Structural insights into tecovirimat antiviral activity and poxvirus resistance

Main
Orthopoxviruses (OPXVs) produce several human diseases, including smallpox and mpox, caused by variola (VARV) and monkeypox (MPXV) virus, respectively. Cowpox (CPXV), camelpox (CMLP), and borealpox (BRPV) also lead to sporadic zoonotic infections1. Smallpox vaccination was discontinued 50 years ago, leaving most of the current population unprotected and raising concerns about the emergence of zoonotic OPXVs or the reintroduction of VARV. Two major mpox epidemics have emerged since 2022. The first, caused by a mild clade II strain, spread rapidly across the globe, resulting in more than 90,000 cases and 179 deaths2. The second, produced by a virulent clade I strain, produced the largest outbreak ever recorded in the Democratic Republic of Congo and spread to neighbouring countries, resulting in hundreds of deaths3. First-generation vaccines do not meet modern safety standards, and attenuated third-generation vaccines, although effective, are difficult to produce on a large scale and do not induce long-term immunity4,5. Most recently, mRNA-based candidate vaccines have shown efficacy in animal models and are currently being tested in clinical trials6,7.
OPXVs have an unusual replication cycle that produces two different virions termed mature and enveloped viruses. Mature viruses are produced in the cytoplasm and formed by a viral capsid surrounded by a single membrane. To disseminate within the host, mature viruses form wrapped virions covered by three membranes, which henceforth fuse the outermost with the plasma membrane to leave the host cell as enveloped viruses, covered by two remaining membranes (Fig. 1a). Two oral drugs have been approved for the treatment of smallpox and mpox, brincidofovir (Tembexa) and tecovirimat (TPOXX). Brincidofovir is an inhibitor of viral DNA polymerase that has been shown to produce side effects in patients8. Tecovirimat is an inhibitor of mature virus wrapping widely used to treat mpox patients infected with clade IIb strains. However, it has a low resistance barrier, and multiple tecovirimat-resistant MPXV strains have been reported9,10. Resistance mapping studies10 have indicated that tecovirimat targets the virus envelope protein F13, a membrane-anchored phospholipase that plays a key role in the production of wrapped virions11,12. Tecovirimat binding to F13 blocks viral wrapping13, but the structural mechanism is poorly understood. Molecular dynamics simulations based on predicted structures have suggested different tecovirimat binding sites14,15, but none of them explain either how tecovirimat blocks wrapping or why resistance mutants escape the drug. This paucity of structural and mechanistic data prevents anticipating which mutations may confer resistance to tecovirimat and the development of better drugs.

a, Schematic representation of the replication cycle of OPXVs. Mature viruses enter the cell, fusing their membrane (in blue) with the cellular one. After DNA replication, immature particles are formed (IV, membrane in red), which give rise to intracellular mature virus particles in the cytoplasm of the infected cell. Mature viruses (MV) can either be released by lysis or wrapping. In the latter, mature viruses acquire two additional membranes (WV, in orange) from the Golgi apparatus or endosomal vesicles to form wrapped virions, fuse the outermost with the plasma membrane and release enveloped viruses (EV). Tecovirimat blocks wrapping, as indicated. The scheme, adapted from Fig. 1 in ref. 86, was created with BioRender.com. b, Crystal structure of the sF13 homodimer represented in cartoon. One protomer is coloured blue and the other green. The N termini and the MIR are indicated on one protomer, and the phospholipase active site is indicated on the other. Bottom panels provide close-up views of the two regions forming the dimer interface, indicated by coloured rectangles in the upper panel. All single escape mutants identified to date are shown as spheres, coloured according to their potency, reported as IC50 fold change. c, Side view of the F13 homodimer interacting with a lipid membrane that mimics Golgi membrane composition, as observed from molecular dynamics simulations. For clarity, water molecules and lipids in the foreground of the membrane are not shown. sF13 chains are coloured as in b, with palmitoylated cysteines and hydrophobic residues in the MIR and N termini depicted as sticks. The bottom panel provides close-up views to show lipid–protein interactions, with the protein residues involved in the interaction depicted as sticks and labelled. Protein carbons are coloured according to the chain, membrane carbons in white. Nitrogen, oxygen, sulfur and phosphate atoms are coloured blue, red, yellow and orange, respectively.
In this article, we elucidate using crystallography and molecular dynamics simulations the structure of F13 with tecovirimat. We showed that F13 forms a homodimer on membranes, and tecovirimat inserts into a cavity formed between the two protomers. Using analytical ultracentrifugation (AUC), size-exclusion chromatography coupled with small-angle X-ray scattering (SEC-SAXS), mass photometry, proximity ligation assay (PLA) and binding free energy calculations, we further report that tecovirimat induces homodimerization of F13 in solution and within cells, but not in resistant mutants, providing a mechanistic basis for drug activity and viral escape.
Results
Tecovirimat binds a pocket between two protomers
F13 is a phospholipase anchored to membranes via two palmitoylated cysteines located in a hydrophobic membrane-interacting region (MIR; Extended Data Fig. 1)16. For the structural studies, we produced a soluble variant of F13 (sF13) by removing the hydrophobic N-terminal tail (amino acids 2–5) and by introducing five mutations in the MIR. We obtained two different crystal forms (Supplementary Table 1). In both, sF13 featured a homodimer stabilized by two helices and a β-hairpin (Fig. 1b) with an interface area of 939 Å2, formed by a network of hydrogen bonds involving residues Y253, N259, N267, Y285, S292 and N300 (Extended Data Fig. 2). The contact region forms a large cavity of 290 Å3. The hydrophobic N termini and MIRs were located on one side of the dimer, and the two phospholipase D (PLD) catalytic pockets were pointed outwards (Fig. 1b). To evaluate whether the dimer was compatible with membrane insertion, we performed molecular dynamics simulations on membranes mimicking the composition of the Golgi membrane (Supplementary Table 2). We observed that the homodimer remained intact during the 1,000 ns of simulation, suggesting that the dimeric interface is stable in the physiological membrane-bound state. sF13 was anchored to the membrane with the two palmitoylated cysteines inserted deeply into the outer leaflet of the membrane, while the N-terminal tail was associated with the lipid head group and glycerol regions (Fig. 1c). Two lines of evidence suggest that the homodimer has a biological role: (a) other membrane-interacting PLDs, such as the human exonucleases PLD3 and PLD4 (refs. 17,18), form similar homodimers with physiological roles; and (b) mapping single escape mutants onto the sF13 homodimer reveals that all lie within or close to the dimer interface. Four escape mutations known to produce higher resistance to tecovirimat (Y258C, A288P, A290V and I372N) were located at the centre of the interface (Fig. 1b and Supplementary Table 3). Moreover, an escape mutant (D280Y) of N1-isonicotinoly-N2-3-methyl-4-chlorobenzoylhydrazine (IMCBH), a selective inhibitor of vaccinia virus (VACV) replication that blocks wrapped virion formation in vitro19,20, also mapped close to the dimer interface. These data indicate that F13 dimerizes when present on membranes and that this homodimer is targeted by tecovirimat and IMCBH.
