Outcomes and prognostic factors in patients with Burkitt lymphoma/leukemia in adolescents and adults: an experience from hematology cancer consortium

Introduction
Burkitt lymphoma and leukemia (BL/L) is a highly aggressive B-cell non-Hodgkin lymphoma (NHL) and constitutes less than 2% of all NHLs [1]. In India, the sporadic and Human Immunodeficiency Virus(HIV) related subtypes are the main subtypes of BL/L [1,2,3]. Treatment protocols like Magrath regimen-, CODOX-M/IVAC [4], hyper-CVAD [5], LMB [6] and BFM [7] are based on the principle of short-course, intensive, non-cross-resistant alternating chemotherapy. In the paediatric setting, these approaches have shown excellent outcomes, with protocols like BFM90 and LMB89 resulting in 6 year event free survival(EFS) of 89%(95%CI: 87–91%) [7] and 5 year overall survival(OS) of 92.5%(95%CI: 90–94%) [8] respectively. On the other hand, similar regimens when used in the adult setting have resulted in inferior outcomes, with the Magrath regimen, hyper-CVAD and LMB protocol resulting in 2 year OS of 72.8% (95% CI: 59.4% to 86.3%) [9], 3 year OS of 49% (95%CI: 38–60%) [5] and 2 year OS of 70% (95%CI: 59–81%) [6] respectively. Addition of rituximab has resulted in an improvement in overall survival in the Magrath regimen, hyper-CVAD protocol and LMB protocol by 11%, 36%, 13% respectively [10,11,12]. Incorporation of rituximab also has given the opportunity to reduce the dose of methotrexate in the Magrath regimen and the GMALL-B-ALL/NHL-2002 protocol without decrease in efficacy [11,12,13,14]. These protocols are associated with increased treatment related toxicities. Use of high dose methotrexate in these regimens makes it challenging in the setting of renal dysfunction, effusions and ascites. Recently, low intensity regimen of dose-adjusted cyclophosphamide, etoposide, prednisone, vincristine, doxorubicin and rituximab(DA-EPOCH-R) has gained increasing interest. Use of this regimen was pioneered by the National Cancer Institute(NCI) based on the concept of maintaining continuous drug exposure, aiming to induce genotoxic stress in the rapidly dividing BL/L cells [15, 16]. The use of DA-EPOCH-R resulted in 86 month OS of 100%(95% CI: 82–100%) in the NCI trial [15] and a 4 year OS of 87.0% (95% CI: 79–92%) in the multicenter trial by Roschewski et al. [17] with lower treatment related mortality rate of 4% [17]. It was noted that in the presence of CNS and bone marrow involvement, this regimen performed inferiorly [17]. The two approaches were compared in a clinical trial by the HOVON-SAKK group. The trial although terminated prematurely, showed that the Magrath regimen(R-CODOX-M/R-IVAC) and the DA-EPOCH-R regimen when used in a population with high risk BL/L(as per Mead’s risk stratification) [9] resulted in 2 year OS of 76% (95%CI: 60–86%) and 75% (95%CI: 59–86%) respectively [18].
Being a rare disease, randomised controlled trials are few. Till now, there have been only two randomised controlled trials in BL/L [12, 18]. Most of the prospective trials in BL/L, have included small number of patients and there is a concern if the trial results can be replicated in the real-world. The recent publication from 30 centres of the United States of America has shown that the outcomes in real world are poorer as compared to those reported in clinical trials [19]. Thus, evaluating the real world outcomes of these patients in our setting becomes relevant, especially because of the unique issues in our health care like delays in treatment initiation, poor tolerance to treatment resulting in dose compromise and treatment abandonment. This analysis can help us identify the appropriate regimen for our patients as well as the relevant research questions that can be addressed by future prospective clinical trials.
Methods
This retrospective multicenter study collected data from eight- member centers of Hematology Cancer Consortium (HCC) (www.hemecancer.org). All the consecutive patients with histologically proven BL/L(including HIV positive patients) aged more than 14 years, who were registered at the participating centres between Jan 2012 to December 2019 were enrolled in the study. Patients who received prior treatment (except steroids for less than 2 weeks and/or cyclophosphamide (≤2 doses) or surgery for obstruction or perforation) were excluded from the analysis.
