A survey-based, quasi-experimental study assessing a high-cannabidiol suppository for menstrual-related pain and discomfort

Introduction
The endocannabinoid system (ECS) is involved in a variety of physiological and psychological processes, including many impacting the gynecological system1. Within the ECS, the endogenous cannabinoids 2-arachidonoyl glycerol (2-AG) and anandamide (AEA) interact with cannabinoid receptors (i.e., CB1R, CB2R), other G protein-coupled receptors, and degradative enzymes (e.g., fatty acid amino hydrolase [FAAH] and monoacylglycerol lipase [MAGL])2. Exogenous cannabinoids also impact ECS function, including Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), the primary intoxicating and non-intoxicating constituents of the cannabis plant, respectively.
Historically, cannabis has been used to alleviate gynecologic conditions and symptoms, including menstrual-related pain and discomfort (e.g., dysmenorrhea)3. Dysmenorrhea is common, with reported prevalence ~71–91% and rates of severe pain as high as 29%4,5, causing significant deleterious effects on quality of life, productivity, and general health. The ECS plays a significant role in the menstrual cycle, with endogenous cannabinoids, cannabinoid receptors, and degradative enzymes found throughout the hypothalamic-pituitary-ovarian axis, fallopian tubes, and uterus1,6,7. Evidence suggests uterine expression of ECS components is modulated by estrogen8. Further, the ECS may also be involved in controlling myometrium contractility; one study demonstrated rodent myometrial concentration of 2-AG and AEA was lower during the oestrus versus dioestrus phase, and administration of a CB1R agonist or FAAH inhibitor (and to a lesser extent a CB2R agonist or MAGL inhibitor) reduced prostaglandin-mediated contractions from dioestrus tissue9. Additionally, estrogen administration in ovariectomized rats yielded anxiolytic and antidepressant-like effects which were modulated by the ECS10.
Additionally, menstrual cycle-dependent endocannabinoid gene expression has been observed in human endometrial tissue11 and is impacted by gynecological issues. A study of healthy, regularly-cycling individuals reported maximal expression of endocannabinoid enzymes during the luteal phase12, whereas a study of individuals with common benign menstrual-related conditions (menorrhagia, leiomyomata, benign adnexal mass, pelvic pain, and cervical intraepithelial neoplasia) reported maximal enzymatic expression during menstruation13. Further, CBR expression may be disrupted in several gynecological conditions including endometriosis14 and adenomyosis15, with altered receptor expression associated with greater severity of dysmenorrhea15. CBR polymorphisms have also been associated with polycystic ovary syndrome (PCOS)16.
ECS involvement in both natural and pathological gynecological functions underscores the potential for cannabinoid-based therapies17,18. In particular, cannabinoid-based therapies may help alleviate menstrual-related symptoms such as pain/discomfort, the impact of pain on daily function, and the need for analgesics to manage pain as well as non-pain symptoms such as issues with concentration, emotion regulation (e.g., mood, anxiety), autonomic reactions (e.g., nausea), water retention (e.g., bloating), and energy19. Both THC and CBD hold promise for addressing pain and inflammation associated with dysmenorrhea19, endometriosis20,21,22, and chronic pelvic pain23, although clinical trial data are lacking. However, observational, longitudinal studies report that medical cannabis (MC) use is associated with improved anxiety, mood, sleep, and pain across various medical conditions24,25,26,27,28,29,30,31. Additionally, surveys assessing MC use for symptoms of premenstrual syndrome (PMS)/premenstrual dysphoric disorder (PMDD)32, endometriosis33,34, chronic pelvic pain35,36, and menopause37 found significantly improved self-reported pain, cramping/muscle spasms, mood, anxiety, sleep, gastrointestinal issues, and quality of life. Further, MC use may help reduce conventional medication use, particularly analgesics26,33, a commonly used class of medications for those experiencing menstrual pain or discomfort.
The current study aimed to expand previous findings via a survey-based, quasi-experimental study assessing pro re nata (PRN) use of a commercially-available, hemp-derived, broad-spectrum, high-CBD (100 mg) vaginal suppository (Foria®) for menstrual-related pain and discomfort over two months (~2 menstrual cycles) compared to a treatment-as-usual (TAU) control group (i.e., no use of CBD suppositories). Primary hypotheses predicted the CBD group would demonstrate significantly improved menstrual-related symptoms, daily functioning, and reduced need for analgesics during premenstrual and menstrual phases relative to the TAU group. Secondary hypotheses predicted that increased suppository use would correlate with greater reduction of menstrual-related symptoms.