To map the tecovirimat binding site, we soaked cubic crystals (Fig. 2a) in a solution containing 1 mM tecovirimat. The resulting maps revealed an additional electron density at the dimeric interface compatible with the size and shape of a tecovirimat molecule (Fig. 2b). However, because the pocket is symmetrical and tecovirimat is an asymmetric molecule, the resulting electron density is featureless, preventing accurate modelling of the molecule. To identify the correct pose and rationalize the featureless electron density, we performed absolute binding affinity calculations based on molecular dynamics simulations and free energy perturbation (FEP) techniques. We generated 15 poses of different rotational states of tecovirimat compatible with the electron density. After equilibration of each pose using free molecular dynamics simulations, we computed the absolute binding free energy (ABFE) using three independent sets of full FEP calculations. Because FEP achieves ABFEs with an accuracy of approximately 1–2 kcal mol−1 (ref. 21), the calculations enabled us to identify high-affinity poses selected by tecovirimat in the experiment. It is worth noting that we identified multiple poses with very low binding free energy values (ΔGbind) of less than −20 kcal mol−1 (Extended Data Fig. 3 and Supplementary Table 4). Averaging over 45 independent simulations, we estimated an exceptionally strong affinity of ΔGbind = −25.1 kcal mol−1, suggesting that tecovirimat strongly stabilizes the homodimer. The presence of multiple conformers with similarly high affinities is compatible with the presence of multiple tecovirimat conformers in the crystal, in agreement with the featureless electron density. To validate our approach, we evaluated a set of seven structurally similar ligands with published half-maximal effective concentration (EC50) values alongside tecovirimat22,23. We aligned each ligand to the tecovirimat best pose and performed ABFE calculations. We obtained good agreement between the computed ABFE values and experimental data (Extended Data Fig. 4).

a, Cubic crystals used to obtain the structure of the sF13/tecovirimat and sF13/IMCBH complexes. b, Crystal structure of the sF13/tecovirimat complex. Left: a Fo-Fc omit map contoured at 3σ showing the density found at the dimer interface in the soaked crystal with the tecovirimat molecule modelled. Centre and right: orthogonal views of the dimerization interface with the tecovirimat molecule modelled and the residues contacting the drug represented as sticks and labelled. sF13 chains are coloured as in Fig. 1. c, Crystal structure of the sF13/IMCBH complex. Left: as in b, an omit map showing the electron density at the dimer interface in the soaked crystal. Centre and right: as in b, orthogonal views showing the sF13/IMCBH contacts.
We used the highest affinity pose to refine the crystallographic data. We observed that the molecule was stabilized by a network of polar contacts involving Y258 and S292 and hydrophobic contacts mediated by Y253, I262, I266 and Y285 (Fig. 2b). Similarly, we soaked sF13 cubic crystals with IMCBH and observed an electron density between both protomers that was compatible with the dimensions of IMCBH (Fig. 2c). Overall, the structural data revealed that both tecovirimat and IMCBH bind to the same pocket between the two protomers of the homodimer. The estimated ΔGbind values suggest that tecovirimat stabilizes F13 homodimers, like a molecular glue.
Tecovirimat stabilizes F13 homodimers
Next, we evaluated the oligomeric state of sF13 with and without tecovirimat by using AUC. In the absence of the drug (Fig. 3a), sF13 was predominantly monomeric in solution (sedimentation coefficient, S = 3.4), with a minor presence of a higher S coefficient species (S = 4.4), which we interpreted as a dimer. Consistent with our hypothesis, tecovirimat shifted the equilibrium to the dimeric form (S = 4.7), with no monomeric protein detected. To confirm that the tecovirimat-induced dimer in solution corresponded to the dimer observed in the crystals, we analysed the sF13/tecovirimat complex using SEC-SAXS (Fig. 3b and Extended Data Fig. 5). In agreement with the AUC experiments, the F13/tecovirimat complex behaved as a monodisperse distribution of dimers in solution with an estimated molecular mass of approximately 76–84 kDa, a maximum distance (Dmax) of 108 Å and a radius of gyration (Rg) of 32.9 Å, matching the calculated values of the crystallographic dimer of 88.6 kDa, 117 Å and 32.3 Å, respectively. For further structural insight, we compared the experimental SAXS curve with the theoretical curves of the F13 monomer and dimer (Fig. 3b) and the ab initio model derived from the SAXS curve with the crystallographic F13 dimer (Fig. 3c). We found in both analyses that the crystallographic dimer fitted well to the experimental SAXS curve. Overall, these results show that tecovirimat induces the dimerization of sF13 in solution and that the resulting dimer adopts the same organization as the dimer observed in the crystal structure.

a, AUC analysis of sF13 without tecovirimat (brown line) and with 10 µM tecovirimat (blue line). Experimentally derived sedimentation coefficient values (Svedberg units (S)) are shown above each peak. c(s), the concentration of protein with sedimentation coefficient s. b, Experimental SAXS profile (green dots) and theoretical profiles (dashed lines) calculated using CRYSOL87 for one monomer of sF13 (blue dashed line) and the dimer shown in Fig. 1 (pink dashed line). I(q), scattering intensity in function of the scattering vector q; q (Å−1), scattering vector; Å, Angstrom; Χ2, chi-squared test. c, Orthogonal views of a representative dummy atom model (green) reconstructed from SAXS data. For comparison, we have included below a model of the crystallographic sF13 dimer with an outline of the dummy model. d, Dose–response curve used to estimate tecovirimat effect in solution. The y axis represents the proportion of dimers in a dilute solution of F13 measured by mass photometry. The x axis represents the concentration of drug (tecovirimat or IMCBH) present in the solution. The EC50 values were determined from a dose–response curve fitted using GraphPad Prism. Data are mean ± s.d. of three independent experiments (n = 3). e, Tecovirimat (blue line) and IMCBH (orange) inhibit plaque formation of MPXV. Vero cells were infected with MPXV clade IIb and treated with the indicated concentrations of tecovirimat or IMCBH. Plaque inhibition is expressed as a percentage, normalized to control conditions. Data are mean ± s.d. of triplicate wells from five independent experiments for tecovirimat (n = 15) and two independent experiments for IMCBH (n = 6).
Source data
Next, we assessed the activity of tecovirimat in solution. To do this, we designed an assay to calculate the concentration of the drug required to induce dimerization of 50% of sF13 (EC50). We used mass photometry, which measures the molecular mass of individual molecules by quantifying the light scattering in a dilute solution. This technique enables a higher throughput than AUC or SEC-SAXS and allows for the evaluation of a full range of tecovirimat concentrations. We compared the EC50 values obtained using the mass photometry assay: 92 nM for tecovirimat and 1,475 nM for IMCBH (Fig. 3d and Extended Data Fig. 6) to the half-maximal inhibitory concentration (IC50) values measured in cells infected with a recently isolated clade IIb MPXV strain24: 17 nM for tecovirimat and 74 nM for IMCBH (Fig. 3e). In both assays, tecovirimat outperformed IMCBH, indicating that the activity measured in solution correlated with the antiviral activity of the drugs. The higher EC50 values in solution can be explained by higher entropy loss during dimerization as, on the membrane surface, F13 diffused in only two translational dimensions and one rotational dimension. Tecovirimat is approximately 15 times more active in solution than IMCBH, but its antiviral activity is 5 times better. This discrepancy could be explained by factors unrelated to their biochemical activities, such as differences in membrane permeability or cellular distribution.
To identify the pathway by which tecovirimat induces dimerization in solution, whether it binds to the monomer and induces dimerization or binds to a pre-formed dimer, we computed the binding affinity of tecovirimat to F13 protomers. Using the best tecovirimat pose, we removed one protomer from the molecular dynamics simulation system and computed the affinity for the remaining protomer. We estimated a ΔGbind of −5.7 kcal mol−1 (Supplementary Table 5), which corresponds to an EC50 value of approximately 68 µM. Thus, at the EC50 value of dimerization of 92 nM (Fig. 3d), only a small fraction of F13 protomers will be occupied by tecovirimat, suggesting that tecovirimat binds and stabilizes a transient dimer.
