A comprehensive review of KCC-1 fibrous silica for water treatment

Introduction
Executive summary
This review provides an in-depth examination of fibrous silica KCC-1 and its significant potential in water treatment, particularly for removing heavy metals (HMs) and organic dyes (ODs) from wastewater. Due to its high surface area, unique fibrous morphology, and customizable porosity, KCC-1 exhibits exceptional adsorption performance, setting it apart from conventional adsorbents. The review focuses on two primary synthesis methods—one-pot and post-grafting techniques—that enable tailored functionalization and enhance KCC-1’s adsorption capacity for diverse pollutants.
In addition to the structural and functional advantages of KCC-1, this review offers a critical assessment of surface modification strategies, shedding light on how different modification techniques impact adsorption efficiency. Key advancements in KCC-1’s applications are outlined, along with existing gaps that point to the need for optimized functionalization methods for practical, real-world applications. Moreover, the review highlights promising future research avenues, including the potential of KCC-1 to target emerging pollutants such as pharmaceutical residues and microplastics, which present new challenges in environmental remediation.
The main topics covered in this review include:
-
Introduction to KCC-1 and its applications in water remediation
-
Chemo-physical characterizations of KCC-1
-
Surface functionalization of KCC-1: methods and implications
-
Application of KCC-1 as an adsorbent for HMs and ODs
-
Comparative analysis of synthesis methods: one-pot synthesis (OPS) vs. post-grafting (PGS)
-
Future research directions and emerging applications.
Challenges of HMs and ODs: an urgent need for efficient removal techniques
The increasing global demand for freshwater, driven by population growth, industrial activities, and agricultural needs, has intensified the search for efficient water treatment methodologies. Water scarcity poses significant challenges, necessitating the development and implementation of advanced treatment techniques to ensure the availability of clean water for various purposes. Among the myriad of techniques employed, including coagulation, flocculation, membrane filtration, and chemical precipitation, the adsorption technique has garnered significant attention due to its numerous advantages1,2,3. The phenomenon of adsorption entails a sequence of events where particles, including atoms, ions, or molecules, originating from a substance identified as the adsorbate, affix themselves to the surface of another material known as the adsorbent through chemical, physical, or chemo-physical interactions. This technique is particularly advantageous due to its high removal efficiency, cost-effectiveness, simplicity, and ability to handle a wide range of contaminants4,5. Recent advancements in adsorption technology have further enhanced its capabilities, making it a pivotal method in water purification. The efficiency of adsorption is affected by several critical factors, including pH, contact time ((t)), temperature ((T)), adsorbent dosage ((W)), and initial adsorbate concentration (({C}_{{rm{i}}})). These parameters must be optimized to achieve maximum contaminant removal.
Water pollution is a pervasive problem, arising from diverse sources such as improper waste disposal, agricultural runoff, and industrial discharges as well as natural processes such as volcanic eruptions and earthquakes. Among the various pollutants, HMs6 and ODs7 are of particular concern due to their toxicity, persistence, and potential for bioaccumulation. HMs, including lead (Pb), cadmium (Cd), and mercury (Hg), are commonly released into the environment from industrial activities, mining operations, and improper disposal of electronic waste. ODs, used extensively in the textile, leather, and paper industries, pose significant environmental hazards due to their complex chemical structures and resistance to biodegradation. This review focuses on the removal of HMs and ODs because they represent significant threats to both environmental and human health. HMs can accumulate in living organisms, leading to severe health problems such as neurological disorders, kidney damage, and various types of cancer8,9 Similarly, ODs can be carcinogenic, mutagenic, and toxic to aquatic life, thereby disrupting ecosystems and posing serious risks to human health through contaminated water sources10,11.
Nanoadsorbents: revolutionizing water treatment technologies with a focus on nanoporous silica materials
A wide array of adsorbents has been investigated for their efficacy in water treatment applications. These adsorbents can be categorized into several types, including powder-type adsorbents, polymeric adsorbents, and composite materials that combine powders and polymers. Each category offers unique benefits and limitations regarding adsorption capacity, regeneration potential, and cost12,13. Recently, nanomaterials have emerged as highly efficient adsorbents due to their large surface area, high reactivity, and adjustable surface properties14,15. Examples of nanoadsorbents include nanoporous silica materials (NSMs)16,17, carbon nanotubes (CNTs)18,19, graphene/graphene oxide20,21, metal-organic frameworks (MOFs)22,23, covalent organic frameworks (COFs)24,25, and layered double hydroxides (LDHs)26,27, which have demonstrated remarkable performance in removing contaminants from water. Among these, NSMs have attracted substantial scientific interest due to their highly porous structure, the ability to functionalize their surfaces, the accessibility of precursor materials, their non-toxic nature, the simplicity of their synthesis protocols, and their remarkable versatility in customizable design. NSMs are categorized based on pore size into three main types: microporous, mesoporous, and macroporous. Microporous materials have pore diameters of less than 2 nm, making them ideal for gas adsorption and separation applications. Mesoporous materials, characterized by pore diameters within the range of 2–50 nm, are widely used in adsorption, catalysis, drug delivery, and as support for various nanostructures due to their larger surface areas and pore volumes. Macroporous materials have pore diameters greater than 50 nm, which facilitate applications requiring high permeability and flow-through properties, such as filtration and chromatography.
Mesoporous silica materials exhibit a wide array of structural configurations, broadly divided into ordered and non-ordered families, along with fibrous silica nanospheres (FSNs). The ordered nanoporous silica category includes the MCM family (MCM-41, MCM-48), the SBA family (SBA-15, SBA-16), the KIT family (KIT-5, KIT-6), and the FDU family (FDU-1, FDU-12)28. Within the non-ordered category, significant types comprise hollow spheres, multi-spheres, and additional morphologies such as porous nanorods, helical fibers, nanowires, gyroids, crystals, thin films, and nanofibers29. NSMs-based adsorbents are distinguished by their high surface area, porosity, and stability, making them especially suitable for adsorption applications. These materials offer significant advantages over other types of adsorbents, including ease of functionalization and high adsorption efficiency, positioning them as promising candidates for advanced water treatment technologies30,31. FSNs, also known as KCC-1 and alternatively referred to as dendritic fibrous nanosilica (DFNS), dendritic fibrous silica particles (DFSPs), and dendritic mesoporous silica nanoparticles (DMSNs)—have emerged as a novel and notable addition for NSMs. Distinguished by their unique fibrous morphology, DMSNs have demonstrated considerable potential as promising NSMs-based adsorbents, warranting further exploration and attention.
Can KCC-1 serve as a game-changer in nanomaterials for water treatment?
KCC-1 represents a significant advancement in the field of nanomaterials. First introduced by V. Polshettiwar32, KCC-1 is characterized by its unique fibrous morphology, providing a high surface area and enhanced accessibility for adsorbates. The exceptional physicochemical properties of KCC-1, including its high thermal stability and versatile functionalization capabilities, make it an effective material for various applications. These applications span across adsorption, catalysis, drug delivery, and sensor technologies. In the context of water treatment, KCC-1’s high surface area and porosity enable efficient capture and removal of contaminants such as HMs and ODs. This review aims to provide a comprehensive examination of the synthesis methods, physicochemical properties, and specific applications of KCC-1 in water remediation, highlighting its potential to address pressing environmental challenges.
Surface modification is essential for enhancing the adsorption properties of nanosilica materials like KCC-1. By introducing functional groups onto the silica surface, the interaction between the adsorbent and the adsorbate can be significantly improved, leading to higher adsorption capacities. Two primary approaches for surface functionalization are one-pot (direct) synthesis and post-grafting (indirect) modification, abbreviated as OPS and PGS, respectively. OPS involves the simultaneous formation and functionalization of the silica material, offering a streamlined process but potentially resulting in less control over the distribution of functional groups. In contrast, PGS allows for precise control over the functionalization process by modifying pre-formed silica, although it can be more labor-intensive and time-consuming. Both methods have their respective advantages and disadvantages, influencing their suitability for different applications. Understanding these functionalization techniques is crucial for optimizing the performance of KCC-1-based adsorbents in water treatment.
Given the increasing interest in KCC-1 and its composites for water treatment, there is a significant need for an updated review that consolidates recent developments and applications in this field. Such a report is crucial for advancing academic understanding and guiding future research efforts. This review aims to fill the gap in the existing literature by providing a detailed analysis of the synthesis, characterization, and application of KCC-1 and its composites as adsorbent materials for the removal of HMs and ODs. By compiling the latest studies and highlighting innovative approaches, this review will serve as a valuable resource for researchers and practitioners in the field of water remediation. The continuous sections of this review will explore the synthesis and properties of KCC-1, as well as its performance in adsorbing various contaminants, offering insights into the potential of this material to contribute to effective and sustainable water treatment solutions.
KCC-1 synthesis
Traditional synthesis
The development of mesoporous silica KCC-1 (King Abdulaziz City for Science and Technology Composite-1) was pioneered by Vivek Polshettiwar in 201032. His groundbreaking work, detailed in the publication “High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology,”32 introduced a novel synthesis approach for creating silica nanospheres with a distinctive fibrous architecture. This structure imparts a high surface area and mesoporosity, making KCC-1 highly suitable for adsorption and catalysis. In Polshettiwar’s method, cetylpyridinium bromide (CPB) served as the surfactant, tetraethyl orthosilicate (TEOS) as the silica precursor and cyclohexane as the solvent. Urea was employed to provide the necessary base environment, facilitating the hydrolysis and condensation of the silica precursor. The reaction mixture was subjected to a microwave (MW) irradiation (400 W maximum power) at 120 °C for 4 h, and subsequent calcination in 550 8 °C for 6 h in air resulted in colloidal spheres (with diameter range from 250 to 450 nm) constituted of dendrimeric fibers possessing a thickness of 8 nm to 10 nm. This synthesis route produced nanospheres with a surface area of up to 641 m2⋅g–1, with the fibrous morphology significantly enhancing their effectiveness in various applications.