Methods
This study was approved by the Mass General Brigham (MGB) Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Participants were recruited through unpaid social media ads (e.g., Facebook, Twitter, Reddit), the study sponsor’s website, and Rally, the MGB online recruitment platform. In addition, the study sponsor conducted targeted email campaigns using their own customer database. Prior to starting study procedures, participants were given information about the study and provided voluntary consent. Study enrollment was conducted between December 2019-January 2022, using voluntary response sampling to generate a non-probability sample. Inclusion criteria included: assigned female at birth; aged 18–55; endorsed menstrual cycling (even if irregular); and endorsed menstrual-related pain and/or discomfort. Exclusion criteria included: current use (≥1×/month) of vaginal suppositories or other products from the study sponsor (Foria®) at baseline. Monetary compensation for participation was not provided.
This quasi-experimental study was conducted as a national survey comprised of online questionnaires at baseline and two monthly follow-ups (i.e., ~2 menstrual cycles). After completing baseline surveys, participants were given information regarding how to request CBD suppositories from the manufacturer, if they were interested. Using the CBD suppositories was not required for study participation. Interested participants contacted Foria® directly to obtain hemp-derived, broad-spectrum, high-CBD (100 mg) vaginal suppositories at no cost. Information about the suppository and guidelines for use were provided by the study sponsor and included a recommendation for use 1–2× per day as needed. General information regarding product specifications and frequently asked questions were also provided on the sponsor website38; the research team did not have any direct clinical contact with study participants. Participants used suppositories at their own discretion; those who reported suppository use were included in the CBD group, while those who did not add CBD suppositories to their typical treatment regimen were included in the TAU group. Therefore, treatment group designation was based entirely on individuals’ personal choice to use the CBD suppositories or not.
Clinical assessments
Following screening questions to determine eligibility, consenting participants were directed to a survey consisting of self-report questionnaires administered via REDCap39,40. During baseline assessments, participants completed questions about demographics and menstrual-related conditions and symptoms. At all timepoints, participants answered comprehensive questions about their menstrual cycle and cannabis/cannabinoid use (if any). Menstrual-related scales included the Numerical Rating Scale (NRS)41 for Menstrual Pain, Verbal Multidimensional Scoring System Assessment of Dysmenorrhea (VMS)42, Retrospective Symptom Scale of Dysmenorrhea (RSS)43, Menstrual Symptom Questionnaire (MSQ)44, and Menstrual Distress Questionnaire (MDQ)45,46.
On the NRS, participants rated their average pain and worst pain during their last period on a scale from 0 (no pain) to 10 (worst imaginable pain). The VMS assesses the impact of menstrual symptoms on daily life, including ability to work, requirement of analgesics, and experience of systemic symptoms (e.g., nausea, fatigue, lightheadedness), and is rated from 0 (none/no impact) to 3 (severe/great impact). The RSS rates overall frequency and severity of 18 menstrual-related symptoms during participants’ last cycle from 0 (did not occur/not noticeable) to 4 (lasted several days/very severely bothersome). Two items querying menstrual-related additional hours spent in bed and number of analgesic pills were also included. The MSQ assesses 12 spasmodic and 12 congestive symptoms rated by frequency from 1 (never) to 5 (always). Spasmodic symptoms refer to spasms of pain typically beginning the first day of menstruation. Congestive symptoms typically occur during the premenstrual phase with dull, aching pains accompanied by lethargy and depression. The MDQ examines 41 symptoms categorized into 7 subscales: Pain (e.g., cramps, headaches), Concentration (e.g., forgetfulness), Behavioral Changes (e.g., avoiding activities), Autonomic Reactions (e.g., nausea/vomiting), Water Retention (e.g., swelling), Negative Affect (e.g., anxiety, depression), and Arousal (e.g., sexual desire, energy). Symptoms are rated by severity and impact from 0 (not experienced) to 5 (very severe, disabling). For this study, MDQ ratings were collected for both the premenstrual phase (1 week before menstruation) and menstruation.
At both follow-up surveys, participants completed questionnaires related to suppository use. Those who used suppositories also completed the Patient Global Impression of Change (PGIC)47 scale to assess perceived change since beginning use, rated 1 (no change or condition has gotten worse) to 7 (a great deal better and a considerable improvement that has made all the difference).