Tecovirimat escape mutants prevent F13 dimerization
Taken together, these structural and functional data suggest that escape mutants circumvent drug activity by preventing the formation of F13 homodimers. To test this hypothesis, we investigated the effect of escape mutants on tecovirimat activity using the mass photometry assay. We tested three escape mutants identified in mpox patients treated with tecovirimat (Supplementary Table 3): A295E, the quadruple mutant N267D, A288P, A290V, D294V (4MUT) and ΔN267. We also assayed one escape mutant (G277C) identified in vitro but never reported in mpox patients. The mutations identified in the clinical MPXV strains were located at the homodimerization interface and were expected to have an impact on tecovirimat-induced dimer formation, whereas G277C was located further away (Fig. 1b). Consistently, the mass photometry assay showed that sF13A295E, sF134MUT and sF13ΔN267 did not form homodimers in the presence of tecovirimat at the range of concentrations tested, whereas sF13G277C did as the wild-type protein (Fig. 4a). To complement the mass photometry data, we repeated the AUC experiments reported above using sF13A295E and sF134MUT (Fig. 4b and Extended Data Fig. 6). In contrast to the mass photometry experiments, which were performed using a dilute solution of sF13 (25 nM), the AUC experiments were performed at a higher concentration (10 μM), which facilitates protein homodimerization, to detect weaker tecovirimat activities. Thus, in the AUC experiments, we observed that the drug induced partial dimerization of sF13A295E, but not of sF134MUT, which aligns with the ΔEC50 values reported for A295E and 4MUT.

a, Mass-photometry-based dose–response curve showing tecovirimat activity against different escape mutants, as indicated. Data are mean ± s.d. of three independent experiments (n = 3). b, AUC analysis of sF13A295E (left panel) and sF134MUT (right panel) without tecovirimat (brown line) and with tecovirimat (black line). Experimentally derived sedimentation coefficient (S) values are shown above each peak. c,d, Orthogonal views showing the dimer interface of sF13A295E (cyan, c) and sF13A295E/tecovirimat (green, d) superimposed on sF13WT (orange). Residues E295, R291, Y285 and N300 are represented as sticks and labelled. Polar contacts are indicated with dashed lines.
Source data
Next, we aimed to elucidate the structural basis of the partial resistance of the A295E mutant. We obtained cubic crystals of sF13A295E and soaked them in tecovirimat. In the absence of tecovirimat, sF13A295E formed a homodimer that resembled that of sF13WT, but with the ends of the α10 helix being more open, so that Y285 was hydrogen-bonded with Q299 instead of with N300, as in the wild-type protein (Fig. 4c). This results in a reduction in the buried surface area from 939 to 882 A2, likely reducing dimer stability. In the presence of tecovirimat, sF13A295E recovered the native conformation, in which Y285 was hydrogen-bonded to N300 (Fig. 4d). We failed to crystallize sF134MUT, but it is likely that the introduction of a proline in the middle of the α10 helix generates a conformational reorganization that prevents F13 dimerization. Overall, we concluded that escape mutants isolated from tecovirimat-treated patients alter the dimerization interface of sF13, making tecovirimat-induced dimerization less efficient.
We then investigated whether tecovirimat induces F13 dimerization in cells. To evaluate this, we performed PLA. This technology is based on two oligonucleotide-labelled antibodies (probes) that bind to the constant region of a pair of primary antibodies targeting the proteins of interest. If the probes are less than 40 nm apart, they hybridize and produce a fluorescent signal that can be visualized and quantified by fluorescence microscopy (Fig. 5a). As we did not have specific antibodies targeting F13, we engineered a version of F13 with a flag tag in the loop connecting β1A to α1A, away from the dimerization and membrane interaction interfaces (Flag–F13WT). We also produced two variants that escape tecovirimat (Flag–F134MUT and Flag–F13A295E). We used two commercial antibodies targeting the flag tag, one with a mouse and the other with a rabbit Fc. Hela cells were transiently transfected with Flag–F13WT, Flag–F134MUT or Flag–F13A295E and incubated with 10 μM tecovirimat or 0.1% dimethyl sulfoxide (DMSO) as a control. PLA signal was quantified after 24 h of incubation. A signal was detected in F13-expressing cells but not in control cells. In the absence of tecovirimat, a signal was observed in cells expressing WT or mutant F13, likely corresponding to basal levels of proteins that were less than 40 nm apart. In line with the AUC experiments, tecovirimat induced a strong increase in the PLA signal in cells expressing Flag–F13WT, a slight increase in cells expressing Flag–F13A295E and none in cells expressing Flag–F134MUT (Fig. 5b and Extended Data Fig. 7). Therefore, we conclude that tecovirimat induces F13 dimerization in cells and that escape mutants interfere with this dimerization.

a, Schematic model representing the PLA experiment. F13 protomers are coloured green and cyan with the approximate location of the flag tag indicated with a blue sphere. The three steps of the assay, dimerization, ligation and amplification, are indicated. b, Upper panel: representative fluorescence microscopy images with the nuclei coloured in blue and the PLA signal in red. Scale bar, 100 µm. Lower panel: quantification of the PLA signal as the average area of PLA fluorescence per cell; 7,000 to 12,000 cells were analysed per data point. Data are mean ± s.d. of two independent experiments performed in triplicate (n = 6). For statistical analysis, two-way analysis of variance was used. NS, non-significant; ****P < 0.0001; **P = 0.0087. NT, non-transfected.
Source data
Dimer-stabilizing mutations render VACV non-viable
Isolation of tecovirimat-resistant MPXV strains revealed only ten mutations that, either isolated or in combination, confer resistance to the drug (Fig. 1a and Supplementary Table 3)9. We wondered why other mutations did not emerge. One plausible explanation is that most mutations in this region are associated with a major loss of fitness, rendering the virus non-viable. To test this, we designed three escape mutants (S292F, S292K and L296Y) by introducing bulky side chains into the tecovirimat binding cavity (Fig. 6a) and studied their sensitivity to tecovirimat in vitro using the mass photometry assay. We confirmed that sF13S292F, sF13S292K and sF13L296Y were totally insensitive to tecovirimat activity (Fig. 6b). sF13S292F and to lesser extent sF13L296Y formed dimers in the absence of the drug, suggesting that filling the cavity with hydrophobic side chains stabilizes the dimer. To assess viral viability, we introduced S292F into VACV using the marker-free vaccinia virus engineering (MAVERICC) system25 to produce the recombinant virus rVACVS292F. As controls, we generated three additional viruses bearing substitutions in F13 known to confer tecovirimat resistance: rVACVG277C, rVACV4MUT and rVACVA295E. As expected, all the control viruses could be rescued and grew to high titres (Fig. 6c). However, despite multiple attempts, we were unable to generate rVACVS292F, suggesting that this substitution is deleterious to viral morphogenesis.
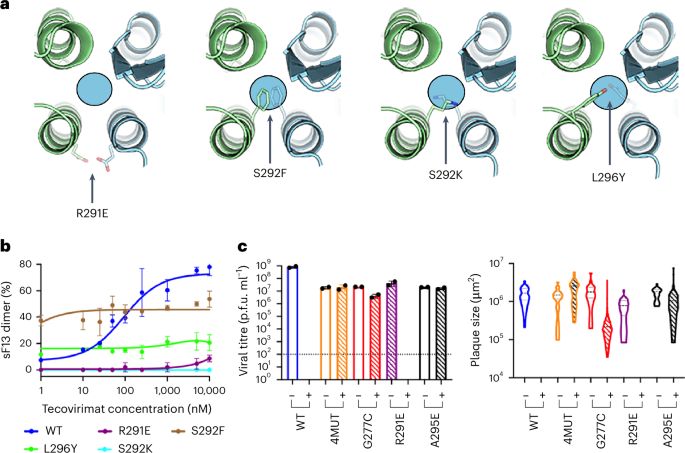
a, Close view of the dimerization interface across the twofold axis showing the designed mutations S292F, S292K and L296Y and the mutation identified in VARV, R291E. The circle indicates the localization of the tecovirimat-binding site. b, Mass-photometry-based dose–response curve showing tecovirimat activity against different mutants, as indicated. Data are mean ± s.d. of three independent experiments (n = 3). c, Viral titres in p.f.u. ml−1 (left panel) and plaque size (right panel) in the presence (+) and absence (−) of 10 µM tecovirimat calculated from plaque assays. Each bar represents the means ± s.d. from two independent experiments (n = 2). The limit of detection (102 p.f.u. ml−1) is indicated with a dashed line.