Subsequent research, such as the study “Size and Fiber Density Controlled Synthesis of Fibrous Nanosilica Spheres (KCC-1)”33, has explored how different synthesis parameters affect the structure and properties of KCC-1. Factors such as the type and concentration of surfactants, the choice of silica sources, solvents, co-solvents, bases, and reaction temperatures play crucial roles in determining the final characteristics of KCC-1 (Fig. 1). In this study, the researchers successfully demonstrated the ability to control and fine-tune key properties of KCC-1 by varying several reaction parameters. By systematically adjusting the concentrations of urea, cetyltrimethylammonium bromide (CTAB), and 1-pentanol (co-surfactant), along with modifying the solvent ratio, temperature, reaction time, and external stirring time, they were able to effectively manipulate the fiber density, particle size, pore volume, and surface area of KCC-1. The study achieved particle sizes ranging from 170 nm to 1120 nm. They also controlled fiber density across a wide range, from low to very high, which allowed for precise tuning of the pore volume, achieving a notable 2.18 cm3⋅g–1, the highest reported for such fibrous materials. Moreover, the researchers significantly increased the surface area to 1244 m2⋅g–1, nearly doubling the previously reported values for KCC-1. This work provides a robust method for synthesizing KCC-1 with customizable properties, enabling precise control over these characteristics and thereby broadening the potential applications of KCC-1 in various fields such as catalysis and adsorption.
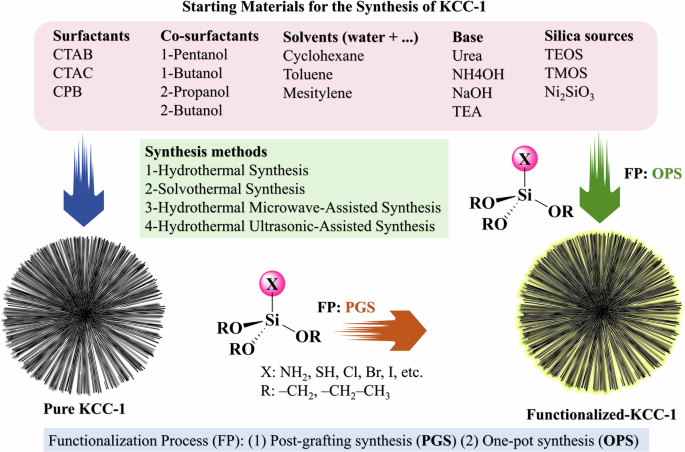
This figure represents the various types of starting materials reported in the literature, which include surfactants, co-surfactants, solvent systems, bases, and silica sources. In addition, it outlines the different synthesis methods employed for the preparation of KCC-1, offering a comprehensive overview of the materials and techniques utilized.
Surfactants
Surfactants are pivotal in forming the fibrous structure of KCC-1. Common surfactants like CTAB, cetyltrimethylammonium chloride (CTAC), and CPB (Fig. 1) facilitate micelle formation, which templates the silica condensation. Higher surfactant concentrations typically lead to more pronounced fibrous structures due to improved micelle formation and subsequent silica organization.
Silica precursor
The silica precursor—viz., TEOS, tetramethyl orthosilicate (TMOS), and sodium silicate (Na2SiO3) (Fig. 1)—also significantly impact the morphology and yield of KCC-1. TEOS is often used for its ability to produce well-defined fibrous structures, while sodium silicate (Na2SiO3) offers a cost-effective alternative, albeit with potential variations in structural properties.
Solvent
The choice of solvent system, such as |cyclohexane+water | , |toluene+water | , and |mesitylene+water| (Fig. 1), influences the oil phase in which the silica precursor and surfactant interact. Cyclohexane generally results in more uniform and fibrous nanospheres compared to toluene, which can lead to less regular structures.
Co-solvent
Co-solvents such as 1-butanol, 1-pentanol, 2-propanol, and 2-butanol (Fig. 1) are utilized to adjust the reaction medium’s polarity, enhancing the dispersion of surfactant micelles and fine-tuning the fiber density and pore structure of KCC-1.
Base
The base—such as urea ((NH2)2C = O), ammonium hydroxide (NH4OH), sodium hydroxide (NaOH), and tetraethylammonium (TEA, (Et)4N+ –Cl) (Fig. 1)—is essential for controlling the hydrolysis and condensation rates of the silica precursor. Urea gradually decomposes to release ammonia, creating a controlled alkaline environment that promotes the formation of the fibrous structure.
Temperature
Temperature is another critical factor in the synthesis process. Elevated reaction temperatures can speed up hydrolysis and condensation reactions, potentially affecting the size and uniformity of the fibrous nanospheres. Maintaining optimal temperature conditions is crucial for achieving the desired fibrous morphology and high surface area. Bayal et al.33 reported that the reaction temperature significantly impacts the nucleation and growth stages of KCC-1, affecting its size and textural properties. At 100 °C, with a reaction time of 1 h, KCC-1 did not form, though very small (20–50 nm) nearly spherical particles were observed. Increasing the temperature to 120 °C and 140 °C resulted in the formation of KCC-1 with average particle sizes of 880 nm and 1120 nm, respectively. Higher temperatures not only increased particle size but also enhanced fiber density, with KCC-1 synthesized at 140 °C being denser and exhibiting a reduced surface area of 486 m2⋅g–1 compared to 711 m2⋅g–1 at 120 °C. Although the N₂ sorption isotherms were similar, the pore volume decreased from 1.0 cm3⋅g–1 for KCC-1 (880 nm) to 0.66 cm3⋅g–1 for KCC-1 (1120 nm). In addition, higher temperatures resulted in a narrower pore size distribution and smaller mesopores in KCC-1 (1120 nm).
Reaction type
KCC-1 can be synthesized through several methodologies (Fig. 1) including (1) hydrothermal synthesis34,35,36, (2) solvothermal synthesis37,38, (3) MW-assisted hydrothermal synthesis39,40, and (4) ultrasonic-assisted hydrothermal synthesis41, each with distinct advantages and disadvantages. The hydrothermal synthesis method, for example, offers a straightforward and cost-effective approach, producing well-defined nanofibers with high surface areas, but it may require long reaction times and high temperatures. This method involves using a Teflon-lined stainless-steel autoclave. In contrast, the solvothermal synthesis method does not utilize a Teflon-lined stainless-steel autoclave; instead, it employs a conventional condenser-round bottom glass system. This method also produces high-quality KCC-1 with controlled morphologies, but the use of organic solvents poses environmental and safety concerns. MW-assisted hydrothermal synthesis requires an MW reactor, which provides rapid heating and efficient energy use, significantly reducing reaction times and energy consumption. Despite its efficiency, this method demands specialized equipment and precise control over MW parameters, which can be challenging for large-scale applications. Finally, ultrasonic-assisted hydrothermal synthesis utilizes an ultrasonic bath or an ultrasonic probe to enhance reaction kinetics and material uniformity. This method benefits from improved dispersion and uniformity of the nanoparticles, although the necessity for ultrasonic equipment increases the complexity and cost of the process. These methods collectively provide a range of options for synthesizing KCC-1, allowing researchers to choose based on the specific requirements of their applications.
The synthesis mechanism
The synthesis mechanism of KCC-1 involves the initial self-assembly of surfactant micelles in the oil phase, followed by the hydrolysis and condensation of the silica precursor around these micelles. This leads to the formation of silica fibers radiating outward from the center of each nanosphere, creating a high surface area and mesoporous structure. The fibrous morphology is stabilized by the surfactant template, which is later removed through calcination, leaving behind the mesoporous silica framework.
Eco-friendly synthesis
Recently, bio-based materials or biogenic sources such as agricultural waste have been increasingly employed as a sustainable and environmentally friendly approach in the synthesis of sodium silicate, serving as a silica source to produce NSMs. In 2022, Soltani et al.16 introduced an innovative bio-based three-dimensional dendritic multi-organo-functionalized silica nanospheres (termed 3D DMF-SNSs), functionalized with amine, thiol, and carboxyl groups. These nanospheres were synthesized via a green chemistry approach, resulting in a hierarchical bimodal micro–mesoporous channel structure, characterized by a combination of micro and mesopores. The 3D DMF-SNSs are characterized by their large surface area (601 m2⋅g–1), substantial pore volume (0.99 cm3⋅g–1), and the presence of numerous active functional groups within their dendritic bimodal micro–mesoporous channels. These unique structural attributes confer significant potential for these nanoparticles to be used as adsorbents, particularly for the simultaneous removal of chrysoidine G and thallium (I). The synthesis process utilized Sedge (Carex riparia), an abundant agricultural waste, to prepare a sodium silicate solution that served as the silica precursor in the formation of the adsorbent. This study highlights the potential of utilizing biogenic sources for the synthesis of KCC-1, offering a cost-effective and sustainable alternative for material production with promising adsorption capabilities.
Hasan et al.42 demonstrated the synthesis of KCC-1 using rice husk ash, a silica-rich byproduct of rice milling. In their study they outlined a synthesis procedure wherein rice husk ash was subjected to a series of treatment steps, including calcination and acid leaching, to obtain silica nanoparticles. These nanoparticles were then utilized in the synthesis of KCC-1 using a modified version of the conventional method. They reported a surface area of ~600 m2⋅g–1 and a pore volume of 1.2 cm2⋅g–1 for the rice husk ash-derived KCC-1. This study highlights the potential of utilizing biogenic sources for the synthesis of KCC-1, offering a cost-effective and sustainable alternative for material production with promising adsorption capabilities.
In 2025, Soltani and colleagues43 introduced an innovative approach to water treatment through the synthesis of a biogenic fibrous silica sphere that incorporates crown ether ionic liquid functionalization (designated as CEIL-S-KCC-1), engineered specifically for the dual-adsorption of methyl orange (MO) and Cd(II) ions from aqueous solutions. The synthesis process utilized sorghum agricultural waste as a sustainable silica source, promoting an environmentally responsible production pathway. In the material’s preparation, thiol-functionalized fibrous silica spheres were efficiently combined with benzo-15-crown-5 ionic liquid via a thiol-ene click reaction, enabling effective integration of the functional groups. Experimental conditions, including a temperature of 298 K, a solution volume of 50 mL, an adsorbent dosage of 5 mg, a pH level of 7, and a shaking speed of 200 rpm, were meticulously maintained to optimize adsorption capacity. Under these conditions, the synthesized material achieved remarkable maximum adsorption capacities of 507.1 mg⋅g–1 for methyl orange and 306.3 mg⋅g–1 for Cd(II). The material’s fibrous and porous architecture, along with its substantial surface area, substantially enhanced its adsorption efficiency by promoting pollutant accessibility and fostering robust surface interactions. This dual-adsorbent material demonstrates significant potential as a multifaceted, cost-effective, and eco-friendly solution for advanced water purification processes. Furthermore, by leveraging agricultural sorghum waste as a raw material, the study aligns with circular economy principles, underscoring the benefits of resource sustainability and waste minimization.
