A novel simplified structural design as an artificial enzyme for efficient hydrolysis of PNPA

Results
Eevaluation of catalytic efficacy of Zn(II)-SMM
p-nitrophenyl acetate (PNPA) was selected as a typical substrate for the evaluation of catalytic efficacy of Zn(II)-SMM. Notably, the slope of reaction rates increased significantly with rising concentrations of PNPA from 20 µM to 100 µM compared to spontaneous reactions under identical conditionsFig. . 2b and Figure S3), indicating that Zn(II)-SMM exhibited unexpectedly high activity for hydrolysis of PNPA hydrolysis in mixtures of HEPES-DMSO and an incremental rate of hydrolysis.
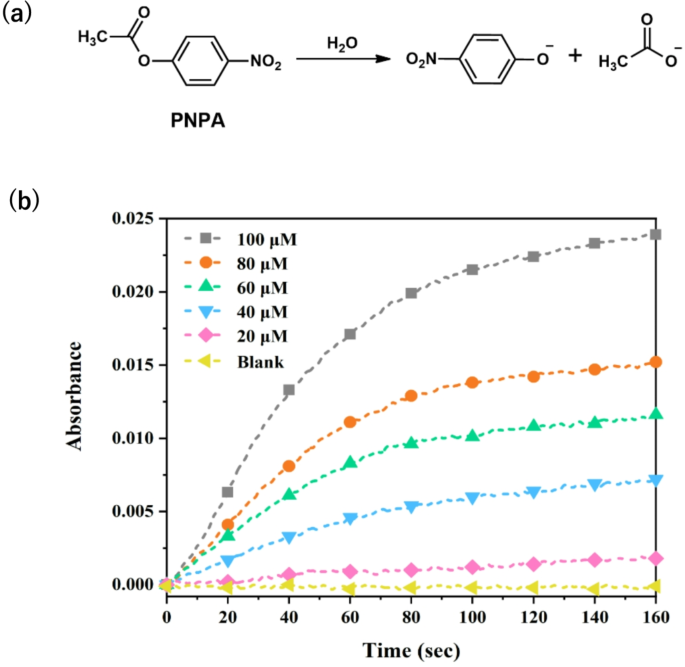
(a) The hydrolysis reaction for the substrate p-nitrophenyl acetate (PNPA). (b) Plots of absorbance vs. time at 400 nm in HEPES of different concentrations for substrate PNPA assisted by 10 µM Zn(II)-SMM in a DMSO/ H EPES mixture (20 : 80 v/v) at 25 ◦C and pH 7.0, Blank: DMSO / HEPES mixture (20 : 80 v/v) at 25 ◦C and pH 7.0 .
Even upon introducing tenfold excess substrate amounts, catalytic hydrolysis consistently concluded within approximately sixty seconds—highlighting the remarkably high catalytic efficiency of this enzyme system (Fig. 2). The apparent rate constant Kobs was determined by varying concentrations of Zn2+ (Fig. 3), Kobs gradually increased until reaching a molar ratio of Zn2+ to SMM at 1:1—suggesting that the hydrolase complex Zn(II)-SMM comprised one mole each of Zn2+ and SMM.
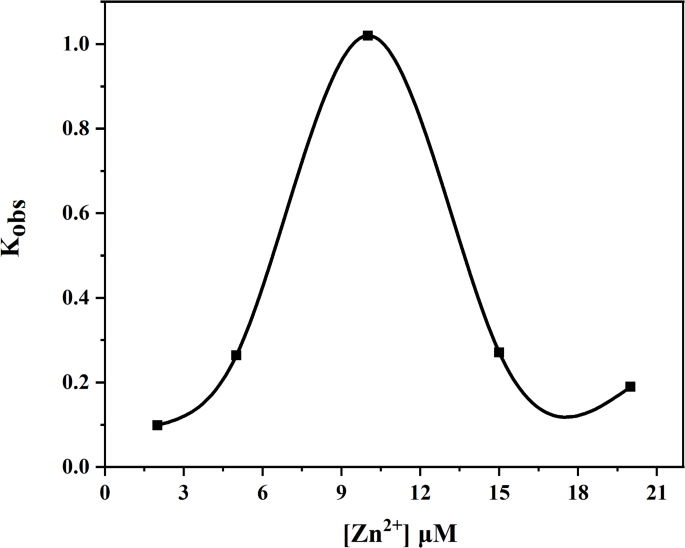
Plots of Kobs for the hydrolysis of PNPA (50 µM) by Zn(II)-SMM (10 µM) in DMSO/HEPES mixture (20 : 80 v/v) with the increase concentration of Zn2+ at 25 ◦C and pH 7.0.
Computational insights
To elucidate the binding mechanism of SMM with Zn2+, energy simulation calculations were conducted. The energy simulations reveal that Zn2+ adopts a characteristic planar structure involving acyl oxygen and nitrogen atoms, achieving maximum stability in a tetrahedral configuration with the nitrogen atom on pyridine Fig. 1). This configuration is energetically favored by 99.62 kJ/mol compared to alkyl-oxygen coordination (Figure S2), utilizing the b3lyp/6–31 g(d, p) method via Gaussian software.
UV-vis monitoring for catalytic effects
The primary architecture of the hydrolase was engineered using a quinoline derivative, facilitating the proximity of PNPA with analogous organic structure and interaction with metal ions. In this study, the hydrolysis of PNPA yielded phenol, which exhibited UV absorption at 400 nm29. To gain deeper insights into the catalytic effect, UV monitoring experiments were conducted (Figure S1). The results indicated that phenol concentration increased over time; however, no hydrolysis occurred with the removal of SMM (Figure S3), implying that Zn(II)-SMM could associate with the substrate and promote the hydrolysis of it.
Kinetics
The kinetics of catalytic hydrolysis of Zn(II)-SMM on PNPA were investigated in detail to elucidate the behavior of the catalytic hydrolysis. The experimental findings showed a characteristic double reciprocal plot and saturation kinetics curve (Figure S4), confirming that the catalytic activity adheres to the Michaelis-Menten Eq. 30, thereby indicating enzyme-like characteristics of the Zn(II)-SMM complex. The calculated kinetic parameter value was 0.8287 min-1 (Kcat), and the hydrolysis rate is 5239 times greater than that of the spontaneous reaction.
Mechanism and pathway
The hydrolysis rate constant of Zn(II)-SMM system was evaluated across various pH levels, and the results (Figure S5) indicated that following the activation of Lewis acid within the system, the monohydroxyl form of the metal ion complex acted as an effective catalyst for.
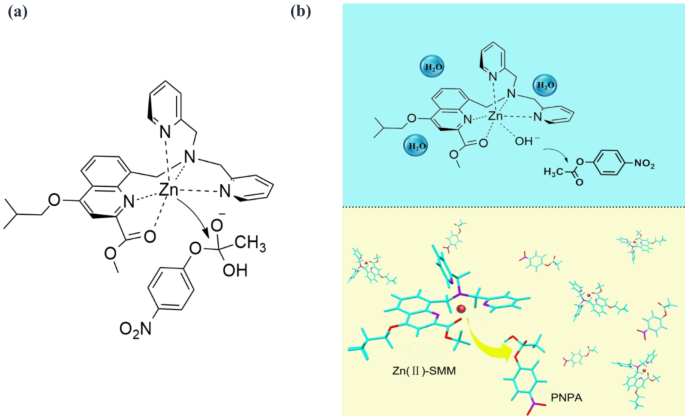
(a) Mechanism of PNPA hydrolysis catalyzed by SMM. (b) Schematic representation of the hydrolysis mechanism for PNPA.
PNPA hydrolysis. Under acidic conditions, the pyridine moiety of the Zn(II)-SMM complex readily.
associates with H+, hindering substrate access and resulting in infrequent hydrolysis events. Conversely, as pH increased, deprotonation of the Zn(II)-SMM complex allowed water to coordinate with Zn2+,
forming a Zn-OH bond that facilitates nucleophilic attack on PNPA, thereby accelerating its hydrolysis.
The proposed hydrolysis mechanism of PNPA under neutral conditions is also illustrated in Fig. 4. Here, the catalytic site of Zn-OH functions as either a general base or nucleophile to activate the C-O carbonyl bond and generate a transition state conducive to cleavage of this bond and subsequent release of p-nitrophenol, the mechanism is hypothesized to align with that of single Lewis acid activation31,32,33.
Discussion
A novel small molecular organic complex capable of coordinating with Zn2+ has been designed, which effectively binds and catalyzes the hydrolysis of the substrate PNPA. This system has been proved to own the ability to mimic the catalytic hydrolysis function of metal hydrolases, and the catalytic rate (kcat/kuncat) exceeds 5239 times that of non-catalytic systems. Furthermore, saturation kinetics studies indicate that the catalytic process of the Zn(II)-SMM complex adheres to the Michaelis-Menten equation, identical to natural enzymes. The hydrolysis mechanism employed by this engineered metal hydrolase is characterized as a single Lewis acid activation. The results of this work elucidates that the artificial metal hydrolase Zn(II)-SMM system is a promising candidate for the design of small molecule hydrolases, and it is believed that this facile route to construct metal hydrolases provides a convenient path for catalytic research, most importantly, it lays the foundation for the further pursuit of our research on chiral metallohydrolase.
Methods
Materials and physical measurements
All chemicals including o-toluidine, bis (pyridin-2-ylmethyl) amine etc. and solvents were of pure analytical grade and obtained commercially from aladdin company. The synthesized small molecule-based metallohydrolase (SMM) prepared via organic synthesis method were characterized through 1 H NMR, 13 C NMR, and MALDI-TOF-MS.
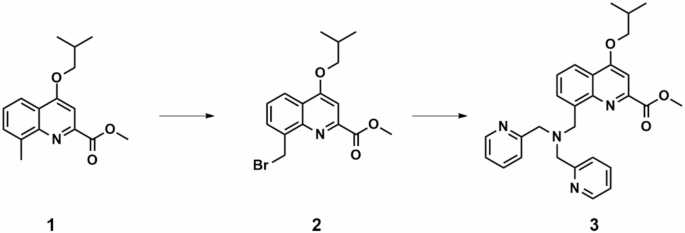
Synthesis of SMM compound and characterizations
Synthesis of compound 2
Compound 1(10 g, 36.6 mmol)), NBS (6.83 g, 38.4 mmol, 1.05 eq) and BPO (9.3 g, 38.4 mmol, 1.05 eq) were dissolved in 100 mL of benzene at 80 °C. The mixture was refluxed for 6 h. the organic solvent was then removed under reduced pressure, the residue was washed with dichloromethane and water. The product was purified by column chromatography with 200–300 mesh silica gel column. The collected product was dried under reduced pressure to give a white solid (9.8 g, 76% yield).
Synthesis of compound 3
Compound 2 (700 mg, 2.06 mmol), 2,2’ -dimethylpyridinolamine (0.82 g, 4.12 mmol), potassium carbonate (1.13 g, 8.23 mmol), Anhydrous DMF(10 mL) was mixed and stirred for 1.5 h, then diluted with 1 M HCl, and washed with AcOEt, the organic phase was dried with MgSO4, and the solvent was removed in vacuum, the target compound 3 (773 mg, 65% yield) was purified by column chromatography ( silica gel, CHCl3/ MeOH ).




Responses