Epidemiology of multimorbidity in childhood cancer survivors: a matched cohort study of inpatient hospitalisations in Western Australia

Introduction
The need for effective survivorship care for childhood cancer survivors (CCS) has increased significantly over the last four decades [1,2,3,4,5]. This is attributed to notable improvements in survival rates for several types of children’s cancers [1, 4, 5], and the mounting evidence demonstrating elevated morbidity [2] and accelerated ageing [3, 6, 7] in the population of CCS. Survivorship care requires efficient structuring to address the adverse effects of cancer treatments on CCS (e.g., the elevated burden of cardiomyopathy, neurocognitive deficits, and anxiety) and the subsequent burden on healthcare services (e.g., the elevated risk of repeated hospitalisations) [8]. CCS experience a significantly higher cumulative burden of chronic health conditions (CHCs) than the general population (p < 0.05) [9]. As the cancer survivor population expands and ages, the need to mitigate the impact of multiple chronic conditions on survivors through personalised healthcare and optimal healthcare delivery will become increasingly important [8, 10].
In CCS, multimorbidity (two or more co-occurring conditions) may develop due to clinical, behavioural, or social factors. These factors include treatment-related complications [11], a higher prevalence of comorbidities [12], side effects of drug-drug interactions [13, 14], social deprivation [13], obesity [15], and physical inactivity [16]. Although the complexity of clinical management increases in cancer survivors with multiple conditions [11], existing clinical care focuses on managing single conditions [17,18,19], which can lead to fragmented healthcare provision and a greater illness burden [13, 20]. Existing barriers, such as the limited capacity of survivorship specialists [2], inadequate care coordination [2, 8], and suboptimal adherence to the recommended follow-up care among survivors [21, 22] can further exacerbate the illness burden due to delayed and inefficient management of complications. A recent systematic review revealed a lack of comprehensive studies on the risk factors for multimorbidity in cancer survivors [23]. This gap in evidence can impede the development of clinical care guidelines that can effectively address the management of simultaneous conditions in this population.
Previous investigations of multimorbidity patterns in CCS have primarily focused on CHCs [9, 12, 24,25,26,27,28]. An increased burden of multimorbidity leading to increased utilisation of inpatient [12, 26, 27] and emergency services [26, 27] was observed in survivors (aged <18) compared to their siblings and the general population, particularly for general paediatric morbidity, neurological deficits, psychiatric disorders, and endocrine conditions [26]. The disproportionate difference observed in the younger population continues into mid-adulthood [9, 24, 28], with evidence showing a higher prevalence of any (37.6% versus 13.1%) [28] and severe/life-threatening (22.5% versus 4.3%) CHCs [24], and a higher cumulative count (an estimated average of 17.1) of CHCs by age 50 [9]. The existing body of evidence needs to be expanded to identify clusters of CHCs in adult CCS [26, 27, 29], provide longitudinal administrative evidence on the prevalence of acute and chronic multiple conditions [9, 12, 24, 28] among the hospitalised population of adult CCS [12], and examine the influence of co-occurring diagnoses on repeated healthcare use and length of stay [12]. Additionally, there is currently no data on the burden of multimorbidity in the Australian CCS population, despite the increase in the number of five-year prevalent cases by 80% between 1979 and 2018 [5].
We aimed to examine the overall and cause-specific multimorbidity in a hospitalised population of five-year CCS in Western Australia (WA) and a matching non-cancer comparison group from the general WA population using recurrent hospitalisation (and community mental healthcare) records. We extended the commonly used operational definition of multimorbidity ‘≥ two CHCs in different organ systems in the same individual’ [30] to ‘≥ two conditions (acute or chronic)’ at the time of hospitalisation. Incorporating acute conditions can help capture the possible cause-effect relationship between acute and chronic conditions over time [30], providing a more comprehensive indication of the illness burden. Chronic disease-specific analyses were also conducted to examine the prevalence of common and severe conditions, and identify frequently co-occurring condition clusters.
Methods
Study design and setting
This is a retrospective examination of whole-population cancer, inpatient, and community mental health service records linked using the WA Data Linkage System, from 1982 to 2019. WA is the largest state in Australia by area, with 2.8 million people in 2019 [31], representing 10.7% of Australia’s population. Eighty per cent of the WA’s population resides in major urban areas, and 3.2% identify as Indigenous Australians [32].
Study participants
The WA Cancer Registry (WACR) and the Perth Children’s Hospital (PCH) Oncology Dataset were used to extract records for children (aged <18) diagnosed with cancer in WA from 1 January 1982 to 30 June 2014. The WACR is a statutory data collection for all histologically and radiologically confirmed neoplasms in WA. The PCH is the tertiary referral centre for all paediatric and adolescent cancers diagnosed in WA. The cancer diagnostic groups were coded and categorised according to the International Classification of Childhood Cancer, Third Edition [33]. The five-year survival status was determined using the death date extracted from the Death Registrations. For each cancer case, we sampled up to 10 sex- and birth/month-year-matched individuals with no history of childhood cancer and a non-deceased status at the corresponding case’s cancer diagnosis date from the WA Birth Registrations. The large non-cancer comparison group enabled matching without replacement, which helped improve the matching quality by increasing the set of possible matches [34].
Multimorbidity ascertainment
In this study, two analyses were performed to assess the burden of multimorbidity among hospitalised participants. The individual instances of inpatient admissions were examined to ascertain the burden of multimorbidity. In the primary analysis, multimorbidity was defined as two or more co-occurring acute or chronic conditions (hereafter referred to as ‘any multimorbidity’) in the same individual. Co-occurring acute or chronic conditions were categorised into major diagnostic groups, including: A) blood and immunology; B) cardiovascular; C) digestive; D) endocrine, nutritional, and metabolic; E) genitourinary; F) infectious and parasitic; G) musculoskeletal and connective; H) neoplasms; I) neurological and sensory; J) respiratory; and K) mental and behavioural disorders. Physical and mental health conditions were identified using the principal diagnosis (i.e., condition chiefly responsible for an inpatient episode) and 21 additional diagnoses (i.e., conditions with significant influence on the treatment of the current episode) fields of records in the Hospital Morbidity Data Collection (HMDC). Since 1980, the HMDC has collected mandatory reported data on all inpatient activities in public and private hospitals in WA. All records (including day and obstetric admissions) were examined for the presence of multimorbidity. The co-existing mental and behavioural disorders were also identified using the Mental Health Information Data Collection (MIND). Since 1966, the MIND has collected mandatory reported data on contacts with specialised ambulatory community-based services. Diagnostic conditions were coded using the International Statistical Classification of Diseases and Related Health Problems, Ninth Revision, Clinical Modification and Tenth Revision, Australian Modification (Supplementary Table 1) [35, 36].
In the secondary analysis, multimorbidity was defined as two or more co-occurring CHCs in the same individual. CHCs were defined using Tonelli’s validated algorithm for identifying multimorbidity in health administrative data (Supplementary Table 2)[37, 38]. This algorithm identifies 30 CHCs with high to moderate validity (i.e., high: positive predictive value (PPV) and ≥70 sensitivity; moderate: ≥70 PPV and <70% sensitivity) [37, 38]. It captures a reasonable number of core CHCs identified in a previously validated algorithm by Barnett et al. [39] and a recent systematic review [40]. Additional CHCs were defined based on conditions published in an international Delphi consensus to measure multimorbidity [41] and publications by the Australian Institute of Health and Welfare (Supplementary Table 2) [42, 43].
The prevalence of CHCs at the end of the study period was determined based on the diagnoses recorded within five years of the primary cancer diagnosis date (or assigned date in the matched comparisons) and throughout the post-survival period. The prevalence of conditions with potential for prolonged remission or cure at the end of the study was assessed using specific observation windows: 24 months for chronic pain, peptic ulcer disease, and severe constipation, and 12 months for depression and anxiety. These observation windows were defined based on Tonelli’s and Barnett’s validated algorithms for measuring multimorbidity.(33–35) The baseline prevalence of chronic multimorbidity was determined using the diagnostic information recorded within five years of the primary cancer diagnosis date (or assigned date in the matched comparisons). Similar lookback periods of 12 and 24 months were also applied to conditions with the possibility of a cure to determine their presence at baseline (Supplementary Table 2).