Chemo-physical characterizations of KCC-1
The unique properties of mesoporous silica KCC-1 are elucidated through various chemo-physical characterization techniques, each providing critical insights into its structural and functional attributes.
X-ray Diffraction (XRD) analysis of KCC-1 reveals distinct differences when compared to other ordered mesoporous silica materials such as SBA, MCM, KIT, and FDU families. Unlike these materials, which exhibit sharp peaks indicative of a long-range ordered structure, the XRD spectrum of KCC-1 lacks sharp peaks. This absence suggests that KCC-1 does not possess a long-range ordered framework, reflecting its unique fibrous morphology rather than a periodic mesoporous structure.
Fourier Transform Infrared (FTIR) spectroscopy is employed to identify the characteristic absorption bands associated with the silica framework of KCC-1. The FTIR spectrum of KCC-1 typically displays several distinct bands34,35. The broad band around 3400 cm−1 is attributed to the O–H stretching vibrations from surface silanol groups and adsorbed water molecules. The band near 1630 cm−1 corresponds to the bending vibrations of molecular water. Key absorption bands related to the silica network include the asymmetric stretching vibrations of Si–O–Si bonds around 1080 cm−1, which is often a strong and broad peak. The symmetric stretching vibrations of Si–O–Si bonds appear near 800 cm−1, and the bending vibrations of Si–O bonds are observed around 460 cm−1. These bands confirm the presence of silica and align with the expected chemical structure of mesoporous silica materials.
Field Emission Scanning Electron Microscopy (FESEM) provides detailed images of KCC-1, revealing its distinctive morphology. The FESEM images show silica spheres with wrinkled surfaces, a hallmark of KCC-1’s structure. This wrinkled surface increases the surface area, enhancing the material’s potential for applications in adsorption and catalysis.
Transmission Electron Microscopy (TEM) offers deeper insight into the internal structure of KCC-1, clearly showing its fibrous nature. The TEM images depict fibers radiating from the center of each nanosphere (Fig. 1), which is a characteristic feature of KCC-1. This fibrous morphology is crucial for its high surface area and mesoporous properties.
Nitrogen adsorption-desorption isotherms further characterize KCC-1, providing quantitative data on its surface area and pore structure. The isotherms typically display a type IV profile with a hysteresis loop, indicative of mesoporous materials. Reported surface areas for KCC-1 range from 500 m2⋅g–1 to 1244 m2⋅g–1, with pore volumes around 1.0 cm3⋅g–1 to 1.3 cm3⋅g–1 33. The hysteresis loop in the isotherms is a signature of the presence of mesopores, corroborating the mesoporous nature of KCC-1.
Ultraviolet–visible (UV–vis) Spectroscopy is used to study the optical properties of KCC-1. Previous studies on pure KCC-1 indicate that it exhibits characteristic absorbance in the UV region, which is typical for silica-based materials. This technique helps in understanding the electronic transitions and band gap of the material.
Surface functionalization of KCC-1
Surface functionalization of mesoporous silica KCC-1 is a crucial modification that enhances its adsorption capabilities for various pollutants44. Functionalization methods can be broadly classified into two categories: the OPS method and the PGS method. The most widely utilized functionalization agents for NSMs are silane coupling agents (SCAs). SCAs are molecules characterized by the presence of alkoxy groups (e.g., –OCH3, –OC2H5, –OC3H7,) attached to a silicon atom along with various organic functional groups, such as -SH, -OH, -NH2, epoxy, allyl, phenyl, etc. (Fig. 2a). These organic functional groups provide versatility, enabling SCAs to be used in a wide range of applications for surface functionalization. The inclusion of these diverse functional groups allows SCAs to interact with different types of materials, facilitating their integration into various chemical processes and enhancing the properties of the resultant nanostructured materials. These agents are readily available commercially, but they can also be synthesized in laboratory settings through the conversion of organic functional groups into desired groups for functionalization purposes. Figure 2 illustrates the SCAs reported in the literature for surface functionalization of KCC-1, particularly for use as adsorbents, since 2010. Various commercially available synthetic SCAs containing organic functional groups (such as −SH, −C ≡ N, and −NH2) have been utilized to functionalize KCC-1 directly, allowing the organic groups to serve as adsorption sites for the removal of HMs and ODs from water. Examples of these SCAs include N-2-(aminoethyl)3-aminopropyltrimethoxysilane (AE-APTMS, (CH3O)3Si-C3H6-NH-C2H4-NH2), which has been employed for the adsorption of Congo red41, and 3-[2-(2-aminoethylamino)ethylamino]propyltrimethoxysilane (AEAE-APTMS, which has shown potential for Cr(VI) adsorption45 (Fig. 2a). These studies demonstrate the potential applications of functionalized KCC-1 materials in the removal of contaminants from water sources.

a SCAs, b, c examples of the OPG method, and d–g examples of the PSG method for preparation of functionalized KCC-1 for use as adsorbents reported in the literature from 2010 to 2024.
One-pot synthesis
The one-pot method involves the simultaneous synthesis and functionalization of KCC-1. This approach integrates functional groups directly into the silica framework during the synthesis process. Typically, silane coupling agents are added to the reaction mixture containing the silica precursor, surfactant, solvent, and co-solvent. This mechanism involves the simultaneous hydrolysis and condensation of SCAs with the silica source (e.g., TEOS or sodium silicate). In the presence of an acidic catalyst or base (e.g., urea), TEOS undergoes hydrolysis, forming reactive silanol (Si–OH) groups. This reaction is crucial as it produces the primary species that will eventually condense into the silica network. The alkoxy groups on the SCAs undergo hydrolysis, forming reactive silanol (Si–OH) groups. These silanol groups then participate in condensation reactions with the silanol groups of the silica precursor (e.g., TEOS or Na2SiO3), resulting in the formation of siloxane (Si–O-Si) bonds. This process integrates the functional groups of the SCAs uniformly into the silica network, creating homogeneously functionalized material. Simultaneously, SCAs, which contain both alkoxy groups and functional organic moieties (e.g., –SH, –Cl, –Br, –NH2), undergo similar hydrolysis to generate silanol groups. These silanol groups from both TEOS (Eq. (1)) and SCAs (Eq. (2)) then participate in condensation reactions, forming siloxane bonds (Si–O-Si) (Eq. (3)) and creating a cross-linked silica network: The reactions can be summarized as follows:
The formation of silica framework typically occurs around surfactant micelles, which act as templates. In an acidic or basic medium, the surfactant molecules organize into micellar structures due to hydrophobic interactions. The hydrolyzed silicate species (both from TEOS and SCAs) then condense around these micelles, forming a silica shell. This process is directed by the surfactant, resulting in the silica structure. The surfactant micelles help to define morphology as well as pore size and distribution within the silica framework.
This method ensures a homogeneous distribution of functional groups within the mesoporous structure, providing enhanced active sites for adsorption. The primary advantage of the OPS method is its simplicity and efficiency, as it combines synthesis and functionalization into a single step. However, this method has its drawbacks. First, the one-pot synthesis approach often results in limited functional group diversity within the silica framework. This limitation arises because the simultaneous co-condensation of silica precursors and functional organosilanes is difficult to control, leading to reduced functional group variety. Additionally, the reaction conditions required to simultaneously incorporate different functional groups are complex, with variations in parameters potentially resulting in inconsistent product quality and reproducibility.
To date, only a limited number of studies have reported the synthesis of organo-functionalized KCC-1 via the OPS method. In 2010, Du et al.46 successfully synthesized a range of dendrimer-like amino-functionalized silica particles with controllable hierarchical porosity. This was accomplished by meticulously adjusting experimental parameters within ethyl ether emulsion systems via a one-pot sol-gel process. In 2020, researchers synthesized 3-mercaptopropyltrimethoxysilane-functionalized mesoporous fibrous silica (SH-MFS) nanospheres via a OPS route47. This approach provided an efficient single-step procedure for producing organo-functionalized fibrous silica-based adsorbents. Furthermore, in 2021, Ven and co-workers48 prepared a bivalent organic cross-linker named silanated DABCO (DABCO-S) by reacting CPTES with DABCO (Fig. 2b). Then, this SCA-bearing DABCO reacted with TEOS and CTAB via OPS method to prepare DABCO-functionalized KCC-1 (DDS) and it was subsequently utilized for the adsorption of methylene blue and orange II dyes from water. In 2022, Soltani and colleagues16 introduced an innovative method to synthesize bio-based, multi-organo-functionalized three-dimensional radial dendritic silica nanospheres (3D DMF-SNSs) featuring a hierarchical micro–mesoporous architecture (Fig. 2c). These structures were synthesized using a one-pot oil–water biphase method, utilizing silica derived from the Sedge plant (Carex riparia). 3D DMF-SNSs possess three types of SCAs on their dendrimeric surface including AE-APTMS, 3-mercaptopropyl triethoxysilane (MPTES), and 3-cyanopropyl triethoxysilane (CyPTES), enhancing the adsorption properties and its functionality. This advanced material demonstrated exceptional capability as adsorbents, effectively removing both chrysoidine G and thallium (I) from aqueous solutions simultaneously.