Source data
Identification of previously undescribed potential tecovirimat-escape mutants
We then sought for other tecovirimat resistance mutations that might have been unnoticed. To identify them, we extracted the F13 sequence from all MPXV genomes available in the GISAID (Global Initiative on Sharing All Influenza Data) database26 and from all OPXV sequences available in the GenBank database. Within each species, we mapped variations in the F13 amino acid sequences at the dimer interface. No escape mutants were identified in MPXV clade I sequences. However, we identified three potential escape mutants in MPXV clade II sequences: L296F, D280Y and P243A, all from an immunocompromised patient treated with tecovirimat27. In addition, we identified R291E in one out of 81 analysed VARV strains. A structural analysis showed that R291E introduced two negatively charged residues facing each other on both sides of the interface (Fig. 6a), creating electrostatic repulsion that may hinder dimer formation and confer tecovirimat resistance. To test this, we produced sF13R291E and studied its sensitivity in vitro to tecovirimat. We showed that the drug had some activity at the highest concentrations in the assay (Fig. 6b). Next, we evaluated the resistance of rVACVR291E to 10 µM tecovirimat by plaque assay (Fig. 6c) using rVACVWT, rVACV4MUT and rVACVA295E as controls. As reported previously28, tecovirimat abrogated plaque formation by rVACVWT. The control mutants completely (A295E, 4MUT) or partially (G277C) escaped tecovirimat, as determined by measurements of both plaque number and plaque area (Fig. 6c), providing evidence that F13 substitutions conferring tecovirimat resistance in MPXV are also transferable to VACV. The mutant R291E remained sensitive to tecovirimat, at least at the high drug concentration we used in these assays.
Discussion
Here we present a structural study of viral phospholipase F13, including its interaction with tecovirimat. Previous studies have identified three important regions for F13 activity: two palmitoylated cysteine residues required for membrane association16, a phospholipase motif29,30,31 and a di-aromatic motif required for interaction with late endosome proteins11. Similar to tecovirimat binding, mutations in any of these regions reduce wrapped virion formation11,32, but none of the escape mutants identified map there. Our structural and molecular dynamics data suggest that F13 may homodimerize on membranes. Comparison with other phospholipases revealed that this homodimer closely resembles that of phospholipases PLD3 and PLD417,18, which are transmembrane proteins with exonuclease activity. Sequence analysis showed that poxviral phospholipase K4, a paralogue of F13 with nuclease activity33, shares 48% sequence identity with human PLD3. Taken together, these findings suggest that poxviruses captured a PLD3-like gene from which both viral phospholipases evolved; K4 became a soluble protein but maintained its nuclease activity, whereas F13 remained in the membrane but acquired broad phospholipase activity31.
Despite this evolutionary link, there is an important difference between the homodimer of F13 and its eukaryotic counterparts. The active form of PLD3 is a stable dimer in solution17, but the soluble version of F13 is mostly monomeric. Structural analysis revealed a large cavity at F13’s dimerization interface, which is absent in PLD3 and PLD4 homodimers. We hypothesized that this cavity reduces the stability of the F13 dimer. Sequence analysis showed that this cavity is conserved across OPXVs, and most of the tecovirimat escape mutants were located around it. Molecular dynamics simulations showed that tecovirimat binds to the cavity with strong affinity, with some poses exhibiting binding free energies lower than −20 kcal mol−1, demonstrating that tecovirimat serves as a highly potent molecular glue. AUC, SEC-SAXS, mass photometry and PLAs confirmed that tecovirimat induced dimerization of F13, both in solution and within cells.
This model provides a molecular framework for understanding most escape mutants identified thus far; they alter the F13 dimerization region and prevent tecovirimat from inducing dimerization. To support this hypothesis, we developed a mass photometry assay to measure the activity of tecovirimat in solution and showed that A295E, 4MUT and ΔN267 did not dimerize in the presence of the drug.
The results presented here have broad implications for public health. Estimating tecovirimat sensitivity of clinical isolates is important for monitoring epidemics. This is currently performed by isolating the virus24,34, which is labour intensive and requires expensive and advanced equipment. The precise mapping of F13/tecovirimat contacts provides the possibility of performing sequence-based estimations of tecovirimat sensitivity. While we were preparing this paper, early results from the PALM007 clinical trial were released35, showing that tecovirimat did not accelerate recovery in patients infected with MPXV clade I. Based on the structure of the sF13/tecovirimat complex, we did not identify any mutations in clade I strains that could explain this lack of activity. Additional clinical trials will examine tecovirimat’s effectiveness on different strains and when administered early in infection. However, these results emphasize the need for new antiviral drugs. The structural data, molecular dynamics simulations and the battery of assays developed here will pave the way for the development of these novel antivirals active against tecovirimat-resistant strains.
Methods
Protein production and purification
To produce soluble F13 (sF13WT and mutants), we cloned a synthetic gene, codon-optimized for expression in Escherichia coli into a pET-28a(+) vector (Novagen) with an N-terminal His- and Strep-tag followed by a thrombin site. The sequence is derived from the Western Reserve strain of VACV (Uniprot code, P04021). We removed the hydrophobic N-terminal tail (residues 2–5) and introduced five mutations in the MIR: W177A, L178A, C181A, C185A and C186A, to remove the palmitoylation sites and the hydrophobic residues around. The point mutants mentioned in the text were introduced into this vector. We transformed E. coli BL21 (DE3) cells (New England Biolabs) and induced protein expression overnight at 16 °C with 0.25 mM isopropyl β-d-1-thiogalactopyranoside. We collected cells from 3 l of culture, resuspended them in 40 ml cold resuspension buffer (Tris–HCl 10 mM pH 8, NaCl 150 mM, EDTA 1 mM) supplemented by one tablet of complete protease inhibitor (Pierce) and froze them at −20 °C. The next day, we thawed and lysed them using a sonicator. After removing the insoluble material by centrifugating at 20,000 g (30 min, 4 °C), we purified the recombinant protein using streptag-based affinity chromatography in a StrepTrapTM HP 5 ml column (Cytiva), treated it with 5 mM Tris(2-carboxyethyl)phosphine hydrochloride (Thermo Fisher Scientific) for 10 min at room temperature to reduce all exposed cysteines and purified the protein using size-exclusion chromatography with a Superdex 75 column (Cytiva) using SEC buffer (Tris–HCl 10 mM pH 8, NaCl 100 mM). The final yields obtained were: sF13WT = 3.5 mg l−1, sF13A295E = 2.5 mg l−1, sF13G277C = 2.8 mg l−1, sF134MUT = 1.4 mg l−1, sF13Δ267 = 0.6 mg l−1, sF13R291E = 1 mg l−1, sF13S292F = 1.9 mg l−1, sF13S292K = 0.2 mg l−1 and sF13L296Y = 0.4 mg l−1. All proteins were analysed by SDS–PAGE to assess their purity (Extended Data Fig. 8a).
Crystallization and structure determination
For crystallization, we digested the purification tags using 1.5 units of thrombin (Cytiva) per 0.1 mg of protein overnight at 4 °C and then treated the protein with Tris(2-carboxyethyl)phosphine hydrochloride for 10 min at room temperature. The digest was loaded to a gel filtration Superdex 75 16/60 column in 10 mM Tris–HCl pH 8.0, 100 mM NaCl, and the fractions of the main peak were pooled and concentrated to 12 mg ml−1 in the same buffer for crystallization trials. Crystallization screening trials were carried out by the vapor diffusion method using a Mosquito TM nanodispensing system (STPLabtech) following established protocols36. Monoclinic crystals of sF13WT were grown after 10 days in 20% (w/v) PEG 3350, 0.1 M HEPES pH 7.5 and 2% (v/v) Tacsimate and were cryoprotected in the same solution supplemented with 20% (v/v) glycerol. Cubic crystals of sF13WT were grown in 1 day in 1 M Na3 citrate and 0.1 M imidazole pH 8 and were cryoprotected using the crystallization solution supplemented with 33% (v/v) glycerol. To obtain the complex with tecovirimat, we soaked cubic crystals, which have a high solvent content (70%), for 5 min into a soaking solution containing 1 mM tecovirimat (BenchChem, catalogue number B611274), 10% (v/v) DMSO, 1 M Na3 citrate and 0.1 M imidazole pH 8. To obtain the complex with IMCBH, we soaked cubic crystals into a solution containing 1 mM IMCBH (BLD Pharmatech, catalogue number BL3H9998EC8C), 10% (v/v) DMSO, 1 M Na3 citrate and 0.1 M imidazole pH 8. After soaking, all crystals were cryoprotected using the soaking solution supplemented with 33% (v/v) glycerol. Similarly, cubic crystals of sF13A295E were obtained in 1 day using 1 M Na3 citrate and 0.1 M imidazole pH 8 and soaked with tecovirimat as reported above.
