A collaborative force for precision medicine progress: the STRIPE pharmacogenomics conference series

The landscape of precision medicine
The field of precision medicine offers a more personalized approach to healthcare, recognizing the unique combination of genetic, environmental, and lifestyle factors that influence each person’s health. This approach aims to tailor treatments to individual needs, potentially making healthcare more effective and reducing unwanted side effects.
Pharmacogenomics, a key part of precision medicine, explores how a person’s genetic makeup affects their response to drugs. This knowledge can lead to optimized therapeutic regimens, ensuring that patients receive medications that are more likely to work well for them while minimizing the risk of adverse reactions.
Implementing pharmacogenomics into everyday medical practice involves several steps and the collaboration of many different parties. The journey from discovering genetic markers that influence drug responses to applying this information in clinical settings is complex. It involves not just the scientists and doctors but also patients who undergo genetic testing and the broader healthcare system that supports this technology.
Despite its potential, the path to integrating pharmacogenomics into healthcare comes with challenges. It requires a collective effort from a wide range of stakeholders, including medical professionals who need to understand and apply genetic information, patients who are central to the testing and treatment processes, and the healthcare infrastructure needed to support these activities. Regulatory oversight also plays a role in ensuring the reliability and safety of pharmacogenomic testing.
The promise of precision medicine, particularly through pharmacogenomics, is to make treatment more personalized and effective. However, reaching this goal involves navigating complexities like standardizing testing practices, making genetic testing accessible to a diverse range of people, and incorporating genetic data into everyday medical decisions. As the field grows, continued collaboration, innovation, and education will be crucial for harnessing the full potential of precision medicine to enhance patient care.
STRIPE: A collaborative force for progress
STRIPE facilitates the consensus of different perspectives and expertise. Precision medicine is inherently multidisciplinary, requiring input from geneticists, clinicians, bioinformaticians, and others. By bringing these diverse voices to the table, STRIPE supports comprehensive and effective practices in pharmacogenomics.
The pharmacogenomics (PGx) testing cycle involves multiple stakeholders (Fig. 1), each playing an important role in ensuring the effective integration and utilization of PGx testing in clinical practice. Physicians and healthcare providers are at the forefront, responsible for identifying patients who may benefit from PGx testing and interpreting the test results to guide personalized treatment plans. Pharmacists collaborate closely with physicians to provide medication management and patient counseling based on PGx data. Laboratory professionals and clinical geneticists conduct and analyze the PGx tests, ensuring accuracy and reliability of the results. Health plans and insurers determine coverage and reimbursement policies, which significantly influence patient access to PGx testing. Patients are central to the cycle, as they provide the genetic material for testing and ultimately benefit from tailored therapeutic strategies. Pharmacogenomics researchers catalyze and lead research in precision medicine for the discovery and translation of genomic variation influencing therapeutic and adverse drug effects [3]. In addition, regulatory bodies and policymakers establish guidelines and standards to ensure the quality, safety, and ethical use of PGx testing. Each stakeholder’s role is integral to advancing the implementation of precision medicine and optimizing patient outcomes through PGx testing. Stakeholders must work together to develop and implement practices that can adapt to the rapid pace of scientific discovery while maintaining rigorous standards of evidence. By aligning on these standards, the industry can enhance the impact of pharmacogenomics on patient care, making personalized medicine a practical reality for more individuals.
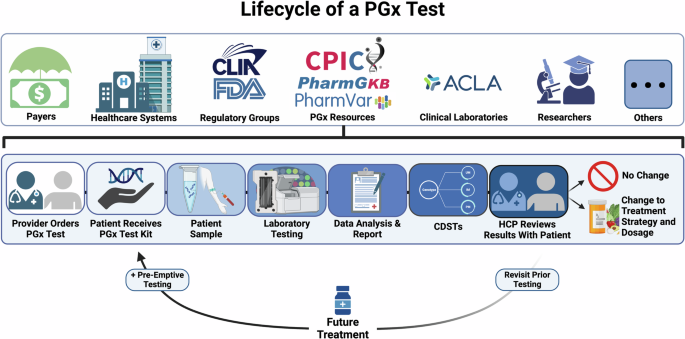
The figure outlines the testing process, including the reuse of previous test results to inform future prescribing decisions. The top section shows stakeholders involved in various stages of the test lifecycle. Every patient test is informed by the contributions of each stakeholder group. CDST Clinical Decision Support Tool, HCP Healthcare Provider, PGx Pharmacogenomics, ACLA American Clinical Laboratory Association, CPIC Clinical Pharmacogenetics Implementation Consortium, CLIA Clinical Laboratory Improvement Amendments, FDA Food & Drug Administration.
The road ahead
As we delve into the conference proceedings, we are met with a compelling narrative of progress and collaboration. The STRIPE community’s dedication to tackling the complex challenges of precision medicine and promoting standardized practices is a testament to the potential that lies within collaborative communities.
In closing, we must recognize that precision medicine is a collective endeavor. It is not the work of a single institution but a global effort that requires the collaboration and consensus of diverse stakeholders. The Partners in Precision Medicine conference proceedings serve as a reminder of the power of collaboration and the importance of standardization in the journey toward realizing the full potential of precision medicine [4]. Together, we can unlock new horizons in patient care and continue to push the boundaries of what is possible in the realm of healthcare.




Responses