Statistical analyses
Participants with data from at least one follow-up survey were included in the final analyses: baseline (CBD n = 77; TAU n = 230), follow-up 1 (CBD n = 76, TAU n = 230), and follow-up 2 (CBD n = 44, TAU n = 92; Fig. 1). Rates of completing all survey assessment measures were high (follow-up 1 = 83.0%; follow-up 2 = 93.4%), but linear mixed model (LMM) analyses with first-order autoregressive covariance structures were conducted to best address missingness.
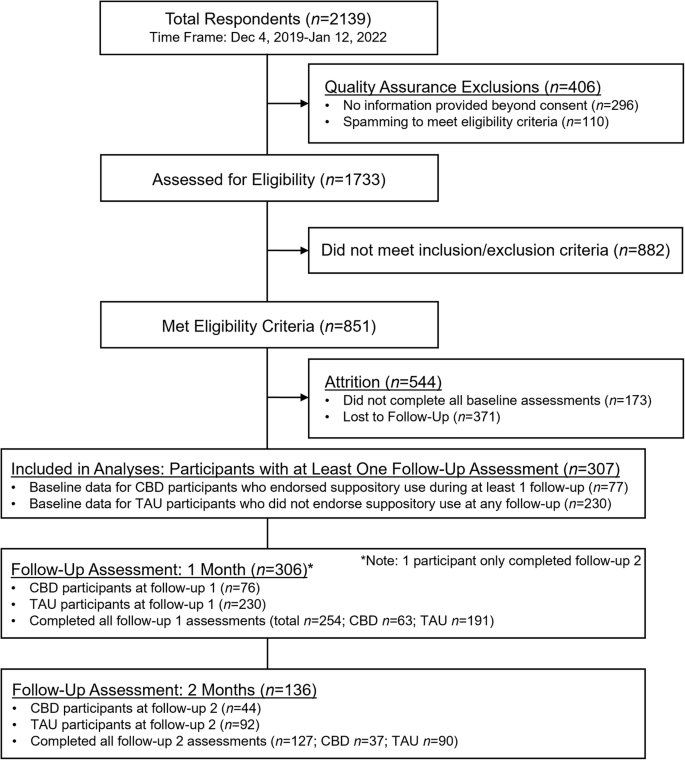
CONSORT flow chart of enrollment for this survey-based, quasi-experimental study examining use of a high-cannabidiol (CBD) vaginal suppository for menstrual pain and discomfort relative to a treatment-as-usual (TAU) control group.
LMM analyses (2 × 3) assessed the effects of treatment group (CBD vs TAU), time (baseline, 1 month, and 2 months), and the group*time interaction. Additionally, analyses of variance (ANOVAs) assessed between-group differences for baseline variables. For all LMM analyses demonstrating significant group*time interactions, post hoc ANOVAs compared the treatment groups at each follow-up timepoint. Within the CBD group, Pearson’s correlations were conducted for variables with significant group*time interactions to assess whether suppository use (i.e., number used) at follow-up 1 was associated with percent change of menstrual scales from baseline to follow-up 1. All statistical analyses were two-tailed (α ≤ 0.050) and conducted using SPSS v28. Corrections for multiple comparisons were conducted by categorizing all subscales into 8 unique domains of menstrual-related symptoms (Pain, Impact on Life, Concentration, Autonomic Reactions, Water Retention, Negative Affect, Arousal, General Symptoms) and correcting for multiple comparisons within each domain.
Results
Baseline characteristics
The treatment groups were well-matched for most demographic variables with no significant differences for age, race, income level, education level, or current cannabis use (Table 1); although, the CBD group had significantly fewer Hispanic participants (p = 0.020). At baseline, the two groups reported similar menstrual cycle length and average flow, but the CBD group endorsed significantly worse menstrual symptoms than the TAU group (Supplementary Table 1). Specifically, participants in the CBD group reported significantly more severe ratings on the VMS (Effect on Work p = 0.009); RSS (Frequency p = 0.012, Severity p = 0.024, Hours in Bed p = 0.050, and Number of Analgesic Pills p = 0.025); and MDQ Menstrual Symptoms (Pain p = 0.034 and Behavioral Changes p = 0.033). Additionally, the CBD group reported significantly greater frequency of menstrual/gynecological conditions and symptoms relative to the TAU group (Supplementary Table 2), including dysmenorrhea (p = 0.007) and menorrhagia (i.e., heavy or prolonged menstrual bleeding; p = 0.043).