X-ray diffraction data were collected on beamlines PROXIMA-1 and PROXIMA-2A at the synchrotron SOLEIL (St Aubin, France) using the beamline control software MXCuBE (version 2). Diffraction images were integrated with XDS (version 10 January 2022)37, and crystallographic calculations were carried out with programs from the CCP4 program suite (version 9)38. To determine the phases, we used a model of F13 obtained using AlphaFold2 (ref. 39) as a template to perform molecular replacement in PHASER (version 2.8.3)40. To obtain the final models, we iteratively built and refined the structures using phenix.refine (Phenix version 1.19.2-4158)41 and Coot (version 0.9.8.95)42 using isotropic B factor and Translation/Libration/Screw groups as refinement strategy. We validated all the models using MolProbity (version 4.5.2)43. The crystallographic statistics are provided in Supplementary Table 1. In crystals soaked with tecovirimat or IMCBH, additional electron density appeared at the dimeric interface that was compatible with the shape and size of the drug, as shown in Fig. 2. To facilitate the modelling, all maps derived from the cubic crystals were corrected using a bulk-solvent mosaic model available in the PHENIX program (phenix.mosaic). The electron density for tecovirimat does not show clear features. We hypothesize that this is because the binding pocket is symmetric, while the molecule itself is not, allowing it to adopt two indistinguishable orientations rotated by 180°. However, it is also possible that this is a crystallographic artefact, with tecovirimat only entering in a single orientation, and the density is featureless because of the presence of a twofold symmetry axis crossing the molecule. To investigate this, we reprocessed the cubic crystals in the space group P1 and refined the tecovirimat molecule in two different ways: in a single orientation with 100% occupancy and in two orientations rotated by 180° with 50% occupancy each, mimicking what is observed in the cubic space group. When comparing the two refinements (Extended Data Fig. 8b), we observed improved R factors and reduced residual densities when using the model with two rotated molecules, supporting our original hypothesis. Coordinates and structure factors have been deposited in the Protein Data Bank. Figures showing the crystallographic models were generated with PyMol v3.0.3 (Schrödinger, LLC).
Molecular dynamics simulations
To assess the stability of the X-ray resolved F13 dimer on the membrane surface, we conducted molecular dynamics simulations. Before simulation, we made several structural modifications to the sF13 dimer. First, the unresolved N-terminal residues (residues 1–5) were modelled as unstructured and integrated into the dimer structure using the Modeller (version 10.4) tool44. Next, the structure was processed using the CHARMM-GUI (accessed on December 2023)45 server to add post-translational palmitoylation on residues C185 and C186 and neutral capping of the N- and C-terminal residues. Subsequently, the F13 dimer was positioned on a membrane (Fig. 1c) mimicking the lipid composition of the Golgi membrane (as outlined in Supplementary Table 2). For the protein force field, we used the CHARMM36m-WYF force field46,47, which includes corrections for cation–pi interactions, while lipids were described using the CHARMM36 force field48. The protein–membrane system was solvated with 52,801 water molecules using CHARMM-modified TIP3P49 water model, and the total charge of the system was neutralized by adding 82 K+ ions. The total system size is 249,131 atoms. The box dimensions were 14.34 × 14.34 × 13.56 nm in the x, y and z directions. The solvated system was energy minimized using the steepest descent algorithm to remove any steric clashes, followed by six short equilibrations ranging from 125 ps to 500 ps with restraints on either protein backbone/side chain atoms or lipid phosphate atoms.
Throughout the equilibration process, we maintained a temperature of 310 K using the Berendsen thermostat50 with a time constant (τt) of 1 ps, while pressure was maintained at 1 bar using the Berendsen semi-isotropic scheme with a time constant (τp) of 5 ps. van der Waals and electrostatic interactions were treated using the cut-off and Particle Mesh Ewald51,52 methods, respectively, with a cut-off of 1.2 nm. Covalent bonds involving hydrogen atoms were constrained using the LINCS algorithm53. For the final production run, we removed all restraints and switched to V-rescale54 and Parrinello–Rahman55,56 semi-isotropic scheme to regulate the temperature and pressure, respectively. The rest of the parameters were consistent with those used during equilibration. The production simulations were conducted for 1 µs with 5 repeats using the GROMACS (version 2021) simulation package57, using a time step of 2 fs. For analysis, we concatenated the last 300 ns from each repeat and examined monomer–monomer contacts within 5 Å. All images and plots were generated using VMD (version 1.8.1)58 and the matplotlib (version 3.10)59 library.
Although the structure of the sF13 dimer was determined at a resolution of 2.6 Å, a crystallographic 2-fold axis passes through the ligand density (tecovirimat), leading to challenges in accurately fitting the ligand. To enhance the accuracy of ligand modelling within the density, we used the recently developed RosettaEMERALD (rosetta release-362)60 protocol. This protocol integrates both RosettaGenFF and genetic algorithm optimization for robust ligand modelling within the density map. The three-dimensional structure of tecovirimat was downloaded from PubChem61 in its endo-isomeric form, which is the favoured product of the Diels–Alder reaction used to synthesize the drug. Next, the sF13 dimer–tecovirimat complex was docked to the density using the ChimeraX (v1.7.1) tool62. Following this, we used the RosettaEMERALD protocol to accurately model tecovirimat within the density. Briefly, an initial pool of 500 ligand conformations, along with protein side chains, undergo genetic algorithm optimization over 10 generations. The top 20 lowest-energy conformations obtained from genetic algorithm optimization were further refined, along with protein side chains, using a cartesian minimization in Rosetta. The protocol was executed in triplicate. Out of the total 60 ligand conformations, redundant poses were eliminated, and 15 poses were selected for binding free energy calculations. These 15 selected poses are indicated by the first number of x-axis labels of Extended Data Fig. 3. The RosettaEMERALD XML script and flags used for refining the ligand within the density are provided in the supplementary material.
ABFE calculation
The selected 15 poses were subjected to binding free energy estimation using an in-house pipeline (publication in preparation). Our ABFE protocol is similar to the one previously described by ref. 63. ABFE calculations were performed in triplicate for each pose. To optimize the ABFE calculations, the membrane was excluded from the simulation. This simplification is justified as the tecovirimat binding pocket is located at 40 Å from the membrane surface, and equilibration simulations show that the interface remains stable throughout the simulation (Extended Data Figs. 9 and 10). The AMBER-ff14sb64 parameters for F13 dimer were acquired through the use of OpenMM (version 8.0)65 and ParmEd (version 4.1.0)66 software, while the OpenFF-2.0.0 (ref. 67) parameters with AM1-BCC charges for tecovirimat were obtained via TOFF68 software v0.1.0. The TIP3P49 water model was used, along with AMBER parameters for ions. GROMACS-2022.4 (ref. 57) simulation package was used as the molecular dynamic engine.
In all cases, the simulation temperature was maintained at 298.15 K using Langevin dynamics with a collision frequency of 2 ps−1. Van der Waals and electrostatic interactions were treated using the cut-off and Particle Mesh Ewald methods51,52, respectively, with a cut-off of 1 nm. Hydrogen bonds were constrained using the LINCS algorithm53. Two different isotropic schemes were used to maintain the pressure at 1 atm: Berendsen50 with a time constant of 1 ps and Parrinello–Rahman55,56 with a time constant of 2 ps. All production simulations used the former. We used a hydrogen mass repartitioning factor of 2.5, which allowed an integration time step of 4 fs for all production simulations. Other molecular dynamics parameters are detailed in the GROMACS input files provided as Supporting Information.
Our in-house pipeline operates as follows (Supplementary Fig. 1): During the ‘Build Simulation System’ phase, the user provides configuration details for the protein–ligand complex, and topologies are generated accordingly. Two neutral solvated systems with 150 mM of NaCl are created for the ligand and protein–ligand complex in an octahedron box with 1.5 nm distance between the solute and the edges’ box with the GROMACS’ solvate module. The ‘Equilibration Setup’ and ‘Equilibration Run’ steps generate molecular dynamics parameters and conduct the corresponding equilibration simulations. For the protein–ligand complex, the process begins with minimization using the steepest-descent algorithm. This is followed by a 1 ns NVT (constant particle number, volume and temperature) phase with a 2 fs integration time step and position restraints on the heavy atoms using a force constant of 2,500 kJ mol−1 nm−2. Next, a 1.05 ns NVT phase and approximately 1 ns NPT (constant particle number, pressure and temperature) phase are conducted, both with a 3 fs integration time step and the same position restraints. The previous NPT phase uses the Berendsen scheme as detailed above. Subsequently, a 5 ns NPT phase with the Parrinello–Rahman scheme with a 4 fs integration time step is performed without restraints. This is followed by a final step of 10 ns under the same conditions. For the ligand alone, the same procedure is followed, except the initial 1 ns NVT phase with a 2 fs integration time step is omitted and the final NPT simulation is performed during 5 ns. The final 10 ns of the of the protein–ligand complex simulation was used to estimate the optimal Boresch restraints for the decoupling phase of the protein–ligand complex simulations.
Similar to the previous two steps of the workflow, ‘FEP Setup’ and ‘FEP Run’ prepare and execute the FEP simulations needed to complete the thermodynamic cycle detailed in the ‘Thermodynamic cycle for FEP’ section. Each window for both the protein–ligand complex and the ligand alone begins with minimization using the steepest-descent algorithm. This is followed by a 10 ps NVT phase with a 2 fs integration time step and position restraints on the heavy atoms using a force constant of 2,500 kJ mol−1 nm−2. Next, a 100 ps NPT phase is conducted with a 4 fs integration time step, the same position restraints and the Berendsen scheme as detailed above. Subsequently, a 500 ps NPT phase with the Parrinello–Rahman scheme and a 4 fs integration time step is performed without restraints. This is followed by a final step of 10 ns under the same conditions.
The free energy contributions of each step are computed using either the multistate Bennett acceptance ratio69 or thermodynamic integration estimators during the ‘Get Contribution’ step. The Python package alchemlyb-2.0.0 (ref. 70) was used for this purpose. Finally, all results are aggregated in the ‘Get Cycle’s ∆G’ step.
Thermodynamic cycle for FEP
The thermodynamic cycle involved decoupling the Coulomb interactions of the ligand in water over 11 λ points, followed by the decoupling of van der Waals interactions over 21 λ points with a soft-core potential activated to prevent numerical instability. Boresch restraints71, chosen from the last 10 ns of the protein–ligand complex free simulation during the equilibration phase (‘ABFE calculation’ section), had their free energy contribution analytically calculated.
Both the selection and energy contribution estimation of Boresch restraints were conducted using the software MDRestraintsGenerator (version 0.2.0)72. The selected restraints were activated for the ligand in complex with the protein, and the van der Waals interactions of the ligand were reactivated in the protein complex over 21 λ points with a soft-core potential to avoid numerical instability. Subsequently, Coulomb interactions were activated over 11 λ points to finally remove the restraints over 12 λ points. The binding free energy is calculated from the contributions of all previously mentioned steps.
Clustering and identification of the most favourable energetic pose
Frames selected by MDRestraintsGenerator and used as input structures for the FEP simulations were clustered based on the protein–ligand interaction fingerprint calculated with ProLIF (version 2.0.3)73. Each bit of the fingerprint represents a pair of atom/atom groups from the protein and ligand involved in a specific class of interaction as defined by ProLIF. This unambiguous definition allows for the separation of potential poses that may be symmetrical. The final fingerprint consists of 956 bits. The similarity among frames is calculated using the Tanimoto metric implemented in RDKit (version 2023.03.2)74 on the constructed protein–ligand interaction fingerprint, resulting in the generation of a similarity matrix.
The similarity matrix was then subjected to the hierarchical clustering algorithm in SciPy using Ward’s variance minimization algorithm75. After constructing the dendrogram, the number of clusters was determined through visual inspection.
ABFE between the most energetic favourable pose and the individual monomers
To investigate whether tecovirimat can bind to the monomer, the ABFE for each individual monomer was calculated for the identified most energetic favourable pose within the dimer. The same methodology previously described was used, with the only difference being that a single monomer was used instead of the dimer.
Calculation of average binding free energies
To estimate the average binding free energy for the dimer and monomer complexes with tecovirimat, we averaged over all independent simulations (N = 45 for the tecovirimat–dimer, N = 6 for the tecovirimat–monomer binding) as shown in equation (1), implying that we average with respect to the binding probabilities (rather than with respect to the binding free energies). Thereby, the average is dominated by the high-affinity binding poses. Here, β is the inverse temperature, and 〈·〉 denotes the average over independent simulations.
The 95% confidence interval was estimated from 1,000 rounds of bootstrapping. In each round, N ({{Delta {{G}}}_{{rm{bind}}}}_{i}) samples were drawn with replacement from our N ({{Delta {{G}}}_{{rm{bind}}}}_{i}) values and averaged according to equation (1). After removing the largest and smallest 2.5% of the 1,000 bootstrapped averages, 95% confidence intervals were obtained from the upper and lower bounds of the remaining 950 averages.
Validation of ABFE calculation via ABFE calculations for seven additional ligands
To provide additional evidence for the tecovirimat binding pose and to validate the ABFE calculations, we carried out additional ABFE calculations with seven structurally similar ligands with available ({{rm{EC}}}_{50}^{{rm{VACV}}}) values alongside tecovirimat22,23. Here ({{rm{EC}}}_{50}^{{rm{VACV}}}) denotes the effective concentration that inhibits 50% of virus-induced cytopathic effects on VACVs. Each ligand was aligned to the tecovirimat pose reported here, and ABFE calculations were conducted using three independent replicates for each of the eight ligands, including tecovirimat. The calculated binding affinity (Delta {G}_{{rm{calc}}}^{circ }) reported for each ligand represents the mean across the three replicates, and the error was taken as the standard error of the mean (s.e.m.).
We assume that the ({{rm{EC}}}_{50}^{{rm{VACV}}}) value is related to the change in free energy upon two reactions, dimerization and ligand binding:
where P denotes the protomer (a single monomer), L the ligand, ({{rm{PP}}}^{{rm{X}}{mbox{-}}{rm{ray}}}) the homodimer observed in the crystal, and ({{rm{PPL}}}^{{rm{X}}{mbox{-}}{rm{ray}}}) is the ternary complex. (Delta {G}_{{rm{dimer}}}^{circ }) denotes the free energy for dimerization of two protomers towards the dimeric crystal structure, and (Delta {G}_{{rm{complex}}}^{circ }) denotes the free energy for ligand binding to the crystallographic homodimer. For the overall reaction, the fraction of protein in ternary complex is
Here, ai denotes the activity of species i defined as ({a}_{i}equiv {C}_{i}/{C}^{^circ }), where C° is the standard concentration of 1 mol l−1. The dissociation constant for equation (2) is:
Equations (3) and (4) yield
Let ({a}^{ast }_{{L}}) denote the ligand activity at which 50% of the protein is in complex (θ = 0.5). Here ‘*’ is used to distinguish the symbol aL, the activity of the ligand at any θ value. Thus, we have:
Assuming that ({a}^{ast }_{{L}}) is proportional to ({{rm{EC}}}_{50}^{{rm{VACV}}}) among the eight ligands, we have ({a}^{ast }_{{L}}=gamma {{rm{EC}}}_{50}^{{rm{VACV}}}/C^circ), where γ is an unknown constant. Thus,
where R is the gas constant and T the temperature. Furthermore, we assume that the activity of the protomer aP on the cell is constant.
In our ABFE calculations, we evaluated only the second step of the two reactions of equation (2):
Thus, (Delta {{G}}_{{rm{calc}}}^{circ }) is offset from ({RT}{mathrm{ln}}left(frac{{{rm{EC}}}_{50}^{{rm{VACV}}}}{{{C}}^{circ }}right)) by two constant contributions:
({Delta G}_{{rm{offset}}}) accounts for (1) the free energy cost of F13 dimerization and (2) our assumption that the intracellular ligand activity is proportional (but not equal) to extracellular ligand concentration in experiment. By comparison of our calculated AFBEs with the experimental ({{rm{EC}}}_{50}^{{rm{VACV}}}) values, we estimated ({Delta G}_{{rm{offset}}}approx leftlangle Delta {G}_{{rm{calc}}}^{circ }-Delta {G}_{exp }^{circ }rightrangle) = −15.2 ± 0.4 kcal mol−1, where 〈·〉 denotes the average over the eight ligands. The error for the quantity ({RT}{mathrm{ln}}left(scriptstylefrac{{{rm{EC}}}_{50}^{{rm{VACV}}}}{{{C}}^{circ }}right)+{Delta G}_{{rm{offset}}}) was estimated by error propagation using the uncertainties Python library76.
Supplementary Fig. 1 correlates (Delta {G}_{{rm{calc}}}^{circ }) with ({RT}mathrm{ln}left(scriptstylefrac{{{rm{EC}}}_{50}^{{rm{VACV}}}}{{C}^{circ }}right)), after correcting for ({Delta G}_{{rm{offset}}}). The reasonable agreement (1) validates the ABFE protocol and (2) suggests that the crystallographic pose of tecovirimat is adopted by the other seven ligands considered in this analysis.
Mass photometry
Mass photometry experiments were done using TwoMP instrument (Refeyn) using filtered (0.22 μm) ‘protein buffer’ (10 mM Tris–HCl pH 8, 100 mM NaCl) to avoid contaminations which would increase the background signal. Contrast-to-mass calibrations were achieved by measuring the contrast of two references (bovine serum albumin (BSA) and urease, both purchased from Sigma Aldrich) diluted in protein buffer, covering mass range from 66 kDa to 272 kDa. Four contrast values were used to generate a standard calibration curve, with the following rounded average masses: 66, 132, 198 and 272 kDa. We performed the experiment using microscope coverslips (24 × 50 mm and 170 ± 5 μm thick) cleaned with isopropanol and Milli-Q water followed by drying with air. Samples were loaded into dried coverslip surface assembled into silicone gaskets. Immediately before mass photometry measurements, 2 μl of sF13 protein stocks, with increasing amounts of tecovirimat or IMCBH, was diluted in 18 μl of ‘protein buffer’ into the gasket hole and mixed twice. In all cases, the final concentration of sF13 was 25 nM. Tecovirimat/IMCBH were at different concentrations between 10 μM and 1 nM. Data acquisition was performed using AcquireMP v2.3 (Refeyn), and movies of 2,936 frames were recorded at 49 Hz framerate, adjusted to maximize camera counts while avoiding saturation. Mass photometry images were processed and analysed using DiscoverMP v2.3 (Refeyn).
Mean contrast values from the BSA and urease calibration were plotted and fitted to a linear function y = bx, where y is the contrast, x is the mass and b is the contrast-to-mass calibration factor. To extract mole fractions (percentage of each species), we converted all particle contrasts obtained from each movie to mass, applied a Gaussian fitting and calculated mole fractions as the area of each Gaussian curve. Finally, sF13 dimer percentage values were plotted against tecovirimat/IMCBH concentration using Prism Graphpad v9.0.2, and EC50 values were extracted using a nonlinear fit function (Extended Data Fig. 6).
AUC
Sedimentation velocity experiments were carried out at 20 °C in an Optima AUC analytical ultracentrifuge (Beckman Coulter) equipped with double-UV and Rayleigh interference detection. Purified sF13 proteins at 0.4 mg ml−1 in the presence or absence of tecovirimat (10 μM) were centrifuged at 42,000 r.p.m. (23,600 g) using an AN60-Ti rotor and 12 mm thick double sector centrepieces. Absorbance and interference profiles were recorded every 5 min. Buffer viscosity (η = 1.016 cP) and density (ρ = 1.0054 g ml−1) at 20 °C were estimated with SEDNTERP 1.09. Partial specific volumes at 20 °C were estimated based on amino acid sequences using SEDNTERP 1.09 software. Data were analysed with SEDFIT 16.1 (ref. 77) using a continuous size distribution c(S) model. Theoretical sedimentations of the complex were generated using hydropro 10 (ref. 78).
SAXS experiments
SAXS data were collected on the SWING beamline at Synchrotron Soleil (France) using the online HPLC system. These experiments have been performed using sF13WT digested with thrombin. sF13WT samples at 4.6 mg ml−1 were prepared in a buffer containing 10 mM Tris pH 8, 100 mM NaCl and 10 μM tecovirimat and injected into a size exclusion column (Superdex 75 increase 5/150 mm) cooled at 15 °C eluting directly into the SAXS flow-through capillary cell at a flow rate of 200 µl min−1. The data were analysed using FOXTROT and PRIMUS from ATSAS 3.2 (ref. 79), from which Guinier was generated. Scattering curves were selected for stable Rg at the apex of the elution profile, the selected curves were averaged, and buffer signal was subtracted. From these corrected scattering curves, the pair distribution function was computed using GNOM (version 5.0)80, and the normalized Kratky plot was generated. Using the structure of sF13WT (PDB 9FHS), the experimental curve was compared to theoretical curve using CRYSOL (version 2.8.3)81. Ab initio models were generated with DAMMIN (version 5.3)82, and for each model, sedimentation characteristic was calculated with hydropro (version 10)78. The SAXS statistics are provided in Supplementary Tables 6 and 7.
F13 transfection for PLA and immunofluorescence staining
To perform the PLA experiment and immunofluorescence staining of F13, HeLa cells (ATTC CCL-2) were transfected with pcDNA 3.1 plasmids coding for either F13WT or F134MUT (N267D, A288P, A290V, D294V), with an internal FLAG tag sequence (GGGDYKDDDDKGGG) inserted within residues D21 and N22. The use of an internal FLAG tag in F13 was necessary, as the N-terminus is buried into the membrane and the C-terminus is part of the dimeric interface. Thus, none of them were suitable for standard N- or C-terminal protein tagging. We selected the best region to insert the FLAG tag based on the sF13 dimer structure reported here. For this, we selected an exposed loop, away from the membrane interaction region and the dimerization interface.
For PLA and immunofluorescence, 1.2 × 104 HeLa cells per well were transfected in suspension using lipofectamine 2000 (Thermo Fisher Scientific) in a 96-well plate (μClear, Greiner Bio-One 655090) with 100 ng of DNA. In each well 50 μl of HeLa cells at 2.4 × 105 cells ml−1 were mixed with 50 μl of transfection mix and 50 μl of DMSO or DMSO/tecovirimat, resulting in tecovirimat at a final concentration of 10 μM and DMSO at 0.1%. Cells were incubated 24 h at 37 °C and 5% CO2; subsequently the cells were fixed with 4% PFA for 10 min.
PLA
PLA was performed using the Duolink PLA Fluoresence kit (DUO92008, Merck). In short, cells were permeabilized at room temperature for 3 min in PBS with Triton 0.1% and washed with PBS. About 40 μl of Duolink blocking solution was added to each well, and the plate was incubated at 37 °C for 1 h. After blocking, cells were incubated at room temperature for 45 min with primary monoclonal mouse M2 (dilution = 1:350, F3165, Sigma-Aldrich) and rabbit D6W5B (dilution = 1:500, 14793, Cell Signaling Technology) anti-FLAG antibodies (diluted in Duolink Antibody Diluent) at a final concentration of 285 ng ml−1. The wells were washed twice for 5 min at room temperature with buffer A (10 mM Tris pH = 7.4, 150 nM NaCl, 0.05% Tween). About 40 μl of PLA probe mix, containing PLA probe PLUS (anti-rabbit, dilution = 1:5, DUO92002, Merck) and PLA probe MINUS (anti-mouse, dilution = 1:5, DUO92004, Merck) was added to the wells following the manufacturer’s instructions. The plate was then incubated at 37 °C for 1 h. After incubation, the wells were washed twice for 5 min with buffer A, then 40 μl of ligase mix was added to the samples following the manufacturer’s instructions; these were incubated 30 min at 37 °C. Wells were washed twice for 5 min with buffer A at room temperature; subsequently, 40 μl of amplification mix (containing a polymerase) were added to each well, following the manufacturer’s instructions. The plate was then incubated for 100 min at 37 °C. Finally, PLA wells were washed once with buffer B (200 mM Tris, pH = 7.5, 100 mM NaCl) for 10 min at room temperature and a second time for 10 min at room temperature with buffer B supplemented with 1 μg ml−1 Hoechst 33342 nuclear staining (Invitrogen). Subsequently, a final wash was performed with 0.01× buffer B for 1 min at room temperature, and cells were then left in fresh PBS. The plates were imaged using an Opera Phenix Plus microscope (Revvity) at ×20. Forty-nine images per well, covering over 90% of the well, were acquired.
Immunofluorescence staining of F13
Immunofluorescence staining of F13 with rabbit and mouse anti-FLAG antibodies was performed in parallel with PLA, in the same plate. Cells were permeabilized at room temperature for 3 min in PBS with Triton 0.1% and washed with PBS. About 40 μl of Duolink blocking solution was added to each well, and the plate was incubated at 37 °C for 1 h. Cells were incubated at room temperature for 45 min with primary monoclonal mouse M2 (dilution = 1:350, F3165, Sigma-Aldrich) and rabbit D6W5B (dilution = 1:500, 14793, Cell Signaling Technology) anti-FLAG antibodies (diluted in Duolink Antibody Diluent) at a final concentration of 285 ng ml−1. The wells were washed twice for 5 min at room temperature with buffer A. Wells were washed twice with PBS for 5 min at room temperature, and Alexa FluorTM 488 goat anti-mouse antibody (dilution = 1:500, A-11001, Invitrogen) and Alexa FluorTM 488 goat anti-rabbit antibody (dilution = 1:500, A-11008, Invitrogen) (diluted in PBS, BSA 1%, Na Azide 0.1%) were added to the respective wells at a final concentration of 4 μg ml−1. The plate was then incubated at 37 °C for 1 h. Wells were washed once for 5 min at room temperature with PBS and once with PBS supplemented by 1 μg ml−1 Hoechst 33342 nuclear staining (Invitrogen). Finally, cells were left in fresh PBS before imaging. The plates were imaged using an Opera Phenix Plus microscope (Revvity) at ×10. Twenty-one images per well, covering over 90% of the well, were acquired.
Viral plaque assay and analysis
Six-well plates were seeded with BSC40 (ATTC CRL-2761) cells 24 h before infection. Confluent BSC40 cells were infected with wild-type VACV-WR25 (provided by J.M. (University of Birmingham)) or rVACV mutants generated from a modified VACV-WR (vNotI/tk) strain (originally provided by B. Moss to K.C., who further modified it by incorporating an mCherry reporter into the tk locus) at a 10-fold dilution in DMEM with 2.5% FBS for 1 h at 37 °C. The infection medium was then removed, and a 0.5% methylcellulose in DMEM media overlay containing 10 µM Tecovirimat was added for 3 days at 37 °C. Afterward, the overlay medium was removed, and the wells were fixed and stained with 1% crystal violet in 20% methanol for 20 min. The crystal violet was removed, wells were washed with PBS, and plates were imaged using a Cytation 7. Plaque counts and diameters were measured to determine titres (plaque-forming units (p.f.u. ml−1)) and plaque sizes (µm) using a program developed on the Cytation 7. The experiments with MPXV were done using the clade IIb strain MPXV/2022/FR/CMIP, which was isolated at the Institut Pasteur (France) in 2022. All experiments were conducted under struct BSL3 conditions according to the French regulations on dual use pathogens. The neutralization assays were done using Vero cells (ATTC CCL-81) plated in a μClear 96-well plate (Greiner Bio-One). The following day, cells were incubated with serial dilutions of Tecovirimat/IMCBH. MPXV was added 4 h later. The cells were fixed after 48 h with 4% paraformaldehyde and washed and immunostained with polyclonal anti-VACV antibodies (PA1-7258, Invitrogen) and an Alexa Fluor 488-coupled goat anti-rabbit antibody (CA-11008, Invitrogen). Images were acquired with an Opera Phenix high-content confocal microscope (PerkinElmer). Infection was quantified by calculating the total area of MPXV-positive cells (MPXV+ area), and the nuclei were counted using the Harmony software v4.9 (PerkinElmer).
Image analysis
PLA and immunofluorescence images were analysed using Signals Image Artist v1.3 (Revvity). For PLA (Extended Data Fig. 6), the PLA area (633 nm) was calculated using an intensity threshold. In parallel the number of cells was quantified using nuclear count on the Hoechst channel. The average PLA area per cell was calculated by dividing the total PLA area by the total number of nuclei per well. For quantification of the number of FLAG (F13) positive cells in each condition, first the number of cells was quantified using nuclear count on the Hoechst channel. Second, the number of nuclei positive for FLAG staining was calculated using an intensity threshold method on the nuclei region of interest. The percentage of FLAG+ cells was calculated by dividing the total number of FLAG+ nuclei by the total number of nuclei per well.
Sequence analysis
We retrieved all MPXV genomes available on the GISAID database26 and all OPXV available on the GenBank database as of 27 May 2024. For each viral species, sequences corresponding to the F13 coding sequence were extracted and aligned using MAFFT v7.505. Alignments were manually curated for accuracy using Geneious Prime v2024.0.5, and sequences covering less than 70% of the coding sequence were discarded. After these steps, we obtained a dataset comprising F13 sequences from 108 MPXV clade I, 8,472 MPXV clade II, 81 VARV, 211 VACV, 13 ECTV, 98 CPXV, 11 CMLP, 1 BRPV, 6 Akhmeta_virus (AKMV), 2 Orthopoxvirus Abatino, 2 Skunkpox virus (SKPV), 2 Taterapox virus (TATV) and 4 Raccoonpox virus (RCNV). Sequences were translated, and all variations to the consensus of each species were extracted. The mutations shown in Supplementary Table 3 were extracted from refs. 9,13,82,83,84. They list all mutations identified so far conferring resistance to tecovirimat. The potential escape mutants identified in this manuscript are described in ref. 27 and are available.
Statistics and reproducibility
Data were collected from at least two independently repeated experiments, as indicated in the figure legends. Data collection and analysis were not performed blind to the conditions of the experiments. Data distribution was assumed to be normal, but this was not formally tested. No statistical method was used to predetermine sample size. No data were excluded from the analyses. Values are shown as mean ± s.d. Prism (GraphPad Software) was used to determine statistical significance. Two-way analysis of variance was used for analysis. n represents the number of independent samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.

Responses