Post-grafting synthesis
The PGS method, on the other hand, involves the functionalization of pre-synthesized KCC-1. In this approach, the silica nanospheres are first synthesized, and then functional groups are grafted onto their surface through a separate reaction. This method allows for greater control over the type and density of functional groups introduced. The functionalization via PGS method is typically performed by reacting the silica surface with SCAs under controlled conditions. However, the PGS method possesses several disadvantages. It typically results in lower surface functionalization density compared to one-pot synthesis due to steric hindrance and limited accessibility of reactive sites on the mesoporous structure. Moreover, post-grafting involves multiple steps, making the process more time-consuming and complex, with each step increasing the risk of material loss and contamination. Furthermore, the distribution of functional groups can be non-uniform, leading to regions of high or low functional density, which can affect the material’s performance in various applications. Additionally, the grafting process can sometimes partially block the mesopores, reducing the material’s surface area and pore volume, thereby diminishing its effectiveness in applications such as catalysis and adsorption. Although KCC-1 does not possess an ordered porous structure and arrays of pores and channels like that of SBA, MCM, KIT, and FDU families, the impact of pore blocking in the PGS method should be considered when selecting an appropriate surface functionalization technique.
Soltani et al. reported a method for the synthesis of multifunctionalized KCC-1, which involved the use of (3-aminopropyl)triethoxysilane (APTES), 3-mercaptopropyltriethoxysilane (MPTES), and bis[3-(triethoxysilyl)propyl] tetrasulfide (TESPTS) as silane coupling agents. They implemented a PGS method and the functionalization of KCC-1 was conducted at a controlled reaction temperature within the range of 70-80 °C. The duration of the reaction spanned ~24 h to ensure adequate interaction between the SCAs and the silica surface. The solvent system employed consisted of toluene and ethanol, providing a conducive environment for the functionalization process. Utilizing CTAB as the surfactant, and TEOS as the silica precursor, the method aimed to yield multifunctionalized KCC-1 with enhanced adsorption properties. Multifunctionalized KCC-1 exhibited enhanced surface area and pore volume. The specific surface area of the functionalized KCC-1 was reported to be around 500–600 m2 g–1, while the pore volume was approximately 1.0 m3 g–1. These values indicate that the functionalization process maintained the mesoporous nature of KCC-1 while introducing additional active sites for adsorption.
When there is a need to introduce a specific organic group onto KCC-1, two primary strategies can be employed based on the desired outcome and reaction environment. The first strategy involves the initial functionalization of KCC-1 with a simple SCA, such as APTES bearing an –NH2 group, 3-chloropropyltriethoxysilane (CPTES) containing a –Cl group, or 3-bromopropyltriethoxysilane (BPTES) featuring a –Br group. These SCAs can be attached to the KCC-1 framework, followed by a secondary chemical reaction, such as a substitution reaction, to introduce the specific functional group onto the pre-functionalized KCC-1. A notable example of surface functionalization is a 2019 study that reported the synthesis of Cys-DFNS-NH249. In this instance, KCC-1 was initially functionalized with APTES using the post-grafting (PGS) method. This was followed by the coupling of L-cysteine to the amine group of KCC-1-NH2, facilitated by the reaction between the –NH2 group of KCC-1-NH2 and the carboxylic group (–COOH) of L-cysteine to form an amide bond. The thiol and amine groups present in the cysteine molecule have the potential to act as chelating agents, effectively capturing cations such as Pb2+, Cd2+, and Ag+ from aqueous solutions. This process exemplifies the modification of functionalized KCC-1 through the attachment of additional molecules, such as cysteine, which is then coupled to the functionalized KCC-1.
Alternatively, the second strategy involves modifying the SCA with the desired functional group prior to grafting onto the pure KCC-1. This pre-modification ensures that the specific functional group is already present on the SCA, which is then introduced to the KCC-1 framework in a subsequent reaction step. This approach can streamline the functionalization process by incorporating the specific group directly during the SCA attachment phase, potentially enhancing the efficiency and specificity of the functionalization.
These methodologies allow for tailored surface modifications of KCC-1, enabling its use in various applications such as adsorption, catalysis, and sensing, where specific functional groups may be required to achieve optimal performance.
In 2015, Yan and colleagues50 developed rhodamine B-functionalized KCC-1 by immobilizing a rhodamine-based receptor (RB-Si), which was synthesized via the reaction between IPTES and Rhodamine B, within the channels of KCC-1 (Fig. 2d). This functionalized material was designed for optical sensing and the removal of Hg(II) ions from aqueous solutions. In the same year, another study by Yan’s team51 involved the synthesis of 8-piperazine acetylaminoquinoline-functionalized SCA (AQ-Si) through the reaction of 8-piperazine acetylaminoquinoline (AQ-N) with IPTES (Fig. 2e). This AQ-Si was subsequently used to functionalize core–shell magnetic KCC-1 (AQ-Fe3O4@SiO2@KCC-1) via the PGS, which was employed for the detection, adsorption, and removal of Zn(II) ions from water. In 2020, Soltani et al.35 reported the addition of 1-methyl-3-(3-triethoxysilylpropyl) imidazolium chloride (MI-Cl SCA) to KCC-1 (Fig. 2f). The MI-Cl SCA was synthesized by reacting CPTES with methylimidazolium. This N-methylimidazolium-functionalized KCC-1 served as an anion exchange adsorbent for the removal of Cr(VI) oxyanions through an ion-exchange process. In 2022, Gao et al.52 reported the selective capture of Pd(II) from aqueous media using ion-imprinted KCC-1. In this study, a Schiff base and pyridine group-containing SCA (SBPCSCA) were grafted onto KCC-1 via the PGS method to adsorb Pd(II) and facilitate subsequent Suzuki reactions in water. The SBPCSCA was synthesized through the reaction between 2-pyridinecarboxaldehyde and AE-APTMS.
Recently, Soltani and colleagues.43 introduced an advanced protocol for synthesizing a dual-function adsorbent specifically designed for the simultaneous removal of anionic MO and cationic Cd(II) ions from aqueous environments. This protocol involves the modification of KCC-1 through post-functionalization with MPTMS, producing thiol-functionalized KCC-1, which was subsequently conjugated with benzo-15-crown-5 ionic liquid (CEIL) using a thiol-ene click reaction to integrate these functional groups effectively (Fig. 3). The resulting CEIL-S-KCC-1 material possesses diverse adsorptive functionalities, including positively charged imidazolium rings and benzo-15-crown-5 crown ether sites, which enhance their affinity for different pollutants. Theoretically, the adsorption of the anionic MO dye is facilitated by the positively charged imidazolium ring through electrostatic interactions and hydrogen bonding, while Cd(II) ions are adsorbed via a chelation mechanism involving the crown ether. These functional groups play an essential role in governing adsorption specificity and efficiency, with the functional chemistry directly influencing adsorbent-pollutant interactions and allowing for targeted pollutant capture. This study represents a highly detailed approach to KCC-1 functionalization, showcasing a tailored adsorbent capable of efficiently capturing both an anionic dye and a cationic heavy metal. Figure 3 provides a schematic illustration of the functionalization strategy.

Preparation of CEIL and its coupling to SH-KCC-1 via thiol-ene click chemistry, creating a dual-function adsorbent for the simultaneous adsorption of MO anions and Cd(II) cations from aqueous solutions. Figure modified from Alsaab et al.43, with permission.
By employing SCAs in conjunction with a variety of organic coupling reactions, researchers can systematically develop and incorporate specific functional groups tailored to interact with specific target species. SCAs act as versatile intermediaries that can bridge the surface of a substrate, like mesoporous silica or other nanostructured materials, with functional molecules possessing desired properties, enabling finely tuned surface characteristics. Through carefully chosen coupling reactions—such as thiol-ene, amide bond formation, and click chemistry—it is possible to integrate functionalities that exhibit selective affinities towards pollutants of interest, including both organic dyes and heavy metal ions.
The work conducted by Soltani’s research group43 exemplifies the potential of such intelligent design. By applying a post-functionalization approach to KCC-1 silica and employing SCAs MPTMS followed by conjugation with a benzo-15-crown-5 ionic liquid, they achieved a dual-function adsorbent with remarkable specificity. The use of imidazolium-functionalized ionic liquids in combination with crown ethers creates adsorption sites that selectively interact with different classes of pollutants through mechanisms like electrostatic attraction, hydrogen bonding, and chelation.
This approach not only enhances adsorption efficiency but also provides a modular framework adaptable to different environmental contaminants. Tailored adsorbents that combine multiple functional groups in a single material platform can address complex pollutant mixtures, making them highly advantageous for real-world applications. Moving forward, further development of such advanced adsorbents is crucial, especially as environmental challenges evolve and new pollutants emerge. By exploring a broader array of SCAs and reaction conditions, researchers can continue to engineer materials that offer selective, high-capacity, and regenerable solutions for water purification, waste treatment, and pollutant remediation. Expanding this research will pave the way for adsorbents capable of responding to application-specific needs, such as higher pollutant loadings, diverse contamination profiles, and the stringent removal standards required for sustainable environmental management.
OPS vs PGS: the need for comprehensive studies on pros and cons
OPS
The following highlights the key advantages and disadvantages associated with the OPS protocol that must be considered when designing functionalized KCC-1.
Advantages:
-
I.
Homogeneous Functionalization: OPS ensures a more uniform distribution of functional groups throughout the structure, as all components are introduced simultaneously during synthesis.
-
II.
Simplicity and Time Efficiency: OPS reduces the need for additional steps, saving both time and resources by incorporating functionalization directly into the synthesis process.
-
III.
Cost-Effective: With fewer steps and reagents, OPS tends to be more economical.
Disadvantages:
-
I.
Limited Control Over Functional Group Placement: Functionalization occurs in situ, making precise spatial control of functional groups challenging.
-
II.
Potential Interference: Certain functional groups can interfere with the structural formation of KCC-1, potentially reducing its porosity and surface area, impacting adsorption performance.
PGS
The following points emphasize the essential advantages and disadvantages of the PGS protocol that should be taken into consideration during the design of functionalized KCC-1.
Advantages:
-
I.
Greater Control Over Functionalization: PGS allows for selective and controlled addition of functional groups following KCC-1 formation, enabling surface tailoring for specific applications.
-
II.
Retention of Structural Integrity: Functionalization after core structure formation helps retain the original porosity and surface area of KCC-1.
-
III.
Versatility in Functional Group Types: PGS allows the introduction of diverse functional groups without impacting initial synthesis conditions.
Disadvantages:
-
I.
Multi-Step Process: PGS requires additional processing, making it more labor-intensive and potentially increasing both time and costs.
-
II.
Potential Non-Uniform Distribution: Surface functionalization may be less uniform compared to OPS, which can affect adsorption efficiency for specific pollutants.
Application suitability
The suitability of KCC-1 for various applications is dependent on several critical factors, including the degree of functionalization (i.e., the proportion of surface coupling agents, or SCAs added), the efficiency of condensation or grafting reactions, the skill of the researcher, SCA type, and synthesis method (e.g., Hydrothermal Synthesis, Microwave-Assisted Synthesis, Ultrasonic-Assisted Synthesis, and Sol-Gel Synthesis).
Currently, a lack of comprehensive studies exploring these variables limits clear guidance on selecting the optimal functionalization method for specific applications. Key unanswered questions include: which method provides the most accessible adsorption sites on the KCC-1 surface for optimized performance?
Future studies to address:
-
I.
Degree of functionalization: The proportion of SCAs influences the number and accessibility of active sites, directly affecting adsorption efficiency.
-
II.
Condensation vs. grafting efficiency: Efficient condensation in OPS can improve functional group homogeneity, while maximizing grafting efficiency in PGS better preserves porosity.
-
III.
Researcher skill level: Handling functionalization reactions, especially in PGS, requires precision, making expertise essential for consistent results.
-
IV.
Type of SCAs and synthesis methods: Different SCAs and synthesis techniques offer varying benefits, such as differing reaction conditions and impacts on structural integrity.
In-depth, comparative studies will aid researchers in selecting functionalization techniques tailored to their specific project needs, ensuring the full potential of KCC-1 is realized in diverse applications.
Application of KCC-1 and its composites as adsorbents for removal of HMs and ODs
Adsorption using KCC-1-based materials has emerged as a promising approach for the removal of HMs and ODs from aqueous solutions. These adsorbents can be categorized into two main groups: powder-type KCC-1 and KCC-1/polymeric composites.
Powder-type KCC-1
Powder-type KCC-1 encompasses both pure KCC-1 and organo-modified KCC-1 variants. Pure KCC-1, synthesized through conventional methods, exhibits inherent mesoporous properties and a high surface area, rendering it effective for adsorption applications. Meanwhile, organo-modified KCC-1 involves surface functionalization with organic moieties, enhancing its affinity towards specific contaminants through tailored surface chemistry.
Table 1 presents the adsorption characteristics of powder-type KCC-1 adsorbents for the removal of HMs and ODs from aqueous environments over the past decade, from 2015 to 2025. The powder-type KCC-1 adsorbents can be categorized into two subgroups based on the source of silica: biogenic-based KCC-1 adsorbents and synthetic-based KCC-1 adsorbents. Biogenic-based KCC-1 adsorbents are prepared from agricultural waste or other natural sources to produce silica particles, while synthetic-based KCC-1 adsorbents are produced using synthetic commercial silica sources such as TEOS and TMOS. In the following sections, to concisely report the adsorption data and facilitate easier comparison, the adsorption parameters will be represented using the following abbreviations (Italic) alongside their units (Roman): the maximum adsorption capacity (Qm, mg⋅g–1), solution volume (V, mL), adsorbent dosage (W, mg or g⋅L–1), contact time (t, min), solution temperature (T, K), stirring speed (SS, rpm).
Biogenic-based KCC-1 adsorbents
In a study reported by Setiabudi et al.42 in 2019, rice husk ash (RHA) was utilized as a low-cost silica precursor to prepare a biogenic-based KCC-1 adsorbent (KCC-1(RHA)) for the removal of Pb(II) from aqueous solutions. The optimum adsorption conditions were achieved at an initial Pb(II) concentration of 322.06 mg L–1, W = 2.4 g L–1, and t = 117 min, resulting in a Pb(II) uptake efficiency of 75%. The experimental data followed the Langmuir isotherm model (R2 = 0.9934) and the pseudo-second-order kinetic model (R2 = 0.9950), indicating the suitability of these models for describing the adsorption process. Furthermore, the KCC-1(RHA) adsorbent demonstrated good performance during five consecutive cycles of adsorption-desorption, highlighting the excellent potential of RHA as a silica precursor for the synthesis of KCC-1 adsorbents with excellent Pb(II) removal capabilities.
In a study published in 2022, Soltani et al.16 successfully synthesized a novel type of “bio-based, three-dimensional, dendritic, multi-organo-functionalized (amine, thiol and carboxyl) silica nanospheres (3D DMF-SNSs)” using an environmentally friendly approach. The synthesized nanospheres displayed distinctive structural and morphological characteristics, such as a dendritic bimodal micro–mesoporous channel configuration decorated with numerous active functional groups, substantial surface area (601 m2 g–1), and high pore volume (0.99 cm3 g–1), which render them highly effective as adsorbents. Comprehensive adsorption experiments were carried out by researchers to assess the simultaneous removal of chrysoidine G and thallium (I) from water using the 3D DMF-SNS adsorbent. The Langmuir isotherm equation determined the maximum adsorption capacities to be 880.6 mg g–1 for chrysoidine G and 806.7 mg g–1 for thallium (I) (pH = 7.0, W = 0.05 g L–1, t = 14 min for chrysoidine G and 20 min for thallium (I), T = 293 K). Regeneration experiments conducted over ten successive adsorption-desorption cycles at 293 K revealed that the adsorption capacities for chrysoidine G and thallium (I) diminished to 745 mg g–1 and 660 mg g–1, respectively, demonstrating the stability and high adsorption efficiency of the 3D DMF-SNS adsorbent.
Synthetic-based KCC-1 adsorbents
A substantial number of studies on the application of KCC-1 in removing HMs and ODs involve the use of synthetic-based KCC-1, where KCC-1 particles are prepared using TEOS as a commercially available silica source (Table 1). For instance, in a 2019 study conducted by Wang et al.53, a phosphonate-functionalized dendritic fibrous nanosilica (PA-DFNS) was developed by modifying the surface of KCC-1 with APTES, epichlorohydrin, and phytic acid. This material demonstrated a remarkable adsorption capacity of 1106 mg g–1 for U(VI) from aqueous solutions under the following conditions: pH = 8, W = 10 mg, V = 20 mL, t = 60 min, and T = 298 K.
In an innovative approach by Soltani and co-workers54, a novel multifunctionalized micro–mesoporous nanosilica KCC-1 (MF-KCC-1) adsorbent was developed through a straightforward PGS method. This powder-type KCC-1 adsorbent, bearing 1-methylimidazolium (MI-Cl-PTES)/tetrasulfide (TESPTS)/amine groups (APTES) (Fig. 2a), revealed a surface area 555 m2 g–1 of and a pore volume of 0.94 cm3 g–1 and demonstrated excellent adsorption capabilities for acid orange II (AO) and acid fuchsin (AF) from water. The pseudo-first-order kinetic and Langmuir isotherm models were found to best represent the experimental data. Under constant conditions (pH=3.0, W = 0.1 g L–1, V = 30 mL, T = 298 K, SS = 180 rpm), MF-KCC-1 exhibited high adsorption capacities of 676.7 mg g–1 and 621.3 mg g–1 for AO and AF, respectively, with a rapid adsorption time of only 20 min. This study showcases the potential of MF-KCC-1 as a highly efficient adsorbent for the uptkae of dyes from contaminated aqueous environments.
Interestingly, it is feasible to grow KCC-1 particles on the surface of magnetic particles, thereby creating fibrous particles with a magnetic core. The benefit of these composite particles lies in the ease of separation after the adsorption process, which is a significant improvement over traditional powder-type adsorbents like KCC-1. Conventional adsorbents typically require separation from aqueous media through time-consuming and laborious processes such as centrifugation or filtration. In contrast, magnetic KCC-1 adsorbents can be efficiently separated by utilizing an external magnetic field, streamlining the process.
However, this innovative approach also presents some challenges. One potential drawback is the decline in the magnetic field’s strength over time, which could impact the efficiency of the separation process. Moreover, magnetic KCC-1 adsorbents possess a greater weight compared to their non-magnetic counterparts due to the presence of a magnetic core. This increased weight could influence the adsorbent dose parameter, necessitating careful consideration of the appropriate adsorbent quantity for optimal performance.
As previously mentioned, AQ-Fe3O4@SiO2@KCC-151 (Fig. 2e) was prepared as a magnetic KCC-1 adsorbent by researchers for adsorption, detection, and removal of Zn(II). The BET surface area, pore size, and pore volume of the adsorbent were reported as 78.8 m2⋅g–1, 7.2 nm, and 0.15 cm3⋅g–1, respectively. Under optimal conditions (pH = 7.2, W = 5 mg, T = 298 K), the maximum Zn(II) removal was determined to be 57 mg⋅g–1 predictively. This research emphasizes the potential of utilizing magnetic KCC-1 adsorbents for effective heavy metal removal in contaminated water.
Zhang et al.55 developed phenylboronic acid-modified magnetic fibrous mesoporous silica (abbreviated as MFMS-P) using a modified oil–water biphasic stratification coating method, resulting in a material with a surface area of 118.2 m2⋅g–1, pore size of 12.7 nm, and good magnetic properties. MFMS-P demonstrated significant potential for catechol removal with an adsorption capacity of 293 μmol⋅g–1.
A recent investigation by Xia et al.56 focused on the development of Fe3O4@DFNS-NH2, a magnetic adsorbent material that demonstrated significant potential for the removal of U(VI) from water. The adsorption capacity of this material reached 255 mg⋅g–1 under optimal conditions (pH = 6, W = 0.4 g⋅L–1, t = 60 min, T = 298 K, and SS = 150 rpm). Notably, this adsorbent exhibited good selectivity for U(VI) even in the presence of competing metal ions, such as Eu3+, Sr2+, Mg2+, Ca2+, Cs2+, and K+.
KCC-1/polymeric composites
KCC-1/polymeric composites are constructed by incorporating KCC-1, either pure or functionalized, within polymer matrices to form hybrid materials. These composites offer synergistic advantages, combining the unique properties of KCC-1 with the versatility and tunability of polymers. Polymers in KCC-1/polymeric composites can be broadly categorized into natural and synthetic polymers. An extensive array of KCC-1/Polymeric Composites, characterized by their innovative integration of fibrous silica spheres with functional polymers, is systematically documented in Table 2, detailing their applications in the realm of water treatment for the elimination of heavy metals and organic dyes, while additionally facilitating a comparative analysis of the various adsorption parameters that govern their performance efficacy.
Natural polymers
Natural polymers, derived from renewable sources such as cellulose, chitosan, alginate, and starch, are environmentally friendly options for composite formation. Their biocompatibility, abundance, and low cost make them attractive candidates for enhancing the adsorption performance and stability of KCC-1-based materials.
In 2019, Zarei et al. conducted a research study in which they developed a novel and environmentally friendly nanocomposite-based adsorbent comprised of triamino-functionalized mesoporous KCC-1 and chitosan-oleic acid (abbreviated as TA-KCC-1/Chi-OLA 5) with 2 and 5 wt.% functionalized KCC-1 nanofiller contents for the adsorption of Pb(II)57. The Langmuir and pseudo-second-order models proved to be the most suitable for predicting the isotherms and kinetics of adsorption, respectively. Under optimal adsorption conditions (pH = 9.0, W = 20.0 mg, V = 30.0 mL, t = 100 min, T = 298 K, initial concentration: 100 mg⋅L–1, SS = 200 rpm), the maximum adsorption capacity of TA-KCC-1/Chi-OLA 5 wt.% was found to be 168 mg g-1 according to the Langmuir model.
In a recent study by Lai et al.58, a chitosan/KCC-1 composite was synthesized utilizing an ultrasound-assisted microemulsion technique for the adsorption of diclofenac. The composite exhibited a surface area of 510 m²/g and a total pore size of 0.492 cm³⋅g–1. Under optimal conditions (pH = 4, W = 150 mg, t = 40 min, ambient temperature, and an initial diclofenac concentration of 160 mg⋅L–1), the adsorption capacity was determined to be 142.01 mg⋅g–1. The Langmuir and Elovich models were identified as the most suitable isotherm and kinetic models, respectively, to describe the adsorption mechanism. This research highlights the potential of chitosan/KCC-1 composites for the effective removal of pharmaceutical contaminants from water sources.
Synthetic polymers
Synthetic polymers, including but not limited to polyvinyl alcohol (PVA), polyethylene glycol (PEG), polyacrylamide (PAM), and polystyrene (PS), offer precise control over composition, structure, and mechanical properties. These polymers can be tailored to meet specific adsorption requirements, such as increased mechanical strength, improved chemical stability, and enhanced selectivity towards target pollutants.
Soltani et al.59 reported in their most recent study a carboxylic acid-functionalized KCC-1/polyamide 6 nanocomposite (COOH-KCC-1/PA6 NC) prepared via a novel ultrasonic-assisted in situ ring-opening polymerization method under organic solvent-free conditions for the adsorption of Cd(II). The composite’s structure, rich in functional groups such as carboxylic, secondary amine, and ketone, offered a promising platform for heavy metal adsorption. Under optimal conditions (pH = 7.0, W = 50 mg, initial concentration = 80 mg⋅L–1, t = 240 min, and T = 298 K), the COOH-KCC-1/PA6 NC demonstrated a Qmax = 109 mg⋅g–1 for Cd(II) removal.
Ershad et al.60 recently developed a polyphenylsulfone membrane modified by KCC-1 (abbreviated as KCC-1-nPr-NH-AcCys) with a surface area of 87.5 m2⋅g–1, pore volume of 1.1 cm3⋅g–1, and mean pore diameter of 4.79 nm. Designed for water treatment, this membrane exhibited excellent performance in heavy metal and dye removal, salt rejection, and pure water flux. The researchers achieved these results by successfully functionalizing the KCC-1 with N-acetylcysteine and incorporating it into the polyphenylsulfone membrane using a phase inversion method.
In 2021, Xia and co-workers conducted a study in which they fabricated a hyper-branched poly(amidoamine) on a KCC-1 substrate, yielding the PAMAM/KCC-1 nanocomposite, with the aim of removing U(VI) from water61. Through EDS and XPS analyses, the adsorption mechanism was elucidated, revealing complexation between amine/amide functional groups and U(VI) ions. Under optimized conditions, specifically, pH = 5, W = 0.5 g⋅L–1, t = 3 h, ambient temperature, and SS = 150 rpm, the adsorbent exhibited an adsorption capacity of 215 mg⋅g–1.
Comparison of KCC-1-based adsorbents and other nanoadsorbents
In the field of nanomaterial-based adsorbents, the search for materials that offer high efficiency, ease of synthesis, and environmental sustainability has gained tremendous momentum. Among these, KCC-1, with its distinctive fibrous morphology and mesoporous structure, has emerged as a particularly attractive material due to its high surface area and tunable properties. However, various other nanomaterials, such as MOFs, COFs, LDHs, ordered mesoporous silica materials (OMSMs), single-layered silica hollow spheres (SLSHS) and multilayered silica hollow spheres (MLSHS), conventional nanoporous carbon materials (such as CNTs, graphene, graphene oxides (GO), reduce graphene oxides (rGO), carbon nitride (C3N4), mesoporous carbon materials, and carbon nanocages) with polymeric and magnetic composites, also exhibit remarkable adsorption capabilities. In this section, we provide a comprehensive comparison between KCC-1-based adsorbents and these nanoadsorbents, focusing on synthesis strategies, material sources, adsorption capacities, cost-effectiveness, and environmental impacts.
Synthesis methods and material sources
MOFs
MOFs comprise porous crystalline materials composed of metal ions or clusters coordinated to organic linkers, and their synthesis methods are as varied and tunable as their structural properties62. The most common synthesis technique for MOFs involves solvothermal or hydrothermal methods, where metal precursors and organic linkers are dissolved in an appropriate solvent and subjected to controlled temperature and pressure conditions, typically resulting in well-defined, highly porous crystals63. In addition to these methods, mechanochemical synthesis—employing minimal solvent through grinding—has gained attention as an environmentally friendly approach, offering similar crystallinity with reduced solvent use. The choice of metal ions, which can range from transition metals such as zinc, copper, and iron, to rare earth elements, plays a critical role in determining the physical and chemical properties of the resulting framework, while the organic linkers, often based on polycarboxylates, phosphonates, or azoles, provide further opportunities for functionalization and structural diversity. Moreover, emerging synthesis techniques such as microwave-assisted methods and electrochemical approaches have been developed to reduce reaction times and energy consumption, thereby addressing some of the scalability challenges associated with traditional synthesis routes. The versatility of MOFs in terms of material source and synthesis method, along with their inherent tunability, has positioned them as prime candidates for a wide array of applications, from gas storage and separation to catalysis and environmental remediation.
Despite the versatility and tunability of MOFs, their synthesis often requires complex procedures involving high temperatures, long reaction times, and the use of hazardous solvents, which can limit scalability and increase environmental concerns. In addition, the cost and availability of certain metal precursors and organic linkers, particularly those involving rare or expensive metals, can pose significant challenges in producing MOFs on an industrial scale.
COFs
COFs represent a unique class of crystalline porous materials constructed through the covalent bonding of organic building blocks, offering high thermal stability, low density, and tunable porosity. The synthesis of COFs is typically achieved through solvothermal or ionothermal methods, where organic monomers such as boronic acids, aldehydes, and amines undergo condensation reactions to form extended 2D or 3D frameworks, with reaction conditions—such as temperature, solvent choice, and catalyst presence—carefully optimized to ensure crystallinity and high yield64,65. In recent years, alternative synthesis approaches, including microwave-assisted and mechanochemical methods, have gained attention for their ability to accelerate reaction times and reduce solvent consumption, aligning with green chemistry principles. The material sources for COFs are largely derived from commercially available or easily synthesized organic molecules, enabling the precise design of frameworks with tailored functionality for applications in gas storage, catalysis, and optoelectronics. However, despite these advantages, COFs suffer from limited scalability due to the sensitivity of their synthesis processes, which often require precise control over reaction conditions to avoid defects and ensure high-quality crystals. In addition, the use of costly organic precursors and the intricate purification steps involved can further hinder the large-scale production and commercialization of COFs.
LDHs
LDHs are a class of anionic clay materials characterized by their unique structure, consisting of positively charged metal hydroxide layers intercalated with anions and water molecules. The synthesis of LDHs typically involves co-precipitation, where metal salts, often divalent and trivalent cations such as Mg²⁺, Al³⁺, or Zn²⁺, are dissolved in an aqueous solution and then precipitated under alkaline conditions, allowing for precise control of metal ratios and the interlayer anions66,67. Other methods, including hydrothermal, sol-gel, and ion-exchange techniques, have been employed to further enhance the crystallinity, morphology, and functional properties of LDHs, enabling their application in areas such as catalysis, drug delivery, and environmental remediation. The material sources for LDHs are relatively abundant and inexpensive, with metal salts and simple inorganic or organic anions serving as readily available precursors, making LDHs attractive for sustainable synthesis and large-scale production. However, one of the major disadvantages of LDHs is the need for precise control over reaction conditions, such as pH and temperature, to avoid phase separation or the formation of undesirable impurities, which can complicate synthesis. Additionally, while the raw materials are inexpensive, scaling up the synthesis of LDHs with high purity and uniformity can be labor-intensive and cost-prohibitive in some applications.
OMSMs
OMSM are an important class of nanostructured materials characterized by their highly ordered pore structures and large surface areas, making them ideal candidates for applications in catalysis, drug delivery, and adsorption68,69. The synthesis of OMSM is primarily achieved through soft-templating methods, where surfactants such as CTAB or block copolymers are used as structure-directing agents to guide the formation of uniform mesopores during the condensation of silica precursors, such as TEOS, under acidic or basic conditions69,70. The removal of the surfactant templates, typically through calcination or solvent extraction, leaves behind a well-defined porous structure. Variations in synthesis methods, including sol-gel and hydrothermal approaches, allow for further control over pore size, morphology, and structural properties, enhancing the versatility of OMSM for a wide range of applications. The material sources for OMSM are generally abundant, with silica precursors derived from inexpensive materials such as silicon alkoxides or even natural sources like rice husk ash, sorghum, sedge, etc offering a sustainable and cost-effective route to synthesis71,72,73. OMSM exhibit less efficient mass transfer due to their rigid, uniform pore structures with long channels, which are prone to pore-blocking phenomena that hinder access to active sites. In contrast, KCC-1’s fibrous morphology avoids such long channels, providing improved mass transfer and better accessibility to active sites, enhancing its performance in adsorption and catalytic applications.