Statistical analysis
Descriptive statistics were reported as counts and percentages for categorical variables and as the means (standard deviations [SDs]) or medians (interquartile ranges [IQRs]) for continuous variables. The baseline prevalence of chronic multimorbidity was calculated and reported as a percentage with a 95% confidence interval (CI). Follow-up for multimorbid conditions started five years after the initial cancer diagnosis date (index date). In many cancer survivors, the risk of tumour recurrence decreases five years after the primary diagnosis, enabling the identification of complications not attributed to the immediate toxic effects of anti-cancer treatment [2, 20]. In the event of a new cancer within five years of the index date, the latest diagnosis date substituted the original index to ensure that acute therapy-related toxicities were not captured. The matched comparisons were assigned to the index date of the corresponding cancer case to ensure that the follow-up period was comparable. The participants were followed up until the date of administrative censoring (30 June 2019) or until the date of death, whichever occurred first.
The overall rate of recurrent hospitalisations with any multimorbidity (per 100 person-years) was quantified using all records with a multimorbidity indicator divided by the total person-time at risk. The cause-specific rate per 1000 person-years was quantified using all records with indicators for multimorbidity and the specific cause being examined. The risk of recurrent hospitalisations for cause-specific multimorbidity in survivors compared with the non-cancer group was estimated using the Andersen-Gill model for recurrent events (an extension of the Cox model) [44]. The regression model accounted for the confounding effects of several demographic and clinical covariates, including diagnosis decade (categorised into the 1980s, 1990s, 2000s, and the 2010s), cancer diagnosis age (continuous and categorised into the age groups <5, 5–9, 10–14, and 15–18 years), sex, the Index of Relative Socio-economic Disadvantage, IRSD (ranging from the most (quintile 1) to the least (quintile 5) disadvantaged), and the Remoteness Areas (RA) derived from the Accessibility/Remoteness Index of Australia (categorised into a major city, inner regional, outer regional, remote, and very remote). Residential remoteness and socio-economic status indicators were assigned at the start of the follow-up period and carried forward. Age was added linearly with the age-squared term to account for the nonlinear effect of age. The cause-specific adjusted hazard ratio (aHR) was reported. A sensitivity analysis was performed to examine the aHR for multimorbidity in participants with no history of a new cancer or a recurrence of the primary cancer during the post-survival period. The overall and cause-specific rates of recurrent hospitalisations with chronic multimorbidity (per 1000 person-years) were quantified. The difference in the length of hospital stays (LoS) between patients admitted with and without any comorbidities was estimated using negative binomial regression models, adjusted for the presence of multiple diagnosis codes (yes/no), sex, age (and age squared term), RA, IRSD, diagnosis age, diagnosis decade and Indigenous status. The mean LoS (in days) was reported by the primary admitting cause.
Hierarchical clustering analysis (‘unsupervised classification’) [45] was conducted to examine the presence of non-random clusters of CHCs in survivors. CHCs were identified using all the diagnostic information recorded within five years of the primary cancer diagnosis and across the post-survival period. The conditions were coded as dichotomous variables (1=condition present, 0=condition absent). Before clustering, the dendrogram and Hopkins statistic (which measures the clustering tendency within the dataset, with values close to 1 indicating a high clustering tendency and values close to 0 indicating a random distribution of data points) [46] were examined to assess the clustering tendency of the data. The Hopkin statistic (H = 0.7) indicated the presence of a moderate clustering tendency within the data. Conditions with a < 5 prevalence were excluded from the clustering analysis. The Jaccard distance method was used to assess the dissimilarity between pairs of conditions. Ward’s minimum variance method was selected to minimise the total within-cluster variance by progressively joining the clusters with the smallest within-cluster distance [45]. The conditions with the highest prevalence within the dominant clusters were reported.
The prevalence of CHCs [37, 38] within subgroups stratified by socio-demographic and clinical factors by study exit was also reported. The temporal change in the cumulative prevalence of chronic multimorbidity (defined as the presence of two or more co-occurring non-cancer diseases, identified using Tonelli’s algorithm) [37, 38] in survivors with no history of multiple CHCs during the transition to adult healthcare services was examined at ages 18–29, 30–39, and 40–55 years. The analyses were conducted using SPSS 29 (IBM Corporation, New York), StataNow-18 BE (College Station, Texas) and R 4.1.2 (R Foundation for Statistical Computing, Vienna), with a two-sided p-value < 0.05 indicating statistical significance.
Ethics approval
The Human Research Ethics Committees at the WA Department of Health, Child and Adolescent Health Service, and the University of WA approved access to the de-identified health data through a consent waiver according to their respective guidelines (References: RGS0000001488; RA/4/20/5340).
Results
The study cohort consisted of 2,938 CCS and 24,792 non-cancer comparisons. The baseline characteristics of the study participants are summarised in Supplementary Table 3. The follow-up period spanned 32.5 years, totalling 38,630 person-years in survivors and 327,075 person-years in comparisons. The duration of follow-up was similar for both groups, with a median of 12.0 years (range, <1–32.4 years). The mean age at the end of follow-up was 27.1 (SD 10.6) years in survivors and 26.9 (SD 10.5) years in comparisons, with the follow-up extending to early adulthood in both participant groups (range, 5–55 years). For survivors, the median time since cancer diagnosis was 13.3 (range, 5.0–37.5) years, and the most common childhood cancer diagnoses were leukaemia (21.1%), other epithelial and skin carcinomas (19.6%), and CNS tumours (14.2%).
Chronic multimorbidity prevalence at study entry
At baseline, survivors had a significantly higher prevalence of chronic multimorbidity (9.1%, 95%CI 8.0–10.1%) compared to the non-cancer group (0.8%, 95%CI 0.7–1.0%), p < 0.05 (Supplementary Table 3). Among survivors, the most common non-cancer CHCs were back problems (3.2%, 95%CI 2.5–43.8%), chronic kidney disease (3.0%, 95%CI 2.4–3.6%), hypertension (2.8%, 95%CI 2.2–3.4%), other liver diseases (2.6%, 95%CI 2.0–3.2%), and peripheral neuropathy (1.7%, 95%CI 1.2–2.1%). Baseline chronic multimorbidity was more prevalent among survivors diagnosed at age 5–9 years (11.2%, 95%CI 8.5–13.9%) than among those diagnosed between the ages of 15–18 years (7.6%, 95%CI 5.7–9.5%) and among survivors of haematological cancers (14.6%, 95%CI 12.3–16.8%) compared to survivors of CNS tumours (8.9%, 95%CI 6.1–11.6%) and solid tumours (7.5%, 95%CI 6.0–9.0%). It was also more prevalent in male (9.3%, 95%CI 7.8–10.8%) than in female (8.8%, 95%CI 7.3–10.2%) survivors.
Coexistence of acute and chronic conditions
During the study period, 31.3% of the survivors and 17.2% of the comparisons were admitted to the hospital with multiple conditions. The overall rate of any multimorbidity per 100 person-years was 10.6 (95%CI 10.2–10.9) in survivors and 3.2 (95%CI 3.2–3.3) in comparisons (Table 1). Among the survivors, the rate/100 person-years was highest in those diagnosed with cancer at age 5–9 years (12.5, 95%CI 11.7–13.3), those diagnosed in 1982–1989 (11.3, 95%CI 10.7–11.9), those from low socio-economic status (13.7, 95%CI 12.9–14.6), and those of female sex (12.2, 95%CI 11.7–12.7) (Table 1). Within the major cancer diagnostic categories, the rate of any multimorbidity per 100 person-years was highest in CNS tumour survivors (16.3, 95%CI 15.2–17.4), followed by haematological (12.2, 95%CI 11.6–12.9), and solid tumour survivors (8.3, 95%CI 8.0–8.7). In CNS tumour survivors, the rate was highest in survivors diagnosed with cancer at age <5 years (22.2, 95%CI 19.9–24.7), and those from low socio-economic status (29.8, 95%CI 26.6–33.4) (Supplementary Table 4).
The adjusted HR (aHR) for any multimorbidity in survivors versus the comparisons was significantly higher (p < 0.05) for all major admitting causes. The aHR was highest for neoplasms (14.6, 95%CI 11.2–19.1), blood (7.3, 95%CI 4.9–10.7), neurological and sensory (5.2, 95%CI 4.2–6.6), cardiovascular (3.6, 95%CI 2.6–4.8), and endocrine (3.0, 95%CI 2.4–3.6) diseases (Table 2). The most prevalent diagnoses within each major diagnostic category are presented in Supplementary Table 5. Overall, the sensitivity analysis indicated a lower aHR for any multimorbidity in participants with no history of a cancer diagnosis in the post-survival period. However, the variability in the risk between survivors and their comparisons remained significantly higher across all diagnostic categories (Supplementary Table 6). The prevalence of comorbidity pairs stratified by major physical diagnostic groups and mental disorders is presented in Fig. 1. Mental disorders were prevalent among admitted survivors, irrespective of the principal cause of admission. Co-occurring mental disorders were most prevalent in patients admitted for neurological and sensory (71.1%), endocrine (61.5%), and digestive (59.3%) diseases. Several comorbid disorders were particularly prevalent in admitted patients, including endocrine diseases in patients admitted for infectious and parasitic diseases (42.6%), genitourinary and musculoskeletal diseases in patients admitted for blood diseases (40.0% and 38.7%, respectively), and digestive diseases in patients admitted for endocrine diseases (34.2%).
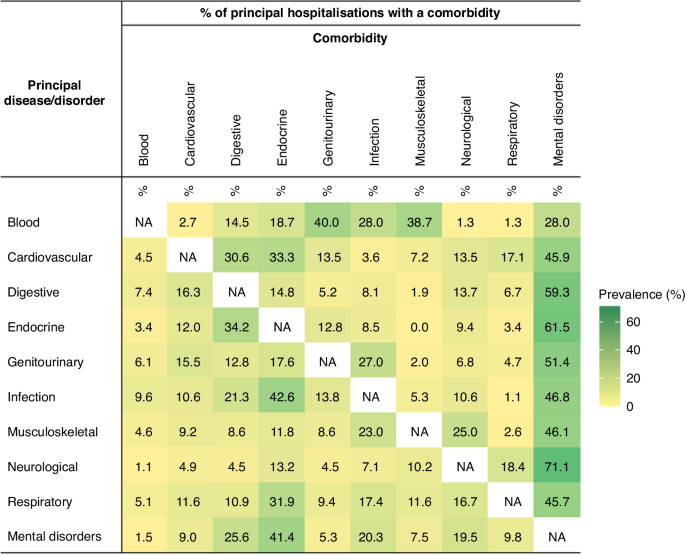
§Mental disorders were identified using the principal diagnosis and 21 additional diagnoses fields in the hospitalisation records, as well as records of contact with community-based mental health services in the same year of hospitalisation (only patients with clinically relevant symptoms or diagnoses were considered).
The average LoS was longer among survivors admitted with any multimorbidity than among those admitted with a single condition (Supplementary Table 7). The mean difference in LoS was significantly greater (p < 0.05) across all diagnostic categories, except for blood diseases (p > 0.05). Comorbidities managed during inpatient care for mental disorders (10.2, SD 8.3), neoplasms (5.9, SD 6.3), and infectious and parasitic diseases (4.5, SD 5.8) had the greatest impact on the duration of hospital stay.
Coexistence of chronic conditions
The overall rate of recurrent hospitalisations with chronic multimorbidity was higher in survivors than in comparisons (rate/1000 PY 16.2, 95%CI 15.0–17.5 vs. 8.6, 95%CI 8.3–9.0) (Supplementary Table 8). Among survivors, the rate of hospitalisation (per 1000 PY) was highest for psychiatric disorders (7.3, 95%CI 6.4–8.2), followed by gastro-intestinal (4.0, 95%CI 3.4–4.6) and kidney disease (3.4, 95%CI 2.8–4.0). By age 55, 14.5% (95%CI 13.2–15.7%) of survivors were admitted to the hospital with chronic multimorbidity (Supplementary Table 9), compared with 5.3% (95%CI 5.0–5.6%) of comparisons (data not shown in the table). The characteristics associated with a higher prevalence of multimorbidity included CNS tumour diagnosis (22.1%, 95%CI 18.1–26.0), cancer diagnosis in the 1980s (20.6%, 95%CI 17.0–24.1), high socio-economic disadvantage (18.6%, 95%CI 15.3–21.8%), and female sex (16.3%, 95%CI 14.4–18.2%) (Supplementary Table 9).
In survivors with chronic multimorbidity, the most common diagnoses included back problems (32.2%, 95%CI 27.8–36.7%), other liver diseases (27.3%, 95%CI 23.1–31.5%), kidney disease (26.8%, 95%CI 22.6–31.0%), hypertension (24.7%, 95%CI 20.6–28.8%), and psychoactive substance dependence (19.1%, 95%CI 15.3–22.8%) (Fig. 2). Hierarchical clustering analysis revealed four key clusters: 1) psychoactive substance dependence, alcohol misuse, and other mental and behavioural disorders; 2) hypertension, diabetes, kidney disease, and musculoskeletal diseases; 3) epilepsy, hypothyroidism, and other liver diseases; and 4) hypertension, kidney disease, and other liver diseases (Table 3). Following the transition to adult healthcare services, the prevalence of CHC multimorbidity increased with age in both survivors and comparisons (Supplementary Fig. 1); however, the prevalence was consistently higher (p < 0.05) in survivors across the three examined age groups <29 (14.8 vs. 5.5%), 30–39 (21.2 vs. 10.2%), and 40+ (16.6 vs. 8.6%).
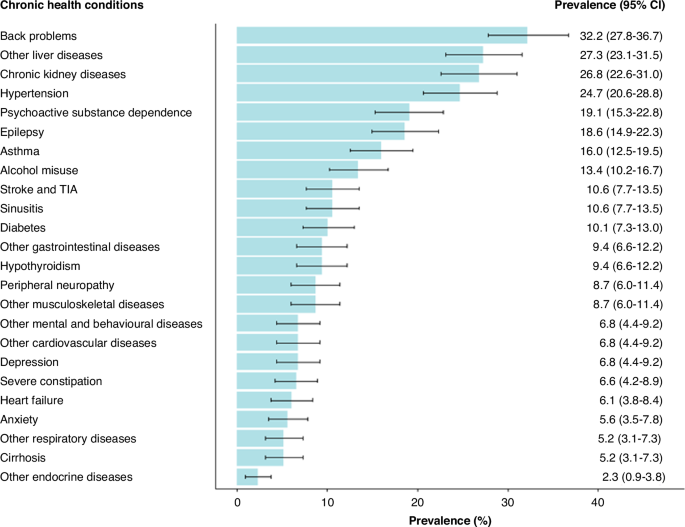
Prevalence of chronic health conditions in 5-year childhood cancer survivors with multimorbidity at study exit, Western Australia, June 2019.
Discussion
This study investigated longitudinal hospitalisation (and community mental health service contact) records of five-year CCS and an aged- and sex-matched non-cancer comparison group to assess the burden of multimorbidity and the clustering of multiple conditions in survivors. In this investigation, the operational definition of chronic multimorbidity was extended to capture acute conditions, which provided novel insight into the burden compared with previous studies [9, 12, 15, 24, 26, 27]. This mechanistic understanding of multimorbidity is needed, as conditions with the possibility of complete remission or with life-long implications can negatively impact survivors’ quality of life and functional capacity [47]. Our findings showed a higher rate of recurrent hospitalisations with any multimorbidity among survivors compared to comparisons. The cause-specific adjusted risk of any multimorbidity was more than 2-fold higher in survivors than in comparisons across all examined diagnostic categories, indicating heterogeneity in outcomes due to the biological differences underlying cancer types [9], variability in therapeutic intensity [9], and socio-behavioural factors [11]. An increased risk across the diagnostic categories was observed in survivors with and without a history of recurrence or new neoplasm during the post-survival period. The duration of hospitalisation was significantly longer in survivors admitted with multiple conditions than in those admitted with a single condition. Comorbidities managed during hospitalisation for a mental disorder or a neoplasm had the most significant impact on the length of stay. The analyses identified characteristics with an elevated burden of multimorbidity (including cancer diagnosis at age 5-9, exposure to oncologic treatments in the 1980s, a history of CNS tumour and haematological cancer diagnosis, female sex, and low social disadvantage) that have been previously identified as predisposing factors for increased morbidity [8, 11, 48] and could benefit from risk-stratified interventions [11, 17].
Examination of conditions comorbid with the principal admitting cause showed a high prevalence of mental disorders, particularly in survivors admitted for neurological, sensory, endocrine, and digestive disorders. The adverse effects of accumulating physical health conditions on psychological functioning can explain this finding [48]. Evidence from the general adult population has identified an association between psychological distress in patients with unmanaged multimorbidity and fragmented healthcare delivery [49, 50]. This study also identified a higher prevalence of comorbidity pairs, suggesting potential disease associations previously documented in the literature. Notable associations include an elevated risk of pathological changes in the endocrine system due to infections [51]; the influence of imbalances in endocrine hormones on the functionality of the gastrointestinal tract [52]; and elevated genitourinary and musculoskeletal [53, 54] morbidity following haematological diseases [54] and haematopoietic cell transplantation [17].
In the chronic condition-specific analyses, a higher prevalence of multimorbidity was observed in survivors than in comparisons. Similar findings were previously reported in five-year CCS aged <18 years (cumulative incidence 5.3 vs. 1.3%) [12] and 18–48 years (relative risk 4.9, 95%CI 4.4–5.5) [28] in comparison with the general population [12] and siblings [28]. These disproportionate differences in prevalence were also observed at baseline, highlighting the consistent pattern of elevated illness among survivors at a relatively young age [12]. Young adult survivors contributed more to the burden of multimorbidity on hospital services, which can be explained by the higher absolute number of survivors aged <30 years. In addition, the higher burden of illness in younger survivors can be a consequence of childhood cancer treatments (such as chest and alkylating agents), which have been linked to accelerated epigenetic ageing and an earlier development of age-related CHCs (such as hypertension and cardiovascular disease) [55]. Compared with those in the general population and siblings, the manifestation of CHCs in survivors can be more severe, irrespective of primary cancer diagnosis [56, 57]. Following the transition to adult healthcare services, hospitalised survivors without a history of chronic multimorbidity exhibited a higher cumulative prevalence of multiple conditions across various age groups compared to their counterparts. This trend can be attributed to the latent health vulnerabilities that increase over time [58] as survivors age [59].
Hierarchical clustering analysis revealed moderate and clinically meaningful clustering of some chronic conditions within individual survivors [45]. This moderate clustering across the cohort of survivors reflects individual-level variability in the conditions they experience. The prevalence of conditions with unshared pathophysiology has been previously reported in survivors and can be attributed to differences in risk factors, treatment exposures and pathophysiological pathways [60]. The co-occurrence of psychoactive substance abuse, alcohol misuse, and other mental disorders highlights the importance of monitoring and educating survivors about risky behaviours. Early interventions targeting these behaviours in adolescent survivors can help mitigate the socio-economic impacts of substance misuse, prevent associated psychiatric and neurotoxic effects [61, 62] and reduce the risk of drug addiction problems in adulthood [63]. The interplay of predisposing risk factors can contribute to the clustering of hypertension, diabetes, and kidney and musculoskeletal diseases. The effect of cranial radiation on the hypothalamic-pituitary axis (HPA) has been linked to endocrine complications (e.g., diabetes) [64] and to subsequent obesity and overweight in CCS [17]. The effects of total body and abdominal irradiation on the HPA, adipose and pancreatic tissues are also linked to high relative fat mass [65]. These cardiometabolic risk factors can, in turn, exacerbate the risk of cardiovascular diseases, including hypertension [66]. Exposure to cancer treatments (including alkylating agents, total body irradiation or abdomen radiation) [17] can also contribute to nephrotoxicity and decline in kidney function [67], which can lead to or be exacerbated by hypertension [67, 68]. The presence of musculoskeletal diseases in this cluster can be attributed to metabolic impairments that may lead to impaired bone growth in CCS [54]. The clustering of hypothyroidism, liver disease and epilepsy can potentially occur in the same survivor as a consequence of therapeutic exposure [28]. The diagnosis of epilepsy and hypothyroidism in CNS tumour survivors can result from surgical resection near or within the hypothalamic-pituitary region [69] and CNS-directed treatment [70]. Additionally, CCS have an increased risk of metabolic syndrome [71, 72], which can lead to hepatic abnormalities, including non-alcoholic fatty liver disease [71] and cellular liver injury [73].