Diagnosis and treatment
Histopathological diagnosis of BL/L was established from an excisional/incisional/core or formalin-fixed paraffin block or peripheral blood in case of leukemic spill. Diagnosis of BL was considered based on the presence of medium sized tumour cells in a diffuse pattern in the biopsy. Moreover, for confirmation, immunohistochemistry and immunophenotyping (in case of Burkitt leukemia in bone marrow or peripheral blood sample) indicating positive expression of germinal centre markers(CD10 and BCl6) and B Cell antigens(CD19, CD20, CD22, CD79a and PAX5) and negative expression of Cyclin D1, CD5, CD23, BCl2, CD138 and TdT [20, 21] was required. Diagnosis was established by the individual institutional pathology team without central pathology review. Staging methods included non-contrast whole body Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) or contrast enhanced computed tomography (CECT) Thorax, Abdomen and Pelvis. In addition, a bone marrow assessment with aspiration, morphology with or without flowcytometry and histopathology was also performed for staging. CNS assessment was performed with cerebrospinal fluid cytology and/or flowcytometric assessment.
Patients received treatment as per the institutional guidelines and treating oncologist’s discretion. Broadly, patients received the following regimens (Supplementary Table 1) –
-
High dose Methotrexate based: modified GMALL-B-ALL/NHL-2002 protocol [14], modified LMB-89 protocol [22], R-MPV [23] protocol.
-
Dose adjusted EPOCH – R: DA-EPOCH-R or short course EPOCH-RR(sc-EPOCH-RR) [15].
-
Others: CHOP, COP, RCHOP, RDHAP, Rituximab+MCP842.
Response was assessed at interim after 2 cycles of chemotherapy and if not in complete response (CR) then at the end of treatment. Response assessment using PETCT and CT scan was reported as per the Lugano response criteria [24]. In patients with Burkitt leukaemia, in addition to the radiological complete response, absence of disease in bone marrow either at the interim or end of therapy was required to document as complete response.
Variables and endpoint
Investigators collected detailed demographic, clinicopathologic and outcome data in an electronic database. Serum LDH was standardized relative to the institutional upper limit of normal (ULN). Other variables which were collected included: Performance status as per Eastern Cooperative Oncology Group(ECOG), Human Immunodeficiency Virus(HIV) status, antiretroviral therapy(ART) usage and CD4 count in the HIV positive patients, baseline B symptoms, surgery prior to presentation, bone marrow and CNS involvement status, extranodal involvement, and albumin values. The Burkitt Lymphoma International Prognostic Index(BL-IPI)was calculated from the variables entered in the database [25]. Primary endpoint for the study was event-free survival (EFS). Secondary endpoints were overall survival(OS) and treatment related mortality(TRM). EFS was defined as the time from the date of diagnosis until the date of progression, lack of response, death due to any cause, or last follow-up. OS was defined as the time from date of diagnosis until the date of death due to any cause or last follow up. TRM was defined as death due to treatment – related adverse event excluding disease related mortality.
Statistical analysis
We described the distribution of continuous variables using mean, median and interquartile ranges and compared them between groups using the two-sample independent t-test or Mann-Whitney U test; categorical variables were tabulated and compared using the chi-square/Fisher’s exact test. OS and EFS were estimated using the Kaplan-Meier method. Median follow-up was determined by reverse Kaplan-Meier method. Parameters which were evaluated for prognostic significance included age, use of rituximab, ECOG PS at presentation, baseline serum LDH value, HIV status, baseline bone marrow involvement, baseline CNS involvement, stage at presentation, BL-IPI risk stratification, type of chemotherapy used, dose compromise or delays and end of treatment response. Univariate analysis for EFS and OS were performed using cox-regression analysis. Parameters independently associated with EFS and OS was determined using multivariable analysis by cox-regression analysis. Univariate and multivariable logistic regression models were used to assess the association between the clinical variables and achievement of CR. Odds ratios and corresponding 95% confidence intervals (CI) were estimated for each variable. A propensity matched (PSM) analysis was performed to compare the survival of patients receiving methotrexate-based protocol and EPOCH-based protocol. For propensity score calculation following variables were selected using a binary regression model: age, gender, HIV status, serum albumin, bone marrow involvement and CNS involvement. Patients receiving methotrexate-based protocol were matched 1:1 with those receiving EPOCH-based protocol using the propensity variable with a caliper control 0.25. All statistical analyses were carried out with IBM SPSS Statistics version 21.0 software.