CBD suppository use
CBD participants reported using ~3 suppositories over the previous month at both follow-ups (Table 2). Suppository use was most common during menstruation followed by the premenstrual phase. At both follow-ups, the CBD group reported a median PGIC rating of 5, “moderately better, and a slight but noticeable change,” and most participants using suppositories endorsed moderate improvement or better (PGIC ≥ 5) since beginning use (follow-up 1 = 72.9%, follow-up 2 = 81.1%).
Changes in menstrual symptoms
LMM analyses demonstrated significant main effects of time, indicating significant reductions in menstrual symptoms over time (Tables 3 and 4). Most importantly, LMM analyses also revealed several significant group*time interactions, indicating the CBD group had significantly greater improvement of menstrual symptoms over time relative to the TAU group (Figs. 2 and 3). Significant interactions were observed for the VMS (Effect on Work p = 0.015, Required Analgesics p = 0.002, and Systemic Symptoms p = 0.017); RSS (Frequency p < 0.001, Severity p < 0.001, Hours in Bed p = 0.014, and Number of Analgesic Pills p < 0.001); MSQ (Congestive Symptoms p = 0.007); MDQ Premenstrual Symptoms (Behavioral Changes p = 0.002 and Autonomic Reactions p = 0.007); and MDQ Menstrual Symptoms (Pain p = 0.001, Concentration p = 0.010, Behavioral Changes p < 0.002, Autonomic Reactions p = 0.019, Water Retention p = 0.032, and Negative Affect p = 0.003). Notably, the majority of significant interaction results survived correction for multiple comparisons (Supplemental Table 3); only VMS Effect on Work and Systemic Symptom, RSS Hours in Bed, and MDQ Menstrual Water Retention results were no longer significant following correction. Post hoc ANOVAs revealed no significant between-group differences at either follow-up.
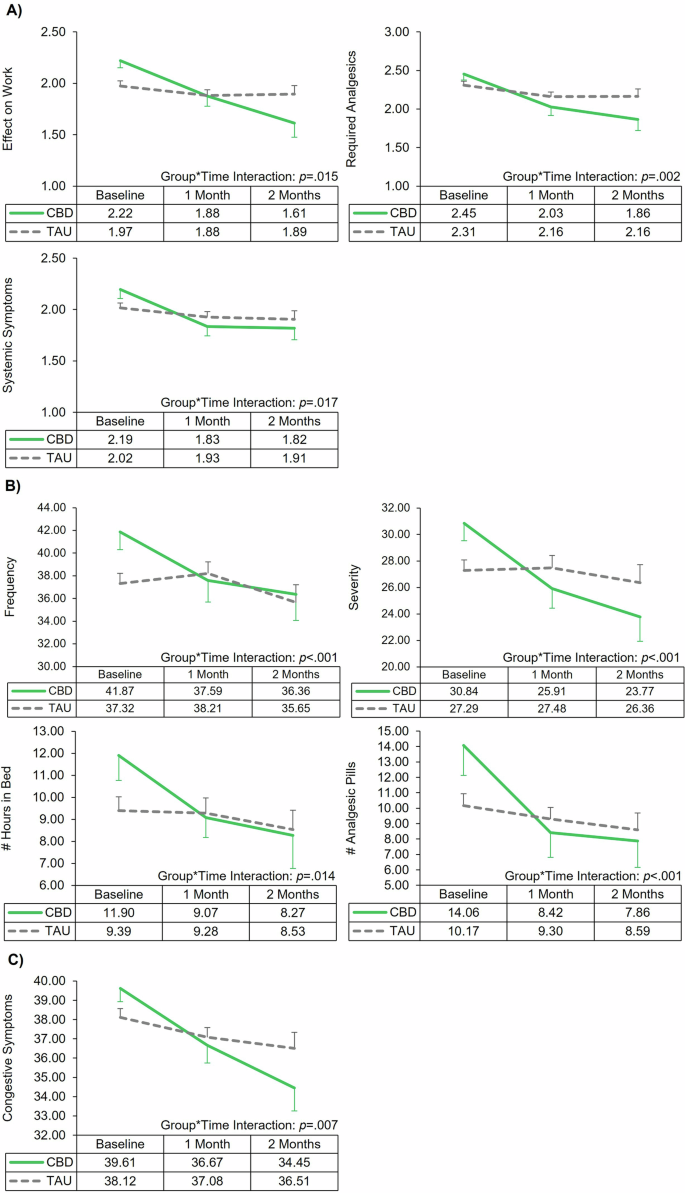
Line graphs of the significant interactions from the linear mixed model analyses assessing change in menstrual-related symptoms by treatment group (cannabidiol [CBD] vs treatment-as-usual [TAU]) over time. Error bars represent standard error. A Verbal multidimensional scoring system (VMS) assessment of dysmenorrhea. B Retrospective symptom scale (RSS) of dysmenorrhea. C Menstrual symptom questionnaire (MSQ).