Policy implications. The findings reported in this study highlight the importance of continuity of care and periodic surveillance to prevent the progression of symptoms and acute conditions to chronic diseases. Evidence shows that integrating care (functional, organisational, and clinical) can help reduce the burden of illness [13, 74] and prevent unnecessary hospitalisations. Managing survivors according to evidence-based guidelines [17, 75] within survivorship clinics that enable access to specialised expertise and patient-centred approaches (including access to self-management tools) can help address the diverse multimorbid conditions observed in CCS. However, the availability of survivorship clinics in overbooked haematology/oncology centres can challenge the provision of recommended follow-up care, highlighting the importance of general practitioners’ involvement in the longitudinal monitoring of treatment outcomes [19]. The development and implementation of avenues (e.g., secure online patient portals) [76] that enhance shared care between specialised expertise within survivorship clinics and general practitioners is needed [20, 77]. A pre-planned multidisciplinary approach needs to be organised around the prevalent clusters of conditions [30] to help reduce the complications arising from the interactions between these conditions and their treatments. The higher prevalence of chronic multimorbidity at baseline and in those aged <30 years emphasises the importance of personalised and holistic healthcare management during and immediately following treatment completion. This approach can ensure early management of existing and newly developed conditions that may impact the course and outcomes of the illness burden in survivors [74, 78]. Furthermore, enabling access to integrated and personalised management during the transition to adult healthcare services is necessary to address the lack of comprehensive transition protocols [79, 80] and ensure continuity of care [60].
Future research. An investigation of the total multimorbidity burden using linked hospitalisation, emergency, primary care, and pharmaceutical health records is warranted. Utilisation of different research methods to understand behavioural and social risk factors [81, 82] is also needed to inform the development of targeted interventions, and reduce the risk of premature death caused by preventable chronic conditions [83]. A review of the availability and effectiveness of the interventions that survivors and caregivers could use to manage multiple conditions is necessary.
This study had notable strengths. First, the mandatory and comprehensive collection of clinically confirmed cancer diagnoses and deaths ensures accurate and complete identification of CCS. Second, the mandatory collection of all admitted activities in public and private hospitals has enabled accurate and complete ascertainment of the inpatient services utilised by the participants. This provides the opportunity to assess how accumulated CHCs can impact the need for hospital services throughout the survivorship period. Third, the 21 additional diagnosis fields have consistently been used to capture conditions that significantly impact the treatments received and resources utilised during inpatient care, which allowed for the examination of severe comorbidities that can have a significant impact on the patient at the time of hospitalisation. Fourth, accessing records of outpatient mental health events provided the opportunity to ascertain psychiatric comorbidities managed at the community level. Fifth, the inclusion of sex- and age-matched comparisons from the general WA population allowed for a more accurate assessment of the potential impacts of childhood cancer on multiple disease burden by providing a control group for comparison. This study had five main limitations. First, comprehensive indicators of ethnicity, social factors (such as employment status and educational level), health-related behaviours, functional deficits, disability, and frailty are not routinely collected in hospitalisation records, limiting the possibility of examining their impact on multimorbidity patterns. Second, the lack of treatment elements and cancer diagnosis stage data prevented the examination of their attribution to comorbidity and total multimorbidity. Third, the lack of access to primary care contacts, outpatient visits, and pharmaceutical dispensing data prevented a more comprehensive quantification of the scale and impact of multimorbidity on survivors. Fourth, the absence of an internationally accepted definition and approach to measure multimorbidity [41] can reduce the comparability of findings. Fifth, the associations between condition severity and observation time [84] and the higher scores of severe CHC observed in survivors [9, 56] can potentially increase the likelihood of identifying multimorbidity in CCS than in the comparisons due to the documented higher severity of their conditions [57].
In summary, this study revealed a higher burden of multimorbidity in hospitalised CCS compared with the matched non-cancer group, which extended from childhood to middle adulthood. The observed morbidity was heterogeneous, reflecting the necessity to ensure holistic management that incorporates disease-oriented specialists, and primary care is available for survivors with an increased prevalence of and susceptibility to multiple conditions. Collaborative international research focusing on the effects of treatment modality, diagnosis type, and diagnosis stage on the risk of developing common multimorbid conditions could help guide the development of prevention strategies for conditions with similar aetiologies. Additionally, it is essential to understand how various clusters of chronic conditions can impact the physical and psychological functioning of survivors.



Responses