Results
Patient and disease characteristics
A total of 320 patients were registered during the study period (Jan 2012 to December 2019). Among them, 265 patients underwent treatment. The baseline characteristics of the treated and untreated populations were similar for most of the parameters (Table 1). In the treated population, median age was 38 years (Range: 14–101 years), 75% were men and 16% had reduced functional status (PS 3 or 4).
Sixty-nine patients (26%) were categorized as stage I/II, while 176 patients (66.4%) were stage III/IV. Bone marrow or peripheral blood involvement was seen in 25% (n = 68 patients), CNS involvement in 12.5% (n = 33 patients) and extranodal involvement in 78.5% (n = 205 patients). Most common extranodal sites apart from bone marrow and CNS were gastrointestinal tract (GI) (19.1%), soft tissue (12.9%) and liver (5.7%). Tumor lysis syndrome at presentation was identified in 48 patients (18.1%).
Out of the total treated patient population(n = 265), 38 (14%) were diagnosed with HIV, with 30 of them having initiated or previously been on ART. The median CD4 count was 228 (IQR: 134–450) cells/mm3. Nearly 90% of the HIV-positive patients were either stage III or IV, whereas this was 60% among those who were HIV negative (p – 0.010). Bone marrow involvement (34.2% vs 23.9%; p – 0.211) and CNS involvement (21.1% vs 10.5%; p – 0.035) was more common in the HIV positive patients than in the HIV negative patients. Raised serum LDH was seen more in HIV positive patients (LDH ≥ 3X ULN 50% vs 32.9%; p – 0.033). Median serum albumin was significantly lower in the HIV positive patients (Median – 3.5 vs 3.8 g/dl; p – 0.006). All other baseline parameters were similar between HIV positive and negative groups (Table 2).
Treatment details
Among 265 patients, 35 patients underwent surgery, 34 of them were performed prior to initiation of chemotherapy. There were 23 and 3 patients who presented with obstruction and perforation respectively. Prephase chemotherapy was administered in 203(76.6%).
Overall, methotrexate-based multiagent protocols were used in 108 patients (40.7%) and EPOCH-based protocol was used in 103 patients (38.8%). The distribution of chemotherapy protocols used in our cohort is summarized in Table 3. Rituximab was incorporated in the chemotherapy for 211 patients (79.6%). Median number of Rituximab doses were 6 (range: 1–11). Dose compromise/delays were seen in 74 patients (27.9%) and the most common reason was toxicity to therapy (58 patients, 74%).
Patients who received EPOCH based protocol were older in comparison to methotrexate-based protocols (median age: 44 versus 28 years). Methotrexate based protocols were preferentially administered in patients with bone marrow involvement, CNS involvement or Burkitt leukemia. Patients who received EPOCH based protocol had lower baseline serum LDH and median albumin levels in comparison to patients who received methotrexate-based protocols. Median number of cycles of chemotherapy administered were 6(Range: 1–6) for methotrexate-based protocols and 6 (Range – 1–8) for EPOCH based protocols. Rituximab was incorporated in 89% and 85.66% of patients receiving methotrexate-based protocols and EPOCH based protocol respectively. Dose compromise/delays were seen in 31.1% and 36% of the patients who received methotrexate-based protocols and EPOCH based protocol respectively. In the patients who received EPOCH based protocol, maximum dose level achieved were -level 1in 77.1%, level 2 in 9.6%, level 3 in 6% and level 4 in 3.6%. Comparison between methotrexate-based and EPOCH based protocol is summarized in Table 4.
Patients with HIV positivity predominantly received EPOCH based protocol (29 patients (76.3%)) which was either short course-EPOCH-RR (sc-EPOCH-RR)(6 patients) or DA-EPOCH-R(23 patients) depending on the institutional practice at the member centers. Rituximab was incorporated in the chemotherapy protocol in 29 (76.3%) out of the 38 HIV positive patients.
Efficacy
Amongst the 265 treated patients, complete response was achieved in 145 patients (CR rate – 54%), with 11 patients achieving partial response, making the overall response rate 58%. Forty-four patients (16.6%) had primary refractory disease and 3 had stable disease. There were 35 patients (13%) who did not continue treatment after initiation of treatment, treatment details were not available in 10 patients (3.7%), and 1 patient developed acute myeloid leukemia (AML) while on treatment. In addition, there were 16 deaths (6.0%) before the first clinical assessment. These details are summarized in the flowchart (Fig. 1).
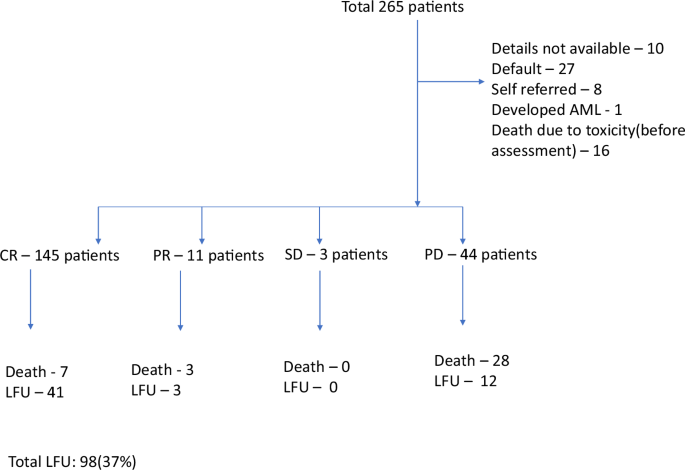
AML Acute myeloid leukemia, CR Complete response, PR Partial response, SD Stable disease, PD Progressive disease, LFU Lost to follow-up.
After a median follow-up of 42 months, there were 99 patients who had an event as per the EFS definition and there were 77 deaths. Amongst the 99 events, 21 were relapse, 30 had progression, 3 had less than partial response and 45 had died. These details are summarized in Fig. 2. Median time to relapse and progression was 4 months (IQR: 3–6 months). Amongst the 77 deaths, 19(24.6%) were due to toxicity and 56(72.7%) were due to progressive/relapsed disease. The 3-year EFS and OS for the overall population was 58% (95% CI: 55–61%) and 66% (95%CI: 63–69%) respectively. The 3-year EFS and OS of the HIV positive population was 54% (95%CI: 46–62%) and 66% (95%CI: 58–74%) respectively. The 3-year EFS and OS for patients with stage I/II were 73% (95%CI: 68–78%) and 78% (95%CI: 73–83%) whereas for stage III/IV were 54% (95%CI: 50–58%) and 63% (95%CI: 59–67%) respectively. Survivals did not differ with the use of methotrexate-based or the EPOCH-based protocols. Comparison of EFS and OS between the different subgroups is summarized in Supplementary Table 2. Kaplan-Meier curves of the overall population and different subgroups are in Fig. 3 and Supplementary Figs. 1–3.
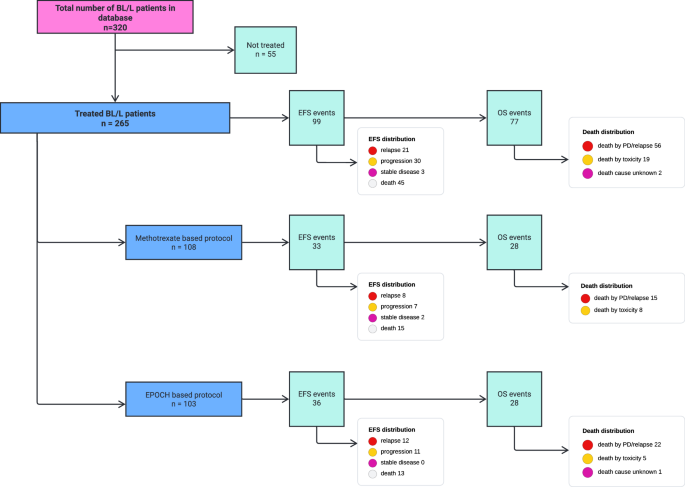
PD Progressive disease, EPOCH Infusional Etoposide, Prednisone, Vincristine, Cyclophosphamide, Doxorubicin, EFS Event free survival, OS Overall survival.
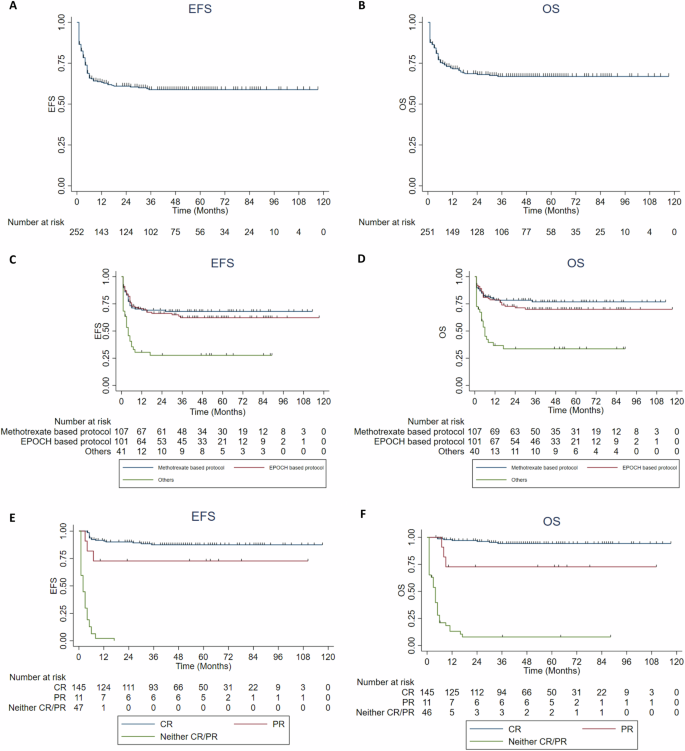
A, B EFS and OS of overall population. C, D EFS and OS according to the chemotherapy protocols used (Methotrexate based vs EPOCH based vs Others). E, F EFS and OS according to the end of treatment remission status (CR vs PR vs no CR/PR). EFS event-free survival, OS overall survival, EPOCH Etoposide, Prednisone, Vincristine, Cyclophosphamide and Doxorubicin continuous infusion, CR complete response, PR partial response, Neither CR/PR includes stable disease and progressive disease.
Toxicity
The most common toxicity encountered in our cohort was febrile neutropenia (58.8%), neuropathy (10.4%), tumor lysis (7.1%), skin toxicity (5.8%), infusion reactions (5.4%) and pulmonary toxicity (3.3%). Cardiomyopathy occurred in 1 patient and venous thrombosis occurred in 2 patients. Data on mucositis was not available from the database. TRM was observed in 19 patients (7.2%) in the overall cohort.
On comparison between the methotrexate-based and EPOCH based protocols, there were more occurrences of febrile neutropenia, infusion reactions, skin toxicity and pulmonary toxicity in the former than in the latter (Table 4). Incidence of neuropathy was similar in both the chemotherapy protocols. TRM in the methotrexate-based and EPOCH-based protocols were seen in 8(7.3%) and 5(4.8%) patients respectively.
Prognostic factors
For determining the prognostic factors, we performed the univariate analysis using cox proportional hazards model in the subset of patients who received chemotherapy protocols which are known to be effective in Burkitt lymphoma/leukemia (EPOCH based protocol and methotrexate-based protocol; N = 208). The factors which were associated with better survival(EFS and OS) included the use of rituximab, baseline PS 0–2, baseline serum LDH value < 3xULN, uninvolved bone marrow, stage of I/II, baseline low and intermediate BL-IPI risk stratification and achievement of CR. Comparison of the methotrexate-based protocols with EPOCH-based protocol by univariate analysis did not show a significant difference in the survivals (Table 5). We performed an additional subgroup analysis to determine the impact of the baseline bone marrow and/or CNS status and the chemotherapy protocols used on the survival. Use of EPOCH based protocol in patients with baseline BM/CNS involvement had an adverse impact on the EFS and OS (Supplementary Table 3). On multivariable analysis, achievement of CR was the strongest prognostic factor for EFS and OS. In addition, a baseline LDH> 3xULN was independently prognostic for the OS (Table 6). We performed logistic regression analysis to determine factors which could predict a CR response. However, none of the baseline factors (age, PS, HIV status, B symptoms, bone marrow involvement, CNS involvement, stage, rituximab use, serum LDH, serum albumin) could predict the occurrence of a CR response by univariate logistic regression.
Propensity matched(PSM) analysis
After propensity matching, 47 patients receiving methotrexate-based protocol were matched to 47 patients receiving EPOCH-based protocol (Supplementary Table 4). There was no difference in the 3-year EFS (68% (95%CI: 62–74%) versus 68% (95%CI: 61–75%); p 0.949) and 3-year OS (72% (95%CI: 66–78%) versus 70% (95%CI: 64–76%); p 0.865) between the two groups, respectively (Fig. 4).
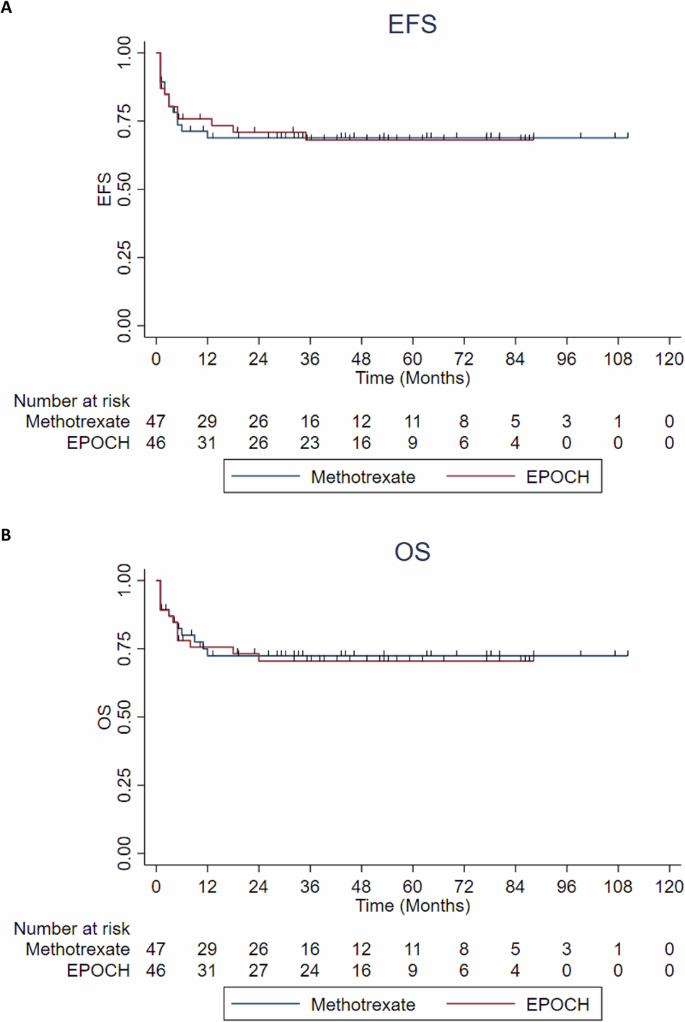
A, B EFS and OS of the propensity matched population comparing the methotrexate-based protocol vs EPOCH-based protocol. EFS event-free survival, OS overall survival, EPOCH Etoposide, Prednisone, Vincristine, Cyclophosphamide and Doxorubicin continuous infusion.