SWSHS and MWSHS
SWSHS (or single-shell SHS) and MWSHS (or multi-shell SHS) are advanced nanostructured materials characterized by their hollow cores and tunable shell thicknesses, which render them highly attractive for applications in drug delivery, catalysis, and adsorption30,74,75. The synthesis of SWSHS is commonly achieved through a templating method, where hard templates like polystyrene or soft templates such as micelles are used to direct the formation of the silica shell, followed by template removal via calcination or solvent extraction, leaving behind a hollow core76,77. For MWSHS, layer-by-layer assembly is typically employed, involving the sequential deposition of silica layers around a core template to produce multiple concentric shells, offering enhanced structural stability and controlled release properties. Both types of hollow spheres utilize silica precursors like TEOS or sodium silicate, which are abundant and cost-effective, while reaction conditions such as pH, surfactant concentration, and temperature are meticulously controlled to ensure uniform shell formation and desired porosity. The versatility of these materials stems from their ability to adjust the wall thickness and porosity by modifying synthesis parameters, making them suitable for various applications requiring high surface area and tailored diffusion properties. However, one of the major disadvantages of both SWSHS and MWSHS is the complexity of their synthesis processes, which involve multiple steps such as template preparation, shell formation, and template removal, increasing both time and energy consumption. Furthermore, the use of hazardous chemicals for template removal and the difficulty in achieving consistent multi-walled structures at large scales present significant challenges in the commercial production of these materials.
Conventional nanoporous carbon materials
Nanoporous carbon materials, encompassing CNTs, graphene, GO, rGO, C₃N₄, mesoporous carbon materials (such as ordered mesoporous carbons (OMCs)), carbon nanofibers, and carbon nanocages, represent a diverse category of nanostructured materials renowned for their exceptional mechanical, electrical, and thermal properties, which facilitate a wide array of applications in energy storage, catalysis, and environmental remediation78,79. The synthesis of CNTs is commonly achieved through methods such as chemical vapor deposition (CVD), where hydrocarbons are thermally decomposed in the presence of metal catalysts, or through laser ablation and arc discharge techniques, which enable the production of high-quality tubes with controlled dimensions and chirality. Graphene is predominantly synthesized via mechanical exfoliation of graphite or chemical reduction of graphite oxide, yielding monolayer sheets that exhibit superior electrical conductivity; GO is synthesized through the oxidation of graphite using strong oxidizing agents, thereby introducing various oxygen-containing functional groups reactive under reducing conditions to form rGO, enhancing its conductivity and versatility. Meanwhile, C₃N₄ is synthesized using techniques such as thermal polymerization of organic precursors, resulting in a polymeric network that can exhibit semiconducting properties. Mesoporous carbon materials are typically produced through template-assisted methods or by carbonizing organic precursors like phenolic resins, followed by activation with gases like CO₂ or steam to create well-defined pore structures, whereas carbon nanocages are often generated via the pyrolysis of MOFs or specific organic templates that decompose to yield hollow spherical structures with tunable porosity. Despite the abundant availability of precursor materials, such as hydrocarbons and organic polymers, the synthesis of nanoporous carbon materials often involve complex, multistep procedures that demand precise control over reaction parameters, such as temperature and time, to achieve the desired structural and functional characteristics. Moreover, the environmental impact associated with the use of toxic reagents during synthesis and the challenges of scaling up production while ensuring consistent quality and reproducibility pose significant barriers to the practical application of these promising nanomaterials.
Magnetic and polymeric composites of the abovementioned materials
Composite forms of nanomaterials, such as OMSM, MOFs, COFs, LDHs, and various carbon-based nanomaterials, when integrated with magnetic nanoparticles and polymers, offer enhanced functional properties that are particularly advantageous for applications in drug delivery, catalysis, environmental remediation, and separation technologies80,81,82. The synthesis of these composites typically involves a multistep process, beginning with the preparation of the core nanomaterial—such as the self-assembly of OMSM or the solvothermal synthesis of MOFs and COFs—followed by the incorporation of magnetic nanoparticles, such as Fe₃O₄, either through co-precipitation or in situ deposition methods, which allows for magnetic responsiveness. Polymer integration, such as with synthetic polymers like polyethylene glycol, polyaniline, Nylon-6 (N6), poly(methyl methacrylate) (PMMA), poly(vinyl alcohol) (PVA), and polystyrene (PS), as well as natural polymers such as chitosan, alginate, cellulose, and gelatin, is often achieved through surface functionalization or grafting techniques, including sol-gel processing, electrostatic adsorption, or covalent bonding70,73,83,84,85,86. These methods are selected depending on the desired interactions between the nanomaterial and polymer matrix, allowing for tailored properties such as enhanced chemical stability, improved mechanical strength, and better biocompatibility in specific applications.
These polymer coatings not only enhance the chemical stability and mechanical properties of the composite but also improve biocompatibility and dispersibility in aqueous media. In addition, the material sources for these composites are relatively accessible, with silica, carbon, and organic linkers being readily available, while magnetic nanoparticles and polymers can be synthesized using cost-effective, scalable methods.
Despite their functional advantages, one of the main disadvantages of these nanocomposites is the complexity of the synthesis process, as it often requires multiple steps, precise control of reaction parameters, and post-synthesis modification, all of which contribute to higher production costs and time. Furthermore, the environmental impact of producing such composites, particularly due to the use of hazardous solvents and the energy-intensive nature of the synthesis, poses significant challenges to the sustainable large-scale manufacturing of these advanced materials. In addition, the magnetic properties of these composites can degrade over time due to oxidation or agglomeration of the magnetic nanoparticles, which diminishes their long-term effectiveness, requiring additional stabilization measures to maintain functionality over extended periods.
Adsorption capacity
Table 3 presents the adsorption capacities and optimal adsorption conditions for several well-known families of nanoporous materials reported in recent years. The adsorption capacities of various adsorbents such as MOFs, COFs, LDHs, OMSM, MWSHS, SWSHS, and carbon-based nanomaterials (like OMC) differ significantly, largely due to inherent factors such as surface area, pore size, pore volume, types of functional groups, and their affinity for specific adsorbates. Environmental factors such as pH, temperature, and the concentration of competing ions also play crucial roles in determining their adsorption performance. Typically, modern materials like MOFs and COFs exhibit superior adsorption capacities, reaching thousands of mg g–1, as a result of their highly tunable surface chemistry and large surface areas. In contrast, traditional adsorbents like LDHs have comparatively lower capacities but can be significantly enhanced by combining them with superabsorbent materials like MOFs, as demonstrated by the research of Soltani’s group15,22,26,87,88. KCC-1-based adsorbents, Tables 1 and 2, exhibit adsorption capacities that vary from tens to thousands of mg g–1, depending on their functionalization and the specific adsorbates involved.
When comparing these materials with respect to adsorption time (t), adsorbent dose (W), and pH, a clear trend can be observed. Generally, adsorption times for many materials, including MOFs and carbon-based nanomaterials, are relatively short, often under 60 min, due to their high surface area and efficient pore structures, while LDHs and OMSM composites tend to have slightly longer adsorption times. In addition, the pH and adsorbent dose required for optimal performance differ significantly across materials. For instance, LDH/MOF composites show improved adsorption capacities at neutral pH values compared to LDHs alone. Although OMSMs and MWSHS composites also display promising performance in heavy metal and dye adsorption, their adsorption capacity remains lower than that of modern adsorbents like COFs. Overall, the incorporation of LDHs with more advanced materials, such as MOFs or polymers, has been shown to dramatically increase their adsorption capacity, providing a clear advantage in practical applications.
Cost considerations
The cost considerations for synthesizing nanoadsorbents are critical in determining their commercial viability, particularly for large-scale environmental applications. MOFs and COFs, while highly efficient in adsorption due to their tunable porosity and surface functionalization, face significant cost barriers. The organic linkers used in their synthesis are often expensive, and the synthesis methods, such as solvothermal and microwave-assisted processes, require specialized equipment and high-energy input, driving up production costs. This has limited their widespread adoption despite their excellent adsorption capacities for heavy metals and organic dyes.
Similarly, carbon-based nanomaterials, including SWCNTs and MWCNTs, necessitate complex synthesis equipment like CVD systems, which further escalate their production expenses. These materials, although highly efficient, are costly to produce at scale, restricting their practical use in cost-sensitive applications such as large-scale water purification.
In contrast, materials such as OMSM, LDHs, and SWSHS and MWSHS offer more economic alternatives. These materials are synthesized from relatively inexpensive precursors, such as TEOS for silica-based materials and inexpensive metal salts for LDHs. In addition, their synthesis methods, including sol-gel and co-precipitation techniques, are less energy-intensive and do not require sophisticated equipment, making them more feasible for mass production. KCC-1-based adsorbents, particularly those synthesized from bio-based silica sources like rice husk ash, are also emerging as cost-effective alternatives due to the abundant availability of raw materials and simple synthesis methods.
To further reduce production costs, many researchers are incorporating polymers into nanomaterial composites. This approach not only lowers the cost by using cheaper polymeric matrices but also enhances the flexibility and applicability of the adsorbents, making them suitable for use as membranes or flexible adsorbents. In addition, polymeric composites allow for easier separation of adsorbents from aqueous solutions, as opposed to powder-type nanomaterials, which often require complex centrifugation or filtration steps. However, while polymer integration reduces costs and improves ease of use, it generally results in a decrease in adsorption capacity compared to pure nanomaterials, as the polymers can reduce the number of active sites available for adsorption. Nevertheless, this trade-off between cost and performance makes polymer-nanomaterial composites attractive for applications where moderate adsorption capacities are sufficient, and cost-effectiveness is prioritized.
Environmental impact
The environmental impact of nanoadsorbents is a critical consideration, particularly given the increasing demand for sustainable and green materials in environmental applications. While there are reports of environmentally friendly synthesis methods for materials like MOFs, COFs, SWCNTs and MWCNTs, many of these materials are still synthesized using toxic solvents, hazardous chemicals, and energy-intensive processes that do not align with green chemistry principles. For instance, MOFs and COFs often require organic solvents like DMF, dioxane, THF, DMSO, xylene, or toluene, which are harmful to both human health and the environment, and their production frequently involves high temperatures and pressures, increasing their carbon footprint. Similarly, the synthesis of CNTs typically involves methods like CVD, which, while efficient, demands substantial energy input and can generate byproducts that are difficult to manage.
In contrast, materials such as LDHs and MSM, including KCC-1, are generally more environmentally friendly. LDHs can be synthesized through co-precipitation methods that require milder conditions and aqueous solutions, reducing the need for toxic reagents. MSMs, particularly those synthesized from bio-based or agricultural waste sources, such as rice husk, sorghum, sedge, or diatoms, offer an even greener alternative. The use of biogenic silica precursors minimizes waste, energy consumption, and environmental toxicity, making these materials more sustainable for large-scale applications.
However, despite these advances, the field of nanomaterials still faces significant challenges in eliminating the use of toxic chemicals during both the synthesis and functionalization stages. For example, many functionalization processes—whether for improving adsorption capacity or tailoring surface properties—still rely on harmful solvents or reagents, posing risks to both environmental and occupational safety. As the field progresses, it is the duty of environmental chemists and materials scientists to develop greener, more sustainable synthesis approaches that reduce these risks. This includes not only minimizing the use of toxic substances but also improving energy efficiency and incorporating renewable or bio-based materials into the design of nanoadsorbents. Such advancements are essential for making nanomaterials a truly sustainable solution for environmental remediation and water treatment applications.
Synthesis complexity
The synthesis complexity of nanoadsorbents varies significantly based on the type of material and the methods employed. MOFs and COFs are known for their highly customizable structures, but their synthesis often requires precise conditions, including high temperatures and pressures in solvothermal or microwave-assisted systems, along with expensive organic ligands and catalysts. The multistep processes involved in controlling crystallinity, porosity, and surface functionalization further add to the complexity. Similarly, the synthesis of carbon-based nanomaterials, such as SWCNTs and MWCNTs, involves intricate procedures like CVD, which require sophisticated equipment and careful control of gas flows, temperatures, and catalysts to achieve uniform structures.
In contrast, LDHs, OMSM, and SWSHS and MWSHS are less complex to synthesize. These materials can be produced using more straightforward methods such as co-precipitation and sol-gel processes, which allow for easier scalability. However, controlling the uniformity of pore sizes and maintaining high surface areas still pose challenges, especially in large-scale production. KCC-1-based adsorbents, with their fibrous morphology, offer relatively simpler synthesis routes, particularly when bio-based silica sources are used, reducing the need for complex reagents and equipment.
Despite the reduction in synthesis complexity for some materials, creating functional composites with polymers or magnetic nanoparticles adds additional steps, including surface modification, polymer grafting, and magnetic incorporation, which require careful optimization. Thus, while simpler than MOFs and COFs, these materials still involve multistep processes to achieve the desired functional properties. Overall, the synthesis complexity depends on balancing precise control of material properties with scalability and cost-effectiveness.
Cost, scalability, and environmental impact of KCC-1-based adsorbents
In this section, we explore the cost implications, scalability potential, and environmental considerations of KCC-1-based adsorbents, with a focus on improving their sustainability, reducing production costs, and enhancing scalability, based on insights from recent literature.
The cost of producing KCC-1-based adsorbents remains relatively low compared to advanced nanomaterials like MOFs and COFs, largely because of the abundant availability of silica. Silica, as one of the most prevalent elements in the Earth’s crust, can be sourced from affordable synthetic precursors such as alkoxy silanes (e.g., TEOS) or sodium silicate. A more sustainable approach involves using bio-based silica from agricultural waste, such as rice husk ash, wheat straw, sugarcane bagasse, or sedge. These renewable sources not only reduce the overall cost but also align with the principles of green chemistry by repurposing waste materials that would otherwise be discarded. For example, studies have shown that rice husk ash contains up to 90% silica, making it a highly efficient and eco-friendly source for KCC-1 synthesis. However, the challenge lies in the functionalization process, where synthetic agents like organosilanes are used. These agents often rely on toxic reagents and complex procedures, which increases both costs and environmental impact. Research should focus on developing bio-based or greener functionalization methods, such as using naturally derived functional groups or more environmentally friendly solvents, to further reduce costs and improve the sustainability of KCC-1 adsorbents.
KCC-1-based adsorbents are also promising for large-scale production due to their relatively simple synthesis processes compared to more complex nanomaterials. Methods such as sol-gel and hydrothermal techniques are well-established and require less energy and fewer resources than the synthesis of MOFs or COFs, which often involve intricate control over reaction conditions and expensive ligands. Large quantities of KCC-1 have been successfully synthesized while maintaining consistent quality, which is crucial for applications in water treatment and environmental remediation. For instance, pilot-scale production of KCC-1 has already been achieved using a 20-liter reactor, producing up to 1 kg per batch89. Despite these advancements, scalability at an industrial level is limited by the use of organic solvents like cyclohexane and toluene, which are not only costly but also pose environmental hazards. To overcome these limitations, developing solvent recovery systems to reuse these solvents during synthesis could drastically reduce both costs and environmental impact. In addition, greener solvents or even solvent-free synthesis methods could be explored, which would further enhance the scalability of KCC-1 production.
The environmental impact of KCC-1-based adsorbents, while generally more favorable than MOFs or CNTs, still has room for improvement. The use of volatile organic solvents like cyclohexane and toluene in the preparation and functionalization stages is a significant concern, as these chemicals are toxic and contribute to environmental pollution. Reducing or eliminating these solvents is essential for making the KCC-1 production process greener. Research has already shown that solvent recovery methods can help minimize environmental impact, allowing solvents like toluene and cyclohexane to be recycled and reused in subsequent production cycles. Moreover, using alternative methods for template removal, such as acid extraction, could eliminate the need for energy-intensive calcination processes, which would further reduce the carbon footprint of KCC-1 production. By continuing to explore more eco-friendly synthesis techniques and reducing the reliance on toxic chemicals, KCC-1-based adsorbents can become an even more sustainable and cost-effective solution for large-scale environmental applications.
Future research directions and emerging applications
KCC-1 and its functionalized derivatives hold immense promise as versatile materials for water remediation, catalysis, drug delivery, and other advanced applications. The unique fibrous structure of KCC-1, characterized by its high surface area, tunable porosity, and ease of functionalization, provides an ideal platform for developing efficient adsorbents. In water treatment, functionalized KCC-1 demonstrates effective adsorption for a range of pollutants, including dyes and heavy metals, due to the enhanced interaction sites provided by surface modifications. Emerging applications such as the removal of pharmaceutical pollutants, microplastics, and persistent and complex contaminants like per- and polyfluoroalkyl substances (PFAS) are particularly promising. For these pollutants, functionalized KCC-1 derivatives can be tailored to exhibit selective interactions through hydrophobic, electrostatic, or hydrogen-bonding mechanisms, thus broadening KCC-1’s scope and utility in addressing contemporary water pollution challenges.
Despite these advantages, there are several challenges that must be addressed to optimize KCC-1’s functionality for specific applications. Achieving consistent functionalization without compromising structural integrity, for instance, remains a key hurdle, particularly when dealing with pollutants requiring different adsorption mechanisms. For instance, microplastics, due to their large size and hydrophobic nature, might necessitate unique surface chemistries that differ from those needed for heavy metal or dye removal. Similarly, capturing PFAS requires highly selective and robust interactions, as these compounds are resistant to conventional removal methods. Beyond adsorptive applications, KCC-1’s potential as a catalyst or drug delivery agent could be further enhanced by refining functionalization techniques to improve stability, control release kinetics, and maintain biocompatibility.
Future studies should focus on developing more efficient functionalization methods that can diversify KCC-1’s applications without compromising its porosity or stability. A comprehensive understanding of pollutant-specific interactions and the development of multi-functional KCC-1-based materials capable of simultaneous adsorption and catalytic degradation could lead to breakthrough technologies in water remediation and environmental management. Addressing these challenges will be critical for expanding KCC-1’s role in sustainable applications, allowing it to effectively respond to evolving environmental and biomedical demands.
Conclusion
In conclusion, as global water scarcity continues to mount, innovative water treatment technologies are essential in safeguarding the environment and preserving human health. This comprehensive review offers a deep insight into the remarkable potential of fibrous silica spheres, notably KCC-1, as an effective adsorbent for the removal of heavy metals and organic dyes from polluted water sources. KCC-1’s unique fibrous morphology, high surface area, and versatile surface modification techniques, including one-pot synthesis and post-grafting synthesis, offer significant advantages over traditional adsorbents. Our systematic exploration of KCC-1’s synthesis methods, properties, and modifications highlights the impressive progress in this field while underscoring the need for further research and development.
Despite the notable advancements in KCC-1-based adsorbents, there is still a pressing demand for better understanding its properties and optimization techniques to maximize efficiency and applicability in real-world scenarios. Future studies could investigate modifications that enhance KCC-1’s selectivity and adsorption capacity for pharmaceuticals, which often require tailored interactions due to their diverse chemical structures. In addition, research could explore methods for capturing microplastics by leveraging KCC-1’s functional groups, which could be tuned to facilitate hydrophobic and electrostatic interactions essential for microplastic removal.
This review serves as a perspective towards fostering a more profound and comprehensive understanding of the significance and potential of KCC-1 in addressing contemporary water pollution challenges. By consolidating recent progress and identifying research gaps, we aim to guide future studies and facilitate the development of sustainable water treatment solutions. As the global demand for freshwater continues to surge, KCC-1-based adsorbents hold immense promise as an integral part of a multifaceted approach to address water pollution and ensure the sustainable management of our vital water resources.





Responses