Associations of plasma phospholipid cis-vaccenic acid with insulin resistance markers in non-diabetic men with hyperlipidemia

Introduction
Fatty acids (FA), as essential components of the lipid structure, play a number of important structural and functional roles in living organisms. They affect the properties of cell membranes and cell signaling, are ligands of nuclear factors, and are precursors of the plethora of biologically active molecules [1]. The composition of FAs in human body compartments depends on nutritional intake, endogenous synthesis, the functioning of enzymes desaturases and elongases (see Figs. 1 and 2), and different degradation mechanisms [2]. Moreover, racial and genetic factors play a role, as well [3, 4]. Dysregulation of FA is involved in the etiopathogenesis of insulin resistance. Inflammation and oxidative stress are important factors affecting insulin signaling, as well [5]. Insulin resistance (IR) was recognized to be associated with metabolic syndrome [6], type 2 diabetes mellitus (T2DM) development [7], and different pathological conditions, such as dyslipidemia, but also some cancers or neuropsychiatric diseases [8,9,10].
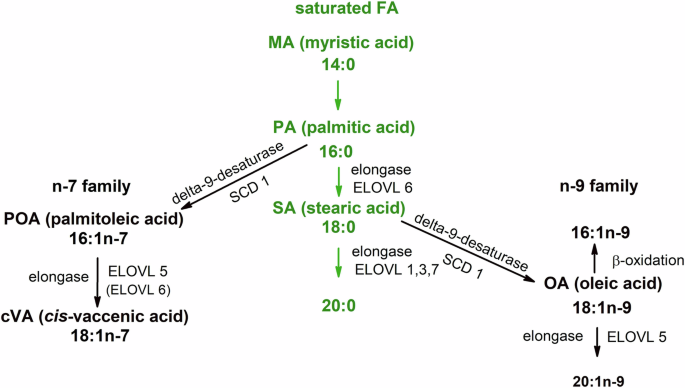
Palmitic acid can be converted into monounsaturated fatty acids of n-9 and n-7 families via activities of several long-chain fatty acid elongases (ELOVL 1,3,5–7) and desaturation with delta-9 desaturase (stearoyl-coenzyme A desaturase, SCD).
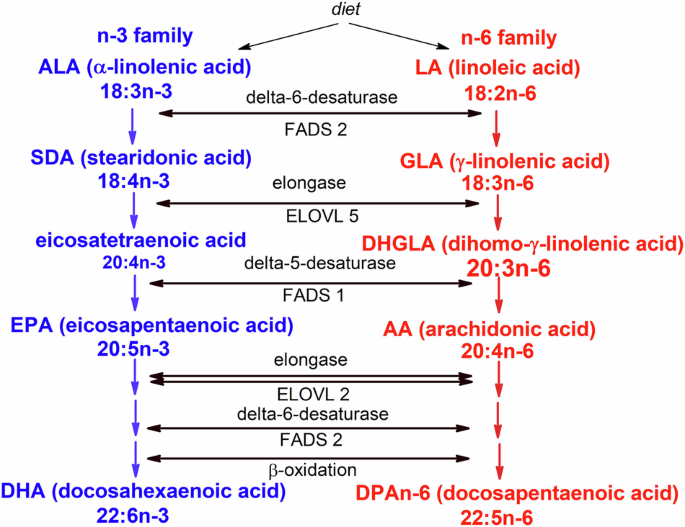
Conversion of polyunsaturated fatty acids (PUFA) into members of PUFA n-3 and n-6 families includes two types of desaturases (FADS 1,2) and long chain fatty acid elongases (ELOVL 2,5). The distal part of the pathway also includes retroconversion by β-oxidation.
The relationships of individual classes of FA to insulin resistance, as well as to inflammation and oxidative stress, are different. Generally, a higher content of saturated fatty acids in the diet (especially palmitic acid) and a lower content of PUFA (polyunsaturated fatty acids) n-3, as well as monounsaturated FA (principally oleic acid) are mostly associated with insulin resistance [11], while the role of the n-6 PUFA class in this regard is less clear. In a recently published analysis of 20 studies, high plasma linoleic acid (LA, 18:2n-6) levels were associated with a significant decrease in the relative risk of T2DM [12], whereas the proportion of dihomo-γ-inolenic (20:3n-6) acid in serum cholesteryl esters positively correlated with the incidence of type 2 diabetes mellitus [13]. The different effects of individual SFA on cardiovascular risk in humans are a well-known phenomenon [14], and it seems that SFA has variable associations with insulin sensitivity, too. These findings show us that the relationship of individual fatty acids to insulin resistance is apparently more complex. For the reasons stated, the specific metabolic effects of individual FAs in insulin resistance pathogenesis became a subject of intensive research. For example, when investigating the effects of palmitoleic acid (16:1n-7, POA), it was demonstrated that POA synthetized in adipose tissue favorably influences lipid and glucose homeostasis in other tissues, such as liver or muscle, and attenuates liver steatosis induced by high-fat diet or diabetes [15, 16]. Nevertheless, the findings on the effects of circulating palmitoleate on cardiometabolic health in humans are still controversial, as the content of POA in plasma lipid classes was an independent marker of abdominal obesity in men [17] or high plasma glucose concentration [18]. Moreover, POA content in erythrocyte membranes has been shown to be associated with inflammation and a higher risk of metabolic syndrome [19].
These discrepant results can be assigned to many factors, including metabolic conversion of POA. The elongation of POA produces cis-vaccenic acid (18:1n-7, cVA), and interestingly, POA, increases the transcription of the genes encoding elongase enzymes, especially ELOVL5 [20], in endothelial cells in a dose-dependent manner [21]. The results of some studies have shown beneficial effects on human health associated with cVA. Erythrocyte membrane cVA was inversely related to the risk of heart failure with antecedent CHD [22], and in one prospective study, lower cVA in PL predicted consistently lower insulin sensitivity and β-cell function over 6 years [23]. In a large prospective cohort study in the US, comprising 3004 participants free of diabetes (men and women) aged >65 years, it was proved that circulating plasma phospholipid cVA was inversely associated with the risk of diabetes mellitus development [24]. These findings indicate that the potential beneficial effects of cVA on cardiometabolic risk in humans should be further studied, also taking into account sex and depot differences between men and women [25]. Hormonal differences are responsible for different metabolism of MUFA and PUFA due to upregulation of desaturases and elongases resulting in higher PUFA n-3 and different deposition and mobilization of fatty acids from visceral and gluteofemoral adipose tissues [26, 27]. The aim of this study was to investigate relations of proportion of cVA in plasma phospholipids to markers of insulin resistance in non-diabetic men with high cardiovascular risk.
Material and methods
Study design and participants
The study population comprised 231 men consecutively examined as outpatients at the 4th Department of Internal Medicine between the years 2020–2022. As a control group, we examined 50 apparently healthy men (volunteers and university staff). The study protocol was approved by the Joint Ethical Committee of the General University Hospital and the first Medical Faculty at Charles University, Prague. Written informed consent was obtained from all participants prior to inclusion. The study was performed in accordance with the Declaration of Helsinki.
These patients were referred to Lipid Clinic to consider the use of hypolipidemic treatment. Subjects studied had an LDL-C level >3.0 mmol/L (7% of probands) and/or triacylglycerolemia >1.70 mmol/L (68.5% of probands). Moreover, we observed low concentrations of high-density lipoprotein cholesterol (HDL-C) (<1.00 mmol/L) in 24% of them. The following exclusion criteria were applied to the study: ongoing lipid-lowering, antidiabetic or antioxidant medication, excessive alcohol consumption (>30 g/day), hormonal replacement therapy, supplementation with polyunsaturated FA (PUFA n-3 and n-6 families), manifestation of cardiovascular disease and/or cerebrovascular disease, diabetes mellitus, liver disease (except for nonalcoholic fatty liver disease), kidney disease (creatinine >130 μmol/L), hypothyroidism, and recent infections and malignancies. Six weeks before the investigation, they were recommended to adhere to the AHA Step One diet and to maintain stable dietary patterns.
Anthropometric and blood pressure measurements
Basic clinical and anthropometric data, including the assessment of body fat, were analyzed using standard methods as described previously [28]. Moreover, the bioelectrical impedance method was used for the body fat mass measurements with Tanita SC-240 (Tanita Corporation, Tokyo, Japan). Blood pressure measurements were performed according to standardized procedures after resting for 10 min, using automated blood pressure devices (Omron M3 Comfort, OMRON Healthcare Europe) by trained study personnel, and the average of two measurements was presented as a result.
Laboratory measurements
Plasma concentrations of biochemical parameters were measured using routine methods, whereas concentrations of nonesterified FA (NEFA) were determined using an enzymatic colorimetric method (Randox Laboratories, UK). The homeostasis model assessment method (HOMA-IR) was used as the index of insulin resistance [29]. The glomerular filtration rate (eGFR) was estimated from serum creatinine according to the 2021 CKD-EPI equation. Fatty acids were analyzed in plasma phospholipids as methyl esters by GC-FID [30] using LN-FAME-HT (60 m × 0.25 mm ID, 0.20 μm df) column (Chromservis s.r.o., Prague, Czech Republic). This setting allowed proper separation of isomers of monounsaturated FA (see Supplementary Fig. 1 for sample chromatogram). The retention times for FA methyl esters were verified using commercially available standards (NuChek Prep Inc., Elysian, MN, USA, Cat. No. GLC-566C and GLC-674). The FID detector performance was checked on the basis of the linearity of the response; the correction factors were calculated using the abovementioned standard mixtures with defined composition. Method variability, presented as a relative standard deviation, ranged from 1.07% for PA (16:0) to 8.60% for 16:1n-9 [31]. Desaturase and elongase activities were estimated using FA product/precursor ratios [32].
Dietary habits
Average intake of energy, proteins, fats, saturated FA, carbohydrates, and fiber were analyzed by a 3-day dietary questionnaire. The nutritional data were analyzed by Nutrimaster SE software, version 1.0 [33].
Statistical methods
The overall group of men was for further comparisons stratified into quartiles according to the content of cis-vaccenic acid in plasma phospholipids; the investigators were blinded according to the quartile assignment. The statistical analysis was performed using the R statistical software Version 4.1.3 [34], or with the STATISTICA® CZ software for Windows (ver. 12). Studies on insulin resistance in subjects with high or low cVA concentrations have not been published to allow sample size calculations; nevertheless, the a posteriori sample size calculations gave power 1-beta 0.76 (HOMA-IR) and 0.66 (insulin) for the current setting.
Categorical data were summarized using absolute and relative frequencies. Continuous data were expressed as the median and interquartile range (IQR, 25th–75th percentile). The normality of the distribution was tested using the Shapiro–Wilks W test. Comparisons between the groups were determined using the independent t-test and the Wilcoxon test, respectively. P-values for both continuous and categorical data were adjusted for multiple comparisons using the Benjamini–Hochberg correction. Multivariate linear regression with backward stepwise analysis was used to distinguish the significance of intercorrelated relevant predictors.
Results
Clinical and biochemical characteristics of the studied group
Basic demographic, anthropometric, and metabolic data of the group of 231 men with hyperlipidemia are shown in Table 1. The median age was 50 years, and the patients were mostly overweight/obese.
Table 2 shows demographic, anthropometric, and metabolic data according to cis-vaccenic acid concentrations in plasma phospholipids. Quartile 4 was compared with Quartile 1 (Q1 and Q4 groups, median [25th–75th percentiles]). Between the groups Q4 and Q1, there were no differences between the daily energy intake and the content of proteins, fats, and carbohydrates, as well. Similarly, we did not observe significant differences in the intakes of dietary FA—saturated FA, monounsaturated FA. and PUFA (as the sum of both PUFA n-3 and n-6) (data not presented).
In comparison with the Q1 group, the Q4 group was characterized by significantly lower values of insulin, apolipoprotein, and HOMA-IR index, while by significantly higher concentrations of plasma NEFA. Persons in the Q4 group had significantly lower concentrations of conjugated dienes in LDL particles (CD-LDL). Differences in all other variables were not significant (age, BMI, waist circumference, waist-to-hip ratio, fat mass, systolic and diastolic blood pressure), percentage of smokers, as well. There were no significant differences in concentrations of total plasma cholesterol, triacylglycerols, HDL-cholesterol, LDL-cholesterol, non-HDL-cholesterol, apolipoprotein A1, fasting glycemia, uricemia, or hs-CRP. When comparing the control and patient groups, no significant differences were found in age, BMI, proportion of hypertensives, smokers, systolic, and diastolic BP. As expected, significant differences were found in plasma lipid levels (patients with hyperlipidemia differed in significantly higher levels of plasma total cholesterol, triacylglycerols, LDL-C, non-HDL-C, apo B), uric acid, glucose, waist circumference, and index values WHCR. Moreover, controls had a higher proportion of cVA and the sum of MUFA (see Supplementary Tables 3 and 4).
Fatty acid profiles
Fatty acid profiles (mol %) in plasma PL of the Q4 and Q1 groups are shown in Table 3. Q4 group (high cis-vaccenic group) had a higher molar percentage of the sum of MUFA caused by a high content of oleic acid, 18:1n-9, palmitoleic acid, cis-vaccenic and minor FA of this family (cis-hexadecenoic and gondoic acids). The lower content of PUFAn-6 in the Q4 group was more pronounced in minor FA (γ-linolenic and dihomo-γ-linolenic acids); we did not observe significant differences in the content of linoleic and arachidonic acids. The remaining differences in FA concentrations were insignificant after the Benjamini–Hochberg correction.
Surrogate markers of estimated activities of FA desaturases and elongases, computed as the (product/substrate) ratio of respective FA in plasma PL of both Q4 and Q1 groups, are shown in Table 4. In the Q4 group we found raised estimated activity of delta-9-desaturase (D9D) for stearic acid (D9D18) and for palmitic acid (D9D16). Furthermore, we found increased estimated activities for ELOVL5 (calculated as 18:1n-7/16:1n-7 ratio), and ELOVL 2/5 (ratio 22:5n-3/20:5n-3) and 22:4n-6/20:4n-6, respectively. Concurrently, the estimated activity of D6D (ratio 18:3n-6/18:2n-6) was decreased in the Q4 group.
Correlations between fatty acid profiles and selected variables
Relevant correlations (Spearman´s coefficients, p values) of investigated variables of the total group of 231 men with dyslipidemia are presented in Supplementary Table 1. Cis-vaccenic acid negatively correlated with insulin (r = −0.183, p < 0.01), HOMA-IR (r = −0.196, p < 0.01), apo-B (r = −0.203, p < 0.01). We observed significant correlations with insulin and HOMA-IR for several FA (Supplementary Table 1).
Because of high intercorrelations between all monounsaturated fatty acids (see Supplementary Table 1), we performed backward stepwise regression analysis for all analyzed MUFA to identify significant determination of parameters of insulin resistance with these MUFA. According to backward stepwise analysis, only association with 18:1n-7c remains significant for insulin (p = 0.015) and HOMA-IR (p = 0.006) (see Supplementary Table 2 for detailed results).
Discussion
In this study, we have found significant differences in the parameters of glucose and lipid homeostasis between the patients belonging to the upper (group Q4) vs. lower (Q1) quartile of the cis-vaccenic acid content in plasma phospholipids (PL). Fatty acid profiles of the Q4 and Q1 groups significantly differed, as well. To our knowledge, it is the first study investigating associations of high vs. low proportions of cis-vaccenic acid in plasma phospholipids (PL) with markers of insulin resistance in men with hyperlipidemia.
The main finding of the study was that individuals in group Q4 differed from those in Q1 by the more favorable profile of insulin resistance markers. They had significantly lower plasma insulin, HOMA-IR values, and apolipoprotein B concentrations. Moreover, both quartiles differed significantly in the composition of FA in plasma PL. The individuals in the Q4 group had lower proportions of FA 14:0, 18:3n-6, 20:3n-6, and the sum of PUFA n-6. On the other hand, the Q4 group had, besides cVA, significantly elevated FA 16:1n-9, 16:1n-7, 18:1n-9, 20:1n-9, 18:3n-3, sum of MUFA and total plasma NEFA.
We did not prove different dietary habits between Q1 and Q4 subjects (data not shown). It is known that dietary assessment methods have many limitations. The accuracy for individual dietary components reaches a maximum of 70–80% [35].
While insulin and HOMA-IR values serve as surrogate markers of insulin resistance, elevated apolipoprotein B, concurrently with elevated serum triacylglycerols, increased small LDL particles, and reduced level of HDL characterize atherogenic dyslipidemia that is typical for insulin resistant states. Interestingly, the whole group of 231 men with hyperlipidemia had a significantly smaller proportion of cVA and oleic acid in their plasma PL, in comparison with a small control group of 50 normolipidemic men (see Supplementary Tables S2, S3).
Our findings of associations of plasma phospholipid cis-vaccenic acid (18:1n-7) with cardiometabolic risk factors are in line with the results of several studies. In participants of a prospective Cardiovascular Health Study, free of diabetes, concentrations of 18:1n-7 in plasma PL were inversely associated with incident T2DM [24]. From another perspective, in a 6-year study in the Canadian population at risk for diabetes, lower cis-vaccenic acid (cVA) predicted lower insulin sensitivity and β-cell function [23]. Ethnic factors may also play a role in the functioning of cVA. In Multi-Ethnic Study of Atherosclerosis (MESA) participants, higher levels of plasma cVA were inversely associated with insulin resistance scores across four races/ethnicities (Caucasian, Black, American Chinese, Hispanic), and related to 17%, 39%, and 32% lower risks of incident T2DM in Black, Chinese American, and Hispanic participants, respectively. In Caucasians, the significant association of cVA with incident T2DM was not found [36]. In other studies, different results were found, too. In the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam study, cVA by quintile of erythrocyte membrane fatty acid proportions did not correlate with the risk of T2DM [37].
The Q4 group differed from the Q1 group by a higher level of individual MUFAs (16:1n-9, 18:1n-9, 20:1n-9) and palmitoleic acid (16:1n-7, POA), which is a precursor of cVA. The levels of α-linolenic acid (18:3n-3, LnA) and total plasma NEFA were higher in the Q4 group, as well.
MUFA, mostly oleic acid, is recognized as beneficial in influencing insulin sensitivity and preventing DM2. However, not all FA in this class have the same effects. Oleic acid (OA) content has been linked with a number of mechanisms, such as reduced expression of pro-inflammatory genes, upregulation of enzymes responsible for fatty acid oxidation, beneficial effects on endothelial dysfunction, and others [38]. Negative correlations with T2DM were found for OA in both plasma CE [39] and plasma PL [13]. On the other hand, some studies also produced opposite results – in one prospective study, oleic and palmitoleic (POA) acids were independently associated with incident T2DM [40].
Q1 differed from Q4 by significantly higher concentrations of γ-linolenic acid (GLA) and dihomo-γ-linolenic acid (DGLA) while lower proportions of sum PUFA n-6 (at the expense of linoleic acid, LA) and α-linolenic acid (ALA, 18:3n-3). In our previous work, higher proportions of POA, GLA, and DGLA, while lower content of LA in plasma phosphatidylcholines were able to identify in the patients with metabolic syndrome those with higher IR or an oxidative stress marker [33]. Also, DGLA was associated with abdominal fat expressed as waist circumference or waist/hip ratio [41].
In this work, we observed higher levels of NEFA in the Q4 vs. Q1 group. It was generally accepted that elevated plasma NEFA is associated with insulin resistance and T2DM in humans [42]. NEFA was elevated in diabetic subjects with overweight/obesity in comparison with healthy subjects [43] and was prospectively associated with an increased risk of both impaired glucose tolerance and T2DM [44]. Nevertheless, individual NEFA may have different effects on the progress of insulin resistance and the development of type 2 diabetes mellitus [45]. Saturated NEFA causes insulin resistance, while monounsaturated FA increases insulin sensitivity in both diabetic [46] and healthy persons [45].
Still, some issues are to be resolved dealing with different NEFA contribution to insulin resistance and other pathologic states: FA profiles in plasma lipid compartments differ significantly between men and women and among different ethnic groups, as well [47], and reference range for main plasma FA in healthy subjects are missing [48].
The reasons for the higher NEFA concentration in the Q4 group are not clear. Some findings suggest that increased content of cis vaccenic acid, a product of ELOVL5 activity, could be related to a decrease in ectopic fat deposition. Supplementation of obese rats with trans-vaccenic acid (replacing oleic acid) led to increased insulin sensitivity, reduced hepatic steatosis, and decreased nonalcoholic fatty liver disease activity scores. The action of cVA on PPARγ (peroxisome proliferator activator receptor γ) in adipose tissue may counteract excessive ectopic lipid accumulation [49]. In our study, in set of men with hyperlipidemia group of patients belonging to the fourth quartile of serum PL cVA proportions had higher values of estimated ELOVL2/5 activity, calculated both as a 22:5n-3/20:5 n-3 and 22:4n-6/20:4n-6 ratios and activity of ELOVL5 calculated as a ratio 18:1n-7/16:1n-7. In experimental work, increasing hepatic ELOVL5 activity improved glycemia, insulinemia, HOMA-IR, and glucose tolerance to normal values in obese mice [50]. In mice with a knockout of the Elovl5 gene, the lack of endogenously formed long-chain PUFA leads to the derepression of SREBP-1c, the activation of lipogenesis, and hepatic steatosis [51]. ELOVL5 activity was also associated with increased catabolism of triacylglycerols, suppressed expression of enzymes involved in gluconeogenesis [52], and induced the expression of fibroblast growth factor 21 (FGF21), which increases hepatic insulin sensitivity, decreases lipogenesis, potentiates fatty acid β-oxidation, reduces hepatic ER (endoplasmic reticulum) stress, and diminishes VLDL (very low-density lipoproteins) delivery to the liver [53] and can improve ectopic lipid deposition in liver and muscle [54].
The patients in the Q4 group had significantly higher estimated activities of delta-9-desaturase (D9D) both for 16:0 (D9D16) and 18:0 (D9D18), whereas lower estimated activity of D6D (calculated as 18:3n-6/18:2n-6 ratio). Elevated activity of D9D is a marker of de novo lipogenesis, and in this study, both D9D16 and D9D18 correlated significantly with the total plasma NEFA levels and with all principal n-9 FA (16:1n-7, 18:1n-9, 20:1n-9, and cVA.
Interestingly, the estimated activity (D9D18) index correlated negatively with markers of insulin resistance, whereas the D9D16 index correlated positively (see Supplementary Table 1). This probably could be related to the possible positive association of POA with insulin resistance in this set of patients.
Proportion of cVA correlated negatively with insulin, HOMA-IR, and apolipoprotein B, showing that in our set of hyperlipidemic patients with obesity/overweight, cVA in plasma PL may function to improve insulin sensitivity. In contrast, POA correlated rather differently both with anthropometric parameters and markers of glucose and lipid homeostasis. Its proportion in plasma PL correlated positively with BMI, WHCR, fat mass, and triacylglycerols. This finding corresponds with some results of previous human studies, indicating unfavorable effects of POA on glucose homeostasis [19], though not all studies did prove such results [24].
Patients of Q4, in comparison with the Q1 group, had significantly lower values of an estimated D6D (18:3n6/18:2n6) (p < 0.001). This finding is in line with the results of other studies, where elevated estimated activity of D6D was described in patients with metabolic syndrome and T2DM [31], impaired fasting glycemia [30], and other pathologic conditions such as cardiovascular disease [55], tumors [9], or depression [10] Inverse correlations have been found between POA and cVA with estimated D6D activity (while POA correlated positively, cVA correlated negatively, both p < 0.001).
We have analyzed in our group of hyperlipidemic men markers of oxidative stress and inflammation (conjugated dienes in LDL and hs-CRP), as these conditions are associated with insulin resistance. Probands in the Q4 group had significantly lower concentrations of CD-LDL in comparison with the Q1 group. Concentrations of CD-LDL are considered a marker of systemic oxidative stress, partly reflecting the levels of minimally modified LDL, in which only the lipid component is oxidatively modified [56]. This finding is in line with the results of our earlier studies dealing with hypertriglyceridemia, severity of metabolic syndrome, or pancreatic cancer, as well [9, 26, 57]. The significance of lipid peroxidation in the pathogenesis of insulin resistance has been proven in experimental and clinical studies [58].
On the other hand, we were not able to find differences in hs-CRP concentrations. To our knowledge, this is the first work describing significant associations of serum PL cVA with parameters of insulin sensitivity in male patients with hyperlipidemia.
The study was conducted in a relatively large set of men with overweight or obesity, who were not treated with lipid-lowering drugs, supplements of n-3 or n-6 PUFA, or antioxidants. Limitations include a cross-sectional type of study, so causality could not be proved. Moreover, the profile of individual plasma NEFA was not investigated, and the content of individual MUFA and PUFA was not assessed in dietary questionnaires, as well. Also, the data about the physical activity were not available.
Our results support the hypothesis that plasma PL cis-vaccenic acid could be associated with insulin sensitivity in men with hyperlipidemia and high cardiovascular risk. Moreover, the results indicate that individual FA in the same class can have different pathophysiological effects. The results should be further studied and applied to other populations, e.g. healthy subjects, women, or type 2 diabetics.





Responses