Discussion
To our knowledge, this is one of the largest reports from LMIC to date detailing the baseline disease characteristics, treatment details and prognostic factors of patients with Burkitt Lymphoma/Leukemia (BL/L). Treatment of BL/L in this real-world cohort yielded a 3-year EFS and OS of 58% (95% CI: 55–61%) and 66% (95%CI: 63–69%) respectively. Predominant protocols utilized were methotrexate-based in 108 (41.1%) and EPOCH based (DA-EPOCH-R, SC-EPOCH-RR) in 103 patients (39.2%) (Table 3). These results are inferior to the landmark trials using these protocols [12, 14, 15, 17]. Reasons for inferior survival in our cohort could be due to the use of regimens which are proven to be of poor efficacy in 48(18.1%), dose compromise/delays in 74(27.9%), treatment related mortality in 19(7.2%) and treatment abandonment in 27(10.2%) patients (Fig. 1). In addition, 34 patients(12%) underwent surgery prior to initiation of chemotherapy which led to delays in the initiation of definitive therapy. As the timing of chemotherapy is of paramount importance in the treatment of BL/L this may have led to poor outcomes. A similar trend of inferior survival in the real world was seen in the real world analysis from United States (US) demonstrating a 3 year PFS and OS of 65% (95% CI: 61–69%) and 70% (95% CI, 66–74) respectively [19].
For the 108 patients treated with methotrexate based protocol, the 3 year EFS and OS were 68% (95%CI: 64–72%) and 76% (95%CI: 72–80%) respectively (Supplementary Table 2). In comparison, the trials which incorporated rituximab with other methotrexate-based protocol like Magrath regimen [11], LMB89 protocol [12], hyper-CVAD protocol [10] and GMALL-B-ALL/NHL-2002 protocol [14] had a EFS/PFS of 74%, 75%, 80% and 75% and the OS of 77%, 83%, 89% and 80% respectively [10,11,12, 14]. Despite the younger age (median – 28 years) and a lower proportion of patients with advanced stage disease (65.1%) in our cohort compared to these trials, survival rate in our cohort was 5–10% lower. Possible contributing factors might include that 11% patients did not receive rituximab and almost 31% patients having dose compromise/delays. Another possible reason could be that LMB89 protocol and GMALL-B-ALL/NHL-2002 protocol used in our cohort were modified versions of the actual protocols (Supplementary Table 1). In our cohort, the intensification components of these protocols were not used. Also, drugs like teniposide and vindesin which were part of the original GMALL-B-ALL/NHL-2002 protocol were not available for use in India.
For the 103 patients treated with EPOCH based chemotherapy, the 3-year EFS and OS were 62% (95%CI: 57–67%) and 70% (95%CI: 66–74%) respectively (Supplementary Table 2). In contrast, the NCI trial reported a PFS and OS of 95% (95%CI: 75–99) and 100% (95% CI: 82–100) with DA-EPOCH-R and 100% (95% CI: 72–100) and 90% (95% CI: 60–98) with sc-EPOCH-RR respectively [15]. Roschewski et al reported a 4 year EFS and OS of 84.5% (95% CI: 76% – 90%) and 87.0% (95% CI: 79% – 92%) respectively [17]. Reasons for the inferior survival in our cohort could be the higher proportion of patients with poor performance status (PS 3 or 4 in 15%), high rates of dose compromise and delays (36%) and 80% patients receiving a maximum dose level of only 1 (Table 4).
Comparison of patients receiving methotrexate-based and EPOCH-based protocols, revealed the 3-year EFS to be 68% (95%CI: 64–72%) and 62% (95%CI: 57–67%) and 3-year OS to be 76% (95%CI: 72–80%) and 70% (95%CI: 66–74%) respectively. The difference in survival between them was not statistically significant (Supplementary Table 2). This was confirmed in the PSM balanced population where the survivals with both the protocols were similar (Supplementary Table 4 and Fig. 4). Similarly, in the US real world study the 3 year PFS and OS was numerically lower in the patients who received DA-EPOCH-R(OS 69%) compared to CODOX-M-IVAC(OS 77%) and hyper-CVAD (OS – 70%) but was not statistically significant [19]. Even in the randomized HOVON-SAKK trial we see a similar trend (2 year OS: 75% with DA-EPOCH-R vs 76% with R-CODOX-M/R-IVAC) [18]. In our cohort methotrexate-based protocols were associated with more febrile neutropenia, infusional reactions, skin toxicity and pulmonary toxicity. The HOVON-SAKK group similarly noticed more hematological toxicity, infections, gastrointestinal complications with R-CODOX-M/R-IVAC than DA-EPOCH-R [18]. Treatment related mortality were similar between the methotrexate based and EPOCH based protocols in our cohort(7.3% vs 5.7%), US real world data(5% vs 8%) [19] and HOVON-SAKK trial(6.5% vs 11.6%) [18] respectively. An important finding suggested by our subgroup analysis was that patients with bone marrow or CNS involvement do worse when treated with EPOCH based protocol (3 year EFS: 32% (95%CI: 21–43%) and 3 year OS: 45% (95%CI: 33–57%) (Supplementary Table 3)). Similar findings were documented in the multicenter study of DA-EPOCH-R by Roschewski et al, where with CNS and bone marrow involvement the 4 year EFS and OS declined to 45.5% (95% CI: 17 to 71%) and 58.6% (95% CI: 39 to 74%) respectively [17]. In conclusion, both methotrexate based protocol and EPOCH based protocol are reasonable options for the treatment of Burkitt lymphoma. Additionally we propose that methotrexate based protocol must be preferred in patients with bone marrow and/or CNS involvement. EPOCH based protocol would be an option in elderly patients and in patients with contraindications for methotrexate like pleural effusion, ascites and renal failure.
For the 38 HIV positive patients with BL/L in our cohort, the 3-year EFS and OS was 54% (95%CI: 46–62%) and 66% (95%CI: 58–74%) respectively(Supplementary Table 2). In the analysis by Roschewski et al., the 4 year EFS and OS for the 28 HIV positive patient were 84.9% (95% CI, 65–94%) and 84.5% (95% CI, 75–91%) respectively [17]. Results from the real world US-UK analysis of 249 patients by Alderuccio et al had a 3 year PFS and OS of 61% (95% CI: 55–67%) and 66% (95%CI: 59–71%), similar to our cohort [26].
Survival rates did not differ based on the age ( ≤ 40 vs >40 years) and HIV status (Supplementary Table 2 and Supplementary Figs. 1–3). Factors like rituximab incorporation, better baseline PS, lower serum LDH, earlier stage, uninvolved bone marrow and achievement of CR were prognostic for better survival (Table 5 and Supplementary Table 2). Recently validated BL-IPI score [25], when used in our cohort distinctly revealed the difference in survival in the three risk groups, making it suitable for risk stratification even in our population(Table 5). The 3-year OS of the low-, intermediate and high-risk BL-IPI groups were 87% (95%CI: 83–91%), 68% (95%CI: 63–73%) and 54% (95%CI: 49–59%) respectively (Supplementary Table 2). However, on multivariable analysis, achievement of CR is the only important prognostic factor, emphasizing the importance of achieving this important milestone in the treatment of BL/L (Table 6). Part of the reason for this also could be the lack of intensification/salvage options for patients who do not achieve CR.
Conclusion
In conclusion, in this large real world, multicenter cohort of adult BL/L patients from India, the survival outcomes are like other real-world datasets. Our analysis shows that both methotrexate-based protocol and DA-EPOCH-R can be used in the treatment of BL/L. However, use of DA-EPOCH-R should be avoided in patients with CNS, bone marrow and peripheral blood involvement. Achievement of CR was the most important prognostic factor impacting the outcome. Attempts are needed to reduce the toxicity while maintaining the efficacy of currently used protocols.




Responses