The effect of a new developed synbiotic yogurt consumption on metabolic syndrome components in adults with metabolic syndrome: a randomized controlled clinical trial

Introduction
Metabolic syndrome (MetS), as a metabolic disorder, is a significant global public health and clinical challenge due to the increasing prevalence of obesity and sedentary lifestyle [1]. Multiple studies have indicated that the incidence of MetS has increased considerably in recent years [2]. According to recent estimates, on average, 20–30% of the adult population in most countries suffer from MetS [3]. In Iran, it has been reported to be 26% [4]. Impaired glucose tolerance, hypertension, dyslipidemia, and visceral obesity are the components of MetS according to the National Cholesterol Education Program (NCEP) criteria [5]. It has been shown that the risk of chronic diseases including type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) is increased in subjects with MetS [6]. Therefore, understanding the pathogenic processes that contribute to the development and progression of MetS, as well as modifying associated risk factors, is crucial to the management and treatment of the disease and the related complications [7].
Insulin resistance is an important contributor to the pathophysiology of the MetS. Abdominal obesity, as one of the components of MetS, is strongly associated with inflammation and insulin resistance [8]. In addition, studies have demonstrated that an increase in visceral adipose tissue releases free fatty acids, which can raise triglycerides and reduce hepatic insulin clearance, which also leads to insulin resistance [9].
It has been suggested that an imbalance in the gut microbiome plays a significant role in the development of metabolic disorders linked to the MetS, such as insulin resistance [10]. Modifying the gut microbiota with probiotics and synbiotics has been demonstrated to minimize the risk of MetS and associated risk factors [11]. Studies have also indicated that probiotics can improve insulin resistance by enhancing hepatic natural killer and T cell receptors and decreasing inflammatory signaling, which consequently reduces systemic inflammation [12, 13]. In addition, probiotics may improve blood pressure by releasing bioactive peptides such as angiotensin-converting enzyme-inhibiting peptides [14].
Probiotic supplements containing Lactobacillus plantarum have been demonstrated in human trials to be effective in improving MetS components including high blood pressure, lipid profile, waist circumference (WC), fasting blood glucose (FBG), and fasting plasma insulin [15]. Animal studies have also shown that probiotics containing L. plantarum can improve insulin resistance and lipid profile [16]. The concept of synbiotic yogurt explored in this study is based on a traditional product from southern Iran that was used in the past, including strains of Lactobacillus plantarum, Lactobacillus pentosus, Chloromyces marcosianos yeast, and various natural herbs such as celery, shallot, chicory, and mint [17]. However, there is no available study evaluating the effect of a synbiotic yogurt containing L. plantarum on measurable metabolic outcomes in patients with MetS. Thus, we aimed to investigate the effects of daily consumption of synbiotic yogurt containing native strains of L. plantarum, L. pentosus, and C. marcusianus along with prebiotics, including celery, shallot, chicory, and mint, on the components of the MetS in adults with MetS.
Materials and methods
Study participants
Forty-four patients with MetS aged between 30 and 50 with body mass index (BMI) of 25–35 kg/m2 were recruited from the health centers affiliated with Yasouj University of Medical Sciences, Yasouj, Iran, from December 2022 to March 2023. MetS was confirmed based on the ATP III criteria when at least three of the following criteria were met: WC > 102 cm in men and >88 cm in women, triglyceride (TG) ≥ 150 mg/dl, high-density lipoprotein (HDL) ≤ 40 mg/dl in men and ≤50 mg/dl in women, blood pressure ≥130 to 85 mmHg, and FBG ≥ 100 mg/dl. Subjects were not included in the study who followed weight loss programs or had weight changes of more than 10% of initial weight during the last 6 months. Professional athletes or subjects with any changes in the intensity and duration of physical activity in the previous 4 weeks, smokers, alcoholic beverage consumers as well as women with pregnancy, lactating, and postmenopausal were also not included. Other exclusion criteria were as follows: having an allergy to dairy products and probiotics; routine consumption of probiotic or synbiotic yogurts; patients with diagnosed diseases including cardiovascular, lung, nervous system, kidney, liver, thyroid, and other endocrine diseases, diabetes, and cancers; taking drugs that affect appetite, body weight, and lipid metabolisms such as corticosteroids, oral contraceptives, antidepressants, antipsychotics, and anti hyperlipidemics, antibiotics; and receiving probiotics and other dietary supplements in the last 3 months.
It is calculated that 22 subjects should be recruited in each group using the sample size method recommended for randomized clinical trials, considering 95% confidence, 80% power, HDL changes in the intervention (0.88 ± 0.18) and control (1.13 ± 0.32) groups as a primary variable [18], and assuming a dropout rate of 20%.
Study design
This randomized, placebo-controlled trial was conducted on 44 individuals with MetS after obtaining written informed consent. Prior to the intervention, participants underwent a two-week “run-in” phase during which we gathered a range of data such as dietary intake, level of physical activity, medical history, and general demographic information. At the end of the run-in period Subjects were stratified into two groups according to gender (male or female) and BMI (25–30 or 30–35 kg/m2). Using a computerized randomization method with a block randomization procedure of sizes 2 and 4, patients were randomly divided into intervention (n = 22) and control (n = 22) groups. Participants in the intervention group consumed 300 grams of synbiotic yogurt, and those in the control group received the same amount of regular yogurt daily for a period of 12 weeks. Every 2 weeks, a package containing a sufficient amount of yogurt for each participant was delivered. We asked participants to consume yogurt alone as a snack between lunch and dinner, avoid consuming any other probiotic or synbiotic products during the study, and not modify their typical dietary habits throughout the study duration. Compliance with the consumption of yogurt was monitored by counting the number of returned unused and used yogurt containers every two weeks. The researchers maintained blinding throughout the study by ensuring that both randomization and outcome evaluation were conducted by individuals who were not directly involved in the study. This study was approved by the ethics committee of the Tehran University of Medical Sciences (ethics number: IR.TUMS.MEDICINE.REC.1401.080) and registered in the Iranian Registry of Clinical Trials (IRCT20220426054667N1).
Origin and composition of the synbiotic yogurt
The synbiotic yogurt that was used in this study contains strains of L. plantarum, L. pentosus (2 × 108 CFU), Chloromyces marcosianos yeast, and 3% of various natural plants (celery, shallot, chicory, and mint) [19]. Skimmed cow’s milk was used to produce both yogurts. First, skim milk was pasteurized and homogenized at 95 °C for 5–10 min, then cooled to 42–43 °C. In the next step, the prepared starter culture containing the mentioned probiotics was added to the mixture of milk and water at the rate of 3%. Then, the mixture of milk and water was incubated for 3–4 h at 42–43 °C to produce yogurt, and the yogurt was kept at 4 °C for 24 h to mature. Herb powder was delivered in a spice container next to yogurts every two weeks. After the full preparation of the yogurts, they were measured in terms of the live microbial population under the supervision of the Food and Drug Administration laboratory in Yasuj, Iran, and their amount was equal to our desired amount. In addition, to assess the quality and safety of yogurt utilizing various techniques like sensory assessment (taste, aroma, visual aspect, consistency, and texture), as well as measuring acidity and pH at different intervals, including 24 h post-production and after four days of refrigerated storage.
Assessment of variables
Anthropometric measurements were obtained at the beginning and the end of the study by qualified nutritionists working in the health centers. The patients’ weight was measured without shoes and minimal clothing with an accuracy of 100 g and the height was measured without shoes with a precision of 0.1 cm using the Seca scale (Hamburg, Germany). A non-stretch tape was used to measure the WC with an accuracy of 0.5 cm. The patient’s blood pressure was monitored twice with a 15-min gap using a mercury barometer. Before the blood pressure was taken, the participants were asked to avoid smoking, drinking coffee, and engaging in heavy physical activity for 30 min.
Relevant questionnaires were collected from patients before the intervention to collect information related to demographic variables, disease history, drug and supplement consumption, food intake, and physical activity levels. In order to monitor yogurt consumption and assess the patients’ nutritional intake throughout the research, the participants’ food intake was reevaluated at the end of the sixth week as well as at the end of the experiment. Using information from food recalls, nutritionist IV software was used to calculate individuals’ nutrient intakes. The International Physical Activity Questionnaire (IPAQ) [20], which has been validated for the Iranian population, was used to measure the level of physical activity during the sixth and final weeks.
Blood samples (10 cc) were collected after a 12-h overnight fasting at the beginning and end of the intervention. Blood samples were centrifuged at 3500 rpm for 10 min to separate the serum. Then the samples were stored at −70 °C until the analyses. Serum concentrations of HDL-C and TG were measured using commercial kits (Pars Azmoun Co., Tehran, Iran) and the photometric method. An autoanalyzer was used to determine HDL-C and TG levels (Biotecnica, BT 1500). The glucose oxidase enzymatic method using a commercial kit (Pars Azmoun Co., Tehran, Iran) was used to measure FBG. The serum levels of insulin were determined using the ELISA technique using a kit from Monobind (the USA). The formula used to determine the homeostatic model assessment for insulin resistance (HOMA-IR) is as follows: HOMA-IR = (FBG (mg/dl) × fasting insulin (μu/ml))/405.
Statistical analysis
The primary outcomes of the present study are the MetS parameters including WC, blood pressure, TG, HDL cholesterol, and FBG. Results were presented as mean ± standard deviation for normally distributed data and as median (IQR) for non-normal data. The data were tested for normality using the Kolmogorov–Smirnov test. Independent samples t-test, Mann–Whitney test, and Chi-square were performed to compare baseline characteristics and values between the two groups, where appropriate. To evaluate variations within the each group, the paired t-test and Wilcoxon test were used. Considering the baseline values of the outcomes and energy intakes as covariates, to explore any differences between the two groups following the intervention, an analysis of covariance (ANCOVA) was performed. SPSS software version 26 was used to analyze data. Statistical significance was defined as p-value < 0.05.
Results
The trial was completed by 41 patients, including 22 participants in the intervention group and 19 participants in the placebo group. Figure 1 illustrates the flowchart of the study, including patient screening, enrollment, and randomization. Dropouts occurred in the placebo group due to unwillingness (n = 2) and travel (n = 1). Participants exhibited high adherence to the prescribed yogurt consumption, and no adverse events, side effects, or complications were reported. The compliance was checked using three 24 h-dietary records throughout the study. In addition, it was checked through phone interviews once a week. Compliance in this study refers to whether the patients adhered to the consumption of 70% of provided yogurts based on the dietary recall data.
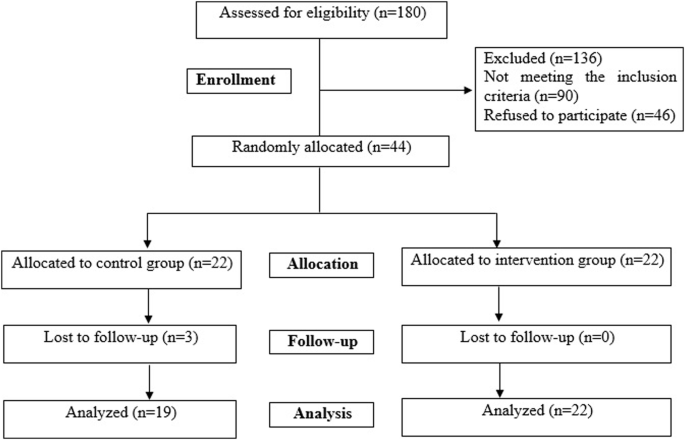
Including patient screening, enrollment, and randomization. Dropouts occurred in the placebo group due to unwillingness (n = 2) and travel (n = 1).
The baseline characteristics of the participants in both groups are presented in Table 1. At baseline, there were no significant differences between the study groups in terms of gender, age, marital status, educational level, and level of physical activity. Table 2 presents the daily dietary intake of the study participants. The average energy (p = 0.009) and carbohydrate (p = 0.03) intakes of the intervention group decreased significantly in the intervention group compared to the baseline. However, there were no significant differences in terms of daily energy, macronutrients, and fiber intake as well as physical activity level between the two groups throughout the study (p > 0.05).
Table 3 shows the anthropometric and blood pressure measurements of the study groups at baseline and the endpoint. There were no significant differences in body weight, BMI, WC, waist-to-hip ratio (WHR), SBP, and DBP between the two groups at baseline. Weight and BMI decreased significantly in the synbiotic yogurt group from baseline (p < 0.05). No significant differences were seen in WC, WHR, SBP, and DBP in the intervention group compared to the baseline (p > 0.05). However, the results of ANCOVA demonstrated a statistically significant difference only in the WHR (p = 0.02) and SBP (p = 0008) in the intervention group compared to the control group. Weight, BMI, WC, and DBP were not statistically significant between the two groups at the end of the study.
Table 4 shows the biochemical characteristics of the study subjects throughout the study. There were no significant differences in FBG, insulin, HOMA-IR, TG, and HDL-C between the two groups at baseline. After 12 weeks of intervention, FBG, insulin, and HOMA-IR levels decreased significantly in the synbiotic yogurt group compared to the baseline (all p < 0.001). Serum FBG levels decreased (p = 0.01), and HDL-C levels increased (p < 0.001) significantly in the regular yogurt group compared to the baseline. Based on the results of ANCOVA, there was a statistically significant decrease in FBG concentration (−12.77 vs. −5.68 mg/dl; p = 0.005), insulin (−2.31 vs. −0.15 mg/dl; p = 0.001), and HOMA-IR (−0.87 vs. −0.14 mg/dl; p < 0.001) in the intervention group compared to the control group at the end of the intervention.
Discussion
In the present study, we investigated the effect of the yogurt enriched with probiotics and prebiotics on anthropometric and biochemical markers in patients with MetS. The findings of our study indicated that the consumption of synbiotic yogurt decreased WHR as well as serum concentrations of FBS and insulin and improved insulin resistance compared to regular yogurt. There were no significant changes in body weight, BMI, WC, blood pressure, and serum concentrations of TG and HDL-C in the intervention group compared to the control.
One of the key findings of this study is the significant improvement in insulin resistance markers in the synbiotic yogurt group. Previous studies have shown that synbiotics have a favorable effect in controlling glycemic parameters in patients with type 2 diabetes mellitus [21]. Esmaeilinezhad et al. found a significant increase in insulin sensitivity after consumption of synbiotic beverages in patients with polycystic ovarian syndrome [22]. It has been suggested that probiotics modulate gut microbiota composition and cause anti-inflammatory effects and by that improve insulin resistance and glucose metabolism [23, 24].
Moreover, the significant improvement in WHR and SBP in the synbiotic yogurt group compared to the control group further supports the potential benefits of synbiotic supplementation in managing MetS complications. Abdominal obesity and hypertension are key features of MetS and are associated with increased cardiovascular risk [25, 26]. The improvement in WHR and SBP observed in the intervention group might be attributed to the anti-inflammatory and vasodilatory effects of probiotics, as well as the potential role of bioactive peptides released by probiotics in regulating blood pressure [27, 28].
While the study demonstrated significant improvements in several key metabolic parameters, such as insulin resistance, WHR, and SBP, it is important to discuss the non-significant findings related to TG and HDL-C levels. Similarly, a meta-analysis investigating the effects of probiotics on metabolic components of T2DM found no significant improvements in lipid profile but observed significant advances in blood glucose and insulin sensitivity [29]. TG and HDL-C are critical components of the MetS and play a pivotal role in cardiovascular health [30]. The absence of significant changes in these parameters in response to the synbiotic yogurt intervention may be attributed to some hypostatized reasons like; alteration in dietary and lifestyle of the participants, strain-specific outcomes, and the duration of the intervention [31]. A meta-analysis that reviewed the effect of synbiotic supplements on patients with MetS [32], showed that the main effect of these supplements was improvement in lipid profile as well as indicators related to the body shape, while the results of our study showed that these effects are more noticeable in improving insulin resistance. On the other hand, Asemi et al. [33] conducted an intervention study with only one bacterium for 6 weeks and showed a non-significant increase in serum total cholesterol, TG, and LDL-C levels, while Bakhshimoghaddam et al. [34] found a significant decrease in these lipid parameters with four types of bacteria during 24 weeks. Based on these results, it seems that using different types of bacteria along with a longer period of intervention could have a significant impact on the results obtained.
The mechanism of action of synbiotics on metabolic pathways has not been clearly explained. But, there are some suggestions in this regard. Synbiotics cause the release of short-chain fatty acids (SCFAs) in the intestine through their probiotic content [35, 36]. SCFAs are the immediate fuel of enterocytes and have been shown to be related to reducing the level of inflammation in the body, increasing the metabolism of glucose and fatty acids, as well as better absorption of dietary nutrients [37,38,39]. By reducing the level of inflammatory markers such as CRP and TNF-α, synbiotics regulate the metabolism of glucose and fatty acids in the mitochondria of the body cells and cause more sensitivity to insulin [40,41,42]. Probiotics also increase the secretion of bile salt hydrolase (BSH) and catalyze the conjugation and excretion of bile salts in the digestive system which leads to increasing cholesterol metabolism in the liver and improving serum lipid profile [43, 44].
Studies have shown that the effects of different types of probiotics on indicators related to the MetS are very different [45]. Among these different types, Lactobacillus acidophilus, Lactobacillus rhamnosus, and Streptococcus thermophilus were among the most effective spices which showed favorable results in interventional studies even in a period of less than 8 weeks [31].
As an evidence supporting our findings, L. plantarum inhibited the expression of fatty acid producing enzymes genes in the liver accompanied by a decrease in the activity of enzymes related to fatty acid oxidation [46].
To the best of our knowledge, this was the first study to evaluate the effects of symbiotic yogurt containing L. plantarum and L. pentosus on MetS components in patients with MetS. The duration of our intervention was 12 weeks and was longer than most of the previously implemented trials [31] and that could contribute to a better assessing certainty. However, it is important to acknowledge that the conceptualization and design of any research endeavor inherently entail certain limitations. Firstly, the selection of adults with MetS may have influenced the generalizability of our findings. Additionally, the choice of clinically diagnosed MetS patients could have introduced biases or limitations in data collection and analysis. Furthermore, the study’s timeframe and resource constraints may have impacted the depth and breadth of our investigation. Also, the methodology employed in our study, while rigorously implemented, is not immune to limitations. For instance, anthropometric assessments such as weight and height or confounding variables like disease history, drug and supplement consumption, and food intake were collected via self-reported questionnaires, and because of its possible recall or under/over report bias more limitations need to be considered upon our method. Moreover, the reliance on a small sample size of patients with MetS recruited from the health centers of a single city constricts the representativeness and validity of this study.
On the other hand, the generalizability of our study findings is an important consideration that warrants discussion. While our research endeavors to shed light on the improving effect of consumption of the probiotic and prebiotic-containing products on the clinical indicators of MetS, it is essential to acknowledge the potential limitations in extrapolating these findings to broader populations or settings. It should be pointed out that our findings cannot be generalized and extended to other types of probiotics. Besides, factors such as age or ethnicity differences may influence the applicability of our results beyond the scope of the study sample, and such variations across different populations or settings may affect the generalizability of our conclusions. Several procedural limitations may also have influenced the interpretation and generalizability of our findings. For instance, it is assumed that the blood samples were taken from the patients while they were fasting, but it is possible that all the participants did not comply with this condition and did not report it. Moreover, factors such as laboratory errors, including changes in the chemistry of samples during transport to the laboratory and storage and testing, are also possible and may have affected the execution of the study protocol and the integrity of the data obtained. Therefore, future research should seek to replicate and validate these findings in diverse populations.
The internal and external validity of our trial are integral aspects of research methodology that warrant careful consideration. Internal validity refers to the degree to which the observed effects can be attributed to the interventions or variables under investigation, while external validity pertains to the extent to which the findings can be generalized beyond the study sample to other populations or settings. In our study, efforts were made to enhance internal validity by blinding the researcher, the laboratory operator, and the analysts of the findings, conducting co-variance analysis and random assignment of samples into two control and intervention groups, as well as simulating the designed yogurt with the yogurt consumed daily by the participants. Furthermore, considerations of external validity were addressed by calculating a suitable sample size using valid formulas for interventional studies. Nevertheless, the external validity of our trial may be influenced by factors such as cultural and genetic factors causing different responses to the same intervention. Future research should aim to further investigate and validate the internal and external validity of our findings across diverse populations and settings to bolster the robustness and generalizability of the evidence presented.
Conclusions
According to the findings of this study, consumption of synbiotic yogurt containing native strains of L. plantarum, L. pentosus, and C. marcosianos yeast in adults with MetS for 12 weeks was associated with improvements in insulin resistance, systolic blood pressure and WHR, which can be beneficial in patients with MetS.





Responses