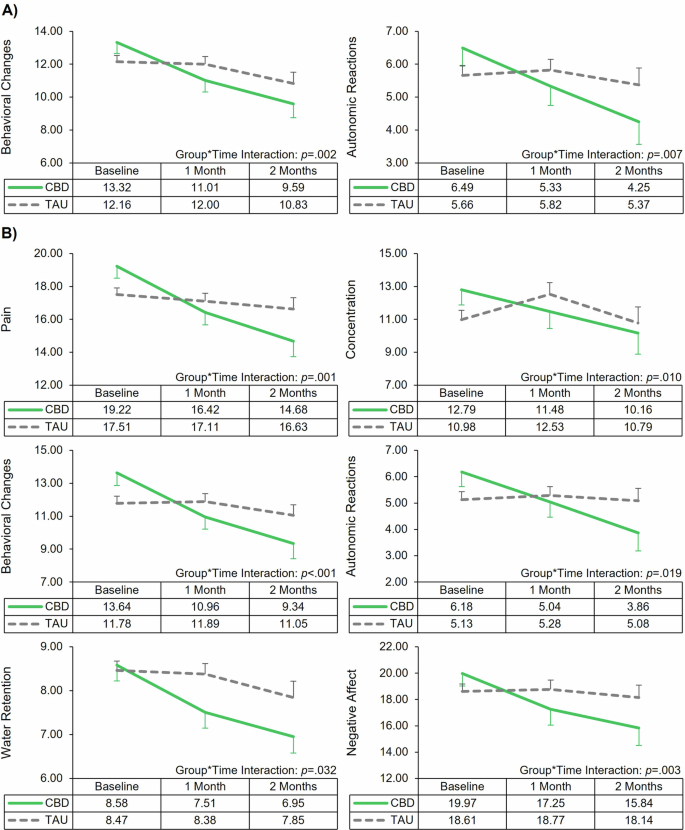
Line graphs of the significant interactions from the linear mixed model analyses assessing change in menstrual-related symptoms during different menstrual phases by treatment group (cannabidiol [CBD] vs treatment-as-usual [TAU]) over time. Error bars represent standard error. A Menstrual distress questionnaire (MDQ): Premenstrual symptoms. B Menstrual distress questionnaire (MDQ): Symptoms during menstruation.
Additionally, suppository use significantly correlated with symptom at follow-up 1 (Table 5, Supplementary Figs. 1 and 2). Increased number of suppositories used was associated with greater percent change improvements on the VMS (Required Analgesics p = 0.020 and Systemic Symptoms p = 0.025); RSS (Frequency p = 0.003, Severity p = 0.003, and Hours in Bed p = 0.014); MSQ (Congestive Symptoms p = 0.003); and MDQ Menstrual Symptoms (Pain p = 0.008, Water Retention p = 0.015, and Negative Affect p = 0.004).
Discussion
This is the first study to assess the impact of a “real-world”, commercially-available, high-CBD suppository on menstrual-related pain and discomfort, and extends prior work suggesting MC has promising potential for alleviating menstrual-related symptoms17,18,19. Previous survey studies have reported MC use significantly reduced pain, cramping/muscle spasms, mood, anxiety, sleep, and gastrointestinal issues as well as improved quality of life in participants with PMS/PMDD32, endometriosis33,34, chronic pelvic pain35,36, and menopause37. Results from the current study indicate that participants who used high-CBD suppositories PRN for menstrual-related pain and discomfort demonstrated significantly greater improvement in their symptoms over two months relative to TAU participants. For the majority of symptoms, both frequency and severity of symptoms as well as the impact of symptoms on daily functioning were more significantly reduced relative to the TAU group, and in the CBD group, the majority of participants perceived at least moderate improvement of symptoms. Analyses of symptom types suggest that CBD suppositories may be more effective for congestive (i.e., dull, aching pains accompanied by energy and mood symptoms) rather than spasmodic symptoms (i.e., sharp spasms of pain). When considering symptoms at various phases of the menstrual cycle, the CBD group demonstrated greater symptom improvement relative to the TAU group for all symptom types during menstruation except for arousal/energy; improvement of premenstrual symptoms was also noted for behavioral changes and autonomic reactions.
Previous work suggests CBD may address pain and inflammation associated with dysmenorrhea19, endometriosis20,21,22, and chronic pelvic pain23, and observational studies indicate that MC use may help reduce the use of conventional medications, particularly analgesics26,33. In the current study, the CBD group reported significantly greater reductions of pain during menstruation, need for analgesics, and number of analgesic pills taken relative to the TAU group. Importantly however, while NRS ratings of pain severity decreased significantly over time for all participants (i.e., main effect of time), the LMM analyses did not differentiate the two groups (i.e., group*time interactions were not significant). Notably, the NRS used in the current study was a 0–10 rating for average and worst menstrual pain, which while important, is a fairly general assessment, and more comprehensive, specific assessments of pain-related symptoms (e.g., VMS, RSS, MDQ) did in fact differentiate the groups, underscoring the importance of utilizing subscales assessing a range of specific symptoms. Additionally, the CBD group did report significant improvement on the impact of menstrual-related symptoms on life (e.g., avoiding activities) as well as reduced use of analgesics, which may indicate that even though pain severity ratings did not differ by group, management of pain (e.g., need for analgesics, impact on daily functioning) was significantly improved in the CBD group relative to the TAU group. Taken together, these results suggest that cannabinoid-based therapies may help to treat menstrual-related symptoms and reduce use of conventional analgesics.
Correlations indicated a potential dose-dependent response, with increased suppository use significantly associated with greater symptom reduction, improved daily functioning, and reduced need for analgesics. These analyses suggest suppository use may be most effective during menstruation, particularly for addressing and managing pain, water retention, and negative affect. While correlations for premenstrual symptoms did not reach statistical significance, most participants reported suppository use during menstruation; only about half reported use during the premenstrual phase. Exploratory correlations utilizing a subset of participants who reported using suppositories during their premenstrual phase indicated that suppository use significantly correlated with improvements in premenstrual behavioral changes (p = 0.042, Table 5). These findings suggest the statistical power for analyses of premenstrual symptoms was likely reduced given fewer participants used suppositories during this phase.
Given expanding legalization and availability of MC products, increasing numbers of individuals are exploring MC to alleviate symptoms of a variety of medical conditions48. Estimates suggest ~13–23% of patients with gynecological conditions (e.g., endometriosis, pelvic pain) use MC to alleviate symptoms33,35. However, cannabinoid-based therapies may not be appropriate for all gynecological indications. For example, increased serum AEA and decreased endometrial FAAH expression may accelerate the progression of PCOS1. Additionally, ECS function in the gynecological system is impacted by aging49, suggesting the potential for differential impacts of cannabinoid-based therapies with age, which should also be explored. Further, exogenous cannabinoids, including CBD, interact with cytochrome P450 enzymes involved in hepatic metabolism, which may result in drug-drug interactions with other medications50,51,52. Caution is warranted for patients taking medications with a narrow therapeutic index (i.e., the ratio between a drug’s toxicity and effectiveness) including some anticoagulants, beta blockers, antidepressants, and antipsychotics. Importantly for the current study, the use of a transmucosal route of administration, with drug absorption occurring through the vaginal mucosa, bypasses first-pass hepatic metabolism which may reduce the risk of potential side effects and drug-drug interactions53.
Previous research has identified several potential sources of ECS dysfunction associated with gynecological conditions and symptoms, including altered expression of ECS genes11, receptors14,15,16, and synthesizing/degrading enzymes12,13. However, more work is needed to identify the specific mechanism(s) of action for cannabinoid-based therapies as well as the most efficacious cannabinoid constituent profiles, dosing strategies, and product types in order to inform clinical practice. Future randomized, placebo-controlled, clinical trials are warranted to determine safety and efficacy for various gynecological conditions and symptoms.
Additionally, some question the use of vaginal applications of cannabinoid-based medicines given concerns regarding absorption, controlled release, and whether cannabinoids reach the uterus using suppositories as a delivery system19. Current results provide preliminary evidence that vaginal suppositories can be an effective route of administration for cannabinoid-based therapies; however, future work should evaluate the impact of different routes of administration, including pharmacokinetic/pharmacodynamic analyses.
This study had several strengