stimulating the expression of pro-inflammatory cytokines. Moreover, increased ROS accumulation leads to oxidative stress, resulting in the senescence of major cellular components (lipids, proteins, and DNA). MDA is a well-established indicator of lipid peroxidation24. Concurrently, the antioxidant defense system protects biological systems by mitigating the destructive effects of ROS. Key components include the antioxidant enzyme SOD and the enzymatic antioxidant GSH, which play crucial roles in maintaining normal ROS levels. Consequently, excessive ROS production is a major factor contributing to the inflammatory response, and effective scavenging of ROS is beneficial for mitigating inflammation25. The results indicated that collagen peptides reduced ROS and MDA production, increased levels of GSH and SOD, inhibited oxidative stress, and alleviated the inflammatory response.
The results of this experiment indicated that administration of a 3.5% DSS solution led to noticeable symptoms such as weight loss, fecal bleeding, and decreased appetite. In addition, the colon was found to be significantly shortened after mouse dissection, which is consistent with the findings of HUANG et al. 26. Compared to the DSS model group, collagen peptides effectively alleviated these symptoms, with the high-dose collagen peptides group showing superior efficacy compared to the low-dose group. In recent years, the incidence of UC has significantly increased worldwide, adversely affecting patients’ quality of life and greatly elevating the risk of colon cancer. Nevertheless, its etiology remains unclear. Current research indicates that ulcerative colitis is closely associated with an imbalance between pro-inflammatory and anti-inflammatory cytokines, impaired colonic epithelial barrier function, and dysbiosis of the intestinal flora. TNF-α and IL-6 are crucial mediators of the inflammatory response in patients with intestinal inflammation, and their levels are closely associated with ulcerative colitis. Consistent with the findings of ZHU et al. 27, we observed that TNF-α and IL-6 levels in the DSS group were significantly higher than those in the control group, and these levels were notably reduced following collagen peptide intervention. Additionally, we observed a significant decrease in IL-10 levels following DSS treatment, which was markedly increased by collagen peptide intervention. Interleukin-10 (IL-10), an anti-inflammatory cytokine primarily secreted by regulatory T cells, has been demonstrated to have a protective effect against ulcerative colitis28. Concurrently, we assessed the changes in MPO activity and NO levels in the colon tissue of mice. MPO activity serves as an indicator of colonic mucosal inflammation and correlates with neutrophil infiltration. MPO is a heme-containing protein abundant in neutrophils, and inflammatory responses can lead to neutrophil aggregation, subsequently releasing myeloperoxidase29. NO is a highly reactive free radical, and its excessive production is associated with inflammatory diseases. The results indicated that MPO activity and NO levels were significantly elevated in the DSS model group, but these changes were markedly reversed by intervention with collagen peptides. These findings are consistent with our in vitro cell experiments. The integrity of the intestinal barrier and a healthy gut microbiota are crucial for host health. These factors are considered intrinsic to colitis and are closely associated with various diseases, including cancer. Intestinal barrier function and the integrity of a healthy gut microbiota are essential for host health. They are considered intrinsic environmental factors in colitis and are closely associated with various diseases, including cancer. Intestinal dysbiosis and impaired barrier function play a pivotal role in the pathogenesis of ulcerative colitis30. Therefore, we examined the changes in tight junction proteins in colon tissue using immunohistochemistry. Studies have demonstrated that tight junctions are the most crucial connections between colonic epithelial cells and serve as the foundation for maintaining barrier function. Claudin-1, Occludin, and ZO-1 are key protein components in intestinal tight junctions. Occludin was the first membrane-associated tight junction protein identified and is involved in various junctional functions. Most members of the Occludin family localize at intercellular junctions and interact with the cytoskeleton via extracellular signal transduction pathways. The interaction between Occludin and ZO-1 proteins regulates intracellular and extracellular signaling, influencing the integrity of tight junction proteins and thereby modulating intestinal mucosal permeability. Claudin proteins are essential for maintaining cell barrier integrity, protective function, proliferation, metastasis inhibition, and signal transduction31,32. Our experimental results indicated that the levels of Claudin-1, Occludin, and ZO-1 were significantly down-regulated in the DSS model group, suggesting that ulcerative colitis severely compromised colonic barrier function and triggered a marked inflammatory response in the host. Collagen peptides treatment reversed this effect, suggesting its protective role in maintaining intestinal barrier function.
Most proteins in the NF-κB pathway regulate inflammation, stress responses, and the immune system. NF-κB typically binds to IκB as a p50-p65 dimer, forming an inactive complex in the cytoplasm. When stimulated by proinflammatory factors, IκB kinase becomes activated, causing IκB to dissociate from NF-κB. NF-κB p65 translocates from the cytoplasm to the nucleus, where it binds specifically to target genes, such as IL-6 and TNF-α, inducing the transcription and release of proinflammatory mediators. NF-κB functions as an activator of inflammatory factors involved in transcription. The MAPK family of serine/threonine protein kinases mediates essential biological processes and cellular responses to external stress signals, regulating the synthesis of inflammatory and apoptotic mediators. The MAPK signaling pathway operates upstream of NF-κB, and its activation contributes to NF-κB activation and nuclear translocation, leading to inflammatory responses33. A previous study by Jin et al. 34 found that the NF-κB/MAPK signaling pathway was activated in the colons of DSS-treated mice. Our results demonstrated that the development of UC led to the activation of the NF-κB/MAPK signaling pathway in mice. In the NF-κB pathway, the phosphorylation levels of IκBα and p65, as well as P38 MAPK in the MAPK pathway, were significantly elevated. However, collagen peptides intervention significantly inhibited this phenomenon.
UC is closely associated with an imbalance in intestinal flora. As intestinal flora plays a crucial role in regulating host immunity, an imbalance in the microbiota can disrupt immune homeostasis, potentially contributing to UC. Therefore, we analyzed the composition and changes in intestinal flora across different groups of mice using 16S rRNA high-throughput sequencing. At the phylum level, Bacteroidetes were significantly decreased, Proteobacteria were markedly increased, and the ratio of Firmicutes to Bacteroidetes was elevated in the DSS model group. Related studies have also shown that UC is associated with a microbiota imbalance characterized by a reduction in Bacteroidetes, which is linked to IBD, particularly during the active phase of the disease35. Additionally, in the presence of immune disorders, the abundance of Proteobacteria, typically low in healthy individuals, significantly increases, promoting intestinal inflammation36. Previous studies have demonstrated that an elevated Firmicutes-to-Bacteroidetes ratio is indicative of significant inflammation, commonly observed in UC patients37. At the genus level, the DSS model group exhibited a marked reduction in Lachnospiraceae NK4A136 and a notable increase in Pseudomonas compared with the control group. Lachnospiraceae NK4A136 is a potential probiotic, whereas Pseudomonas is linked to inflammatory responses to some extent. Pseudomonas can adhere to the mucosa, invade intestinal epithelial cells, and exacerbate inflammation38,39. Intestinal microbiota plays a critical role in the pathogenesis of ulcerative colitis. Regulating intestinal microorganisms presents a unique therapeutic potential for treating ulcerative colitis. Collagen peptides significantly regulate the balance of intestinal microbiota, protect intestinal health, and alleviate ulcerative colitis.
Taken together, our study demonstrates that collagen peptides derived from cod skin can protect RAW264.7 cells from LPS-induced inflammation and oxidative stress in vitro. Furthermore, it was confirmed that collagen peptides improve DSS-induced ulcerative colitis in mice by regulating cytokine secretion, ameliorating pathological damage, enhancing intestinal barrier function, inhibiting activation of the NF-κB/MAPK signaling pathway, and modulating the composition of the intestinal microbiota. However, while the anti-inflammatory effects of collagen peptides were confirmed both in vitro and in vivo, the study primarily focused on their anti-inflammatory properties without fully elucidating the underlying mechanisms driving these effects. The specific molecular pathways and cellular processes through which collagen peptides exert their anti-inflammatory and protective actions require further investigation. Although involvement of the NF-κB/MAPK signaling pathway was indicated, more comprehensive mechanistic studies are necessary to identify the precise molecular interactions and targets of collagen peptide activity. Future studies will aim to explore these mechanisms in greater detail, potentially incorporating proteomics and transcriptomics to gain a deeper understanding of how collagen peptides regulate inflammatory responses at the molecular level. Additionally, the long-term effects of collagen peptides on immune cell populations and gut microbiota will be examined to assess their therapeutic potential for UC and other inflammatory diseases.
This study suggests that collagen peptides from cod skin exhibit anti-inflammatory properties and may prevent the onset of UC. Collagen peptides from cod skin significantly inhibit LPS-induced inflammation in RAW264.7 cells in vitro. Additionally, it reduces inflammation by inhibiting the activation of the NF-κB/MAPK signaling pathway and down-regulating proinflammatory cytokine gene expression. Moreover, by regulating the intestinal microbiota, the abundance of harmful bacteria is decreased, while the relative abundance of probiotics is increased, thereby maintaining intestinal health. Thus, collagen peptides from cod skin may represent a novel therapeutic strategy for treating intestinal inflammation. Further research should focus on exploring its in vivo bioavailability and underlying mechanisms.
Materials and methods
Experimental materials
Cod skin was purchased from Xinping Fishery (Jilin, China), pepsin (1:3000), sodium chloride, sodium hydroxide, n-butyl alcohol, calcium chloride, and activated carbon were sourced from Solaibao Biotechnology Co., Ltd (Beijing, China). PBS buffer, DMEM high-glucose medium, fetal bovine serum (FBS), and 0.25% trypsin EDTA were obtained from Gibco (USA). The detection kits for SOD, MDA, GSH-Px, NO, MPO, IL-1β, IL-6, and TNF-α were obtained from Shanghai Enzyme Linked Biotechnology Co., Ltd. CCK-8 and ROS kits were sourced from Biyuntian Company (Shanghai, China), and dextran sulfate sodium (MW: 36,000–50,000) was sourced from Shanghai Yisheng Biotechnology Co., Ltd (Shanghai, China). Mesalazine was obtained from Shanghai Aidifa Pharmaceutical Co., Ltd (Shanghai, China). Rabbit anti-occludin, anti-claudin-1, anti-ZO-1, anti-p38, anti-phosphorylated p38, anti-p65, anti-phosphop65, and anti-phosphoIκBα monoclonal antibodies, as well as the protease inhibitor mixture, were sourced from Beijing Boorson Biological Technology Co., Ltd (Beijing, China). The hematoxylin-eosin staining kit was obtained from Shanghai Qiyi Biotechnology Co., Ltd (Shanghai, China).
Preparation of collagen peptides from cod skin
The preparation of collagen peptides in this study referred to the preparation method of HOU et al. 40, with appropriate modifications based on previous experiments in our laboratory. with slight modifications. Fresh cod skin was washed thoroughly, cut into small pieces, and dried for later use. The dried cod skin was immersed in 0.1 M NaOH at a ratio of 1:10 (M) at 4 °C for 24 h, during which the alkaline solution was changed every 8 h to remove non-collagenous materials and impurities, before being rinsed with deionized water until neutral pH was achieved. The pretreated cod skin was immersed in 0.75 M acetic acid at a ratio of 1:10 (M), and collagen was extracted at 4 °C for 9 h. After extraction, the solution was filtered to remove insoluble materials. Collagen was enzymatically hydrolyzed using pepsin (1000 U/g) for 4 h in a water bath maintained at pH 2 and 37 °C. The collagen peptides solution was centrifuged at 6000 r/min for 20 min, and the supernatant was purified with acetic acid. The purified collagen peptides solution was frozen at −80 °C for 4 h, followed by vacuum freeze-drying. After 24 h, the collagen peptides powder was obtained for subsequent experiments.
Characterization of collagen peptides from cod skin
The collagen peptides were characterized using Fourier transform infrared spectroscopy (FT-IR), ultraviolet spectroscopy, high-resolution mass spectrometry, and scanning electron microscopy. Characterization of collagen peptides using FT-IR: Potassium bromide was heated at 110 °C for 24 h to eliminate moisture and potentially other volatile contaminants, after which collagen peptides were blended with potassium bromide, ground into a fine powder, and compressed. The samples were then placed in analysis chambers and scanned within a wavenumber range of 4000 to 500 cm−1, using potassium bromide as a baseline41. Characterization of collagen peptides using UV: The lyophilized collagen peptides were dissolved in 0.5 M acetic acid to a concentration of 1 mg/mL. Scanning was performed at room temperature with 0.5 M acetic acid as the baseline, and the scanning wavelength ranged from 200 to 400 nm. High-resolution mass spectrometry was conducted following the method of Alfieri et al. 42, at a wavelength of λ = 337 nm and an accelerating voltage of 25 kV. A 1:1 mixture of matrix solution and ultrapure water (containing 0.1% trifluoroacetic acid) served as the solvent, and the collagen peptides were dissolved in the solvent at a concentration of 1 mg/mL. The sample and matrix solutions were mixed in a 1:1 ratio (v/v), and 1 µL of the mixture was applied to the sample plate. After air drying, the plate was placed into the instrument for analysis. The morphology of collagen peptides was characterized using a scanning electron microscope. Samples were prepared and freeze-dried at −50 °C to remove residual moisture, then cut into 1×1 cm square pieces and coated with gold. The microstructures of the samples were observed at 3.0 Kv43.
In vitro anti-inflammatory activity of collagen peptides from cod skin
Cell culture
The recovered RAW264.7 cells were seeded into sterile culture flasks, and a high-glucose DMEM medium containing 10% fetal bovine serum and 1% antibiotics (streptomycin and penicillin) was added. The cells were cultured in a cell incubator at 37 °C with 5% CO2, with the culture medium being replaced regularly.
CCK-8 assay was used to detect the effect of collagen peptides on the viability of RAW264.7 cells
After the cells reached the logarithmic growth phase, 100 μL of a uniform cell suspension at a density of 3000 cells per well was added to each well of a 96-well plate. The outer wells were filled with PBS, and the plate was incubated for 24 h. Collagen peptides were added to the cultures at final concentrations of 0, 12.5, 25, 50, 100, 200, and 400 μg/mL for 24 h. The culture medium was then aspirated, and the wells were washed twice with PBS. The CCK-8 solution was prepared by diluting it with a complete medium, and 100 μL of the solution was added to each well. Cell viability was assessed after incubating for 3 h.
LPS-induced inflammation in RAW264.7 cells and experimental grouping
A total of 1.5 × 104 cells per well were seeded into six-well plates and incubated for 24 h. The cells were subsequently divided into a blank group, an LPS model group (1 μg/mL), a low-dose collagen peptides group, a medium-dose collagen peptides group, and a high-dose collagen peptides group, and incubated for an additional 48 h.
Effects of collagen peptides on NO release and cytokine secretion of RAW264.7 cells stimulated by LPS
The cells were incubated under the same conditions as previously described. The effects of different concentrations of collagen peptides on NO secretion in LPS-induced RAW264.7 cells were detected using the Griess reagent method. The cell culture medium was transferred into centrifuge tubes and centrifuged at 2500 rpm for 20 min. The supernatant was collected, and the levels of TNF-α, IL-6, and IL-1β were measured using ELISA kits.
Effect of collagen peptides on ROS content and SOD, MDA, GSH-Px levels in RAW264.7 cells stimulated by LPS
The cells were incubated under the same conditions as previously described. The ROS content was measured using the DCFH-DA fluorescent probe method, as per the instructions provided in the ROS detection kit. Images were captured using a fluorescence microscope, and a semi-quantitative analysis of fluorescence intensity was conducted using ImageJ software. The levels of SOD, MDA, and GSH-Px in the cells were measured according to the manufacturer’s protocol provided with the respective detection kits.
Effect of collagen peptides on the mRNA expression of iNOS and COX-2 in RAW264.7 cells stimulated by LPS
qRT-PCR was used for detection, and the cells were incubated under the same conditions as previously described. The cells were harvested through enzymatic digestion, and total RNA was extracted using an RNA extraction kit and reverse transcribed into cDNA according to the reagent instructions. Subsequently, PCR amplification was conducted. The PCR amplification conditions included predenaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s, and annealing and extension at 60 °C for 30 s. The expression levels of iNOS and COX-2 in the cells were quantified using the 2-ΔΔC method, with GAPDH serving as the reference gene. Primers for the target genes were synthesized by Shenggong Bioengineering (Shanghai) Co., LTD, according to the design. The primer sequences and product sizes are provided in Table 1.
Improvement mechanism of collagen peptides from cod skin on DSS-induced UC mice
Animal experiments
Fifty male Babl/c mice (20–25 g) were purchased from the Experimental Animal Center of Yanbian University (Jilin, China). All relevant regulations of the China Animal Protection Committee were followed, and approval was obtained from the Experimental Animal Ethics Committee of Yanbian University (Ethical approval number: YD20040319001). Following a one-week acclimatization period, the mice were randomly divided into five groups: the control group, the DSS model group, the low-dose collagen peptides group, the high-dose collagen peptides group, and the positive drug group. During the first seven days of the experiment, based on preliminary dosing studies, the low-dose group received 300 mg/kg of collagen peptides, the high-dose group received 600 mg/kg of collagen peptides, and the positive drug group received 100 mg/kg of mesalazine. From days 8 to 14, mice models of UC were established and modified based on previous studies44. Except for the control group, the other groups drank 3.5% DSS solution. Simultaneously, the daily activity, mental state, stool characteristics, and body weight of the mice were monitored. Fecal samples were collected daily for fecal occult blood analysis. On the final day of the experiment, the mice were fasted for 12 h, and blood samples were collected from the orbital sinus. After being kept at room temperature for 2 h, serum was obtained by centrifugation at 3000 rpm for 15 min and stored at −80 °C for serological analysis. The mice were sacrificed by cervical dislocation, and their colons were removed, measured, photographed, rinsed with precooled sterile phosphate buffer, and stored in tissue fixative and at −80 °C for further analysis.
Disease activity index score
The DAI is a comprehensive evaluation that combines body weight changes, stool consistency, and fecal bleeding45. The body weight of each group of mice was measured and recorded daily, fecal samples were assessed using an occult blood kit, and stool consistency was also observed, as shown in Table 2. The following formula was used to determine the DAI score:
The colon tissues of mice were stained with hematoxylin-eosin
Following the dissection of the mice, a small segment of colon tissue was collected, rinsed with PBS, fixed with 4% paraformaldehyde for 24 h, and embedded in paraffin. The sections were stained with hematoxylin-eosin (H&E), and pathological changes were examined under a light microscope, with images captured for analysis.
Determination of IL-6, TNF-α, and IL-10 in serum and MPO and NO in colon tissue of mice
Blood samples were collected from the mice via the retro-orbital route and kept at room temperature for 2 h. Following stratification, the supernatant was collected by centrifugation at 3000 r/min for 15 min. Serum concentrations of IL-6, TNF-α, and IL-10 were measured using an ELISA kit according to the manufacturer’s instructions. After the colon tissue was washed with PBS and blotted dry with filter paper, forty milligrams of tissue were weighed and minced to prepare the homogenate, which was then centrifuged at 4 °C and 12000 r/min for 10 min. The supernatant was collected, and MPO and NO levels were quantified according to the kit’s protocol.
The protein expressions of ZO-1, Occludin and Claudin-1 in colon tissue of mice were detected by immunohistochemistry
The tissue sections were deparaffinized, immersed in 3% methanol hydrogen peroxide for 10 min at room temperature, and washed three times with PBS for 3 min each. The sections were then incubated with primary antibodies (ZO-1, Occludin, and Claudin-1) overnight, followed by incubation with secondary antibodies at 37 °C for 30 min. The sections were washed three times with PBS for 3 min each. Color development was achieved using diaminobenzidine, and the sections were photographed using light microscopy. Positive area quantification was conducted using ImageJ software.
The related protein expressions of NF⁃κB/MAPK signaling pathway in colon tissue were detected by Western Blotting
Western Blotting was performed to detect key proteins of the NF-κB/MAPK signaling pathway, including p65, p-p65, IκBα, p-IκBα, p38, and p-p38. Proteins were separated by 10% SDS-PAGE, transferred to a PVDF membrane, and blocked using a blocking solution (0.5 g skim milk powder in 10 mL TBST) at room temperature for 30 min. Rabbit monoclonal antibodies against p65, p-p65, IκBα, p-IκBα, p38, and p-p38 (1:1000 dilution in 1×TBST) were added and incubated overnight at −4 °C. After washing four times with TBST for 8 min each, HRP-labeled goat anti-rabbit IgG secondary antibody (1:8000 dilution in 1×TBST) was added and incubated for 2 h at 4 °C. The samples were washed four more times with TBST, and detection was performed using ECL and visualized.
Effects of collagen peptides on intestinal microbiota in mice
Mouse feces were collected aseptically and stored in lyophilized tubes at −80 °C. Mouse fecal 16S rRNA gene sequencing analysis was performed by Shanghai Qiyi Biotechnology Company. Subsequent bioinformatics operations were conducted using QIIME2, while statistical analysis and graphing were primarily carried out using R, Python, and Java.
Statistical analysis
The experimental data were statistically analyzed and plotted using Prism9 software, and differences between groups were analyzed using one-way ANOVA followed by Tukey’s test. Data in the figures are presented as the mean ± SD. P-values of less than 0.05 were considered statistically significant.

Responses