Revisiting ADT in mCRPC: balancing oncologic control and mitochondrial implications

To the Editor:
We read with great interest the article by McManus et al. [1], which offers an excellent overview of therapeutic sequencing for metastatic castration-resistant prostate cancer (mCRPC). Their comprehensive review highlights the evolution of treatment options, particularly the integration of genetic testing and prostate-specific membrane antigen (PSMA)-targeted imaging to guide newer therapies such as poly(ADP-ribose) polymerase (PARP) inhibitors and [177Lu]Lu-PSMA-617. While their recommendations provide critical insights into optimizing treatment strategies, they reaffirm that androgen deprivation therapy (ADT) remains a cornerstone in the management of prostate cancer across its various stages.
However, the metabolic and cardiovascular complications associated with ADT warrant attention. Moryousef et al. [2] highlighted the increased burden of metabolic syndrome and cardiovascular disease (CVD) among prostate cancer patients undergoing ADT. This phenomenon may be linked to low testosterone-induced mitochondrial dysfunction, as discussed by Prasun [3]. Mitochondrial dysfunction not only exacerbates CVD and metabolic syndrome but is also implicated in neurodegenerative diseases and carcinogenesis, as demonstrated by Zong et al. [4]. The Warburg effect, driven by mitochondrial dysfunction, may contribute to cancer progression and potentially the emergence of new prostate cancer clones during prolonged ADT.
These findings underscore the delicate balance between controlling existing prostate cancer and inadvertently fostering conditions for new oncogenic events. This hypothesis aligns with the promising potential of bipolar androgen therapy (BAT), as reviewed by Leone et al. [5]. By intermittently elevating testosterone levels, BAT may counteract the adaptive mechanisms of cancer cells to low-androgen environments, thus delaying or even reversing resistance to ADT.
As depicted in Fig. 1, the red arrows represent pathways exacerbated by ADT, including mitochondrial dysfunction and the Warburg effect, which contribute to cancer progression. In contrast, the green arrows highlight the modulatory role of BAT, which may restore mitochondrial integrity and counteract metabolic reprogramming. This interplay underscores the potential of BAT to offer a balanced approach, mitigating the adverse metabolic effects of prolonged ADT while maintaining oncologic control.
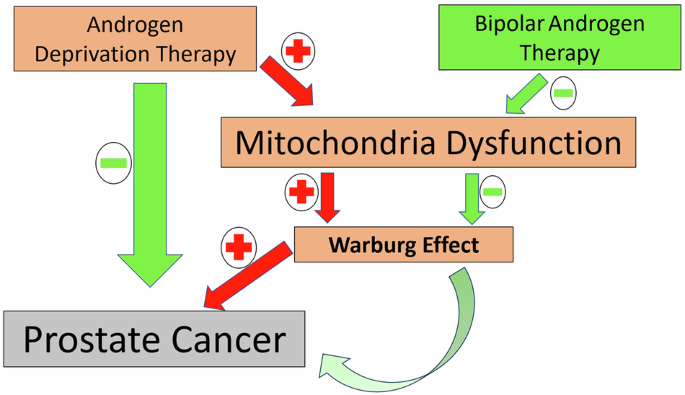
This figure illustrates the intricate interactions between ADT, BAT, mitochondrial dysfunction, and prostate cancer (PC) progression. ADT induces mitochondrial dysfunction by disrupting energy metabolism, contributing to the Warburg effect (a shift to glycolysis in cancer cells) and fostering cancer progression. The red arrows indicate positive regulation (e.g., ADT promoting mitochondrial dysfunction and the Warburg effect), while green arrows signify inhibitory effects (e.g., BAT mitigating these pathways). BAT intermittently elevates androgen levels, counteracting the adaptive resistance mechanisms of cancer cells in low-androgen environments, potentially restoring mitochondrial function and delaying PC progression. This schematic underscores the dual roles of ADT and BAT, highlighting their opposing impacts on mitochondrial metabolism and PC management.
We propose further exploration into the relationship between prolonged ADT, mitochondrial dysfunction, and cancer metabolism to better elucidate the broader implications of current therapeutic strategies. Such insights could refine approaches to prostate cancer management, offering dual benefits of oncologic control and mitigation of systemic side effects.
The insights derived from Fig. 1 emphasize the necessity of a tailored therapeutic approach in mCRPC management. By integrating mitochondrial dynamics into treatment strategies, we may better address the dual challenges of oncologic control and systemic side effects, paving the way for improved patient outcomes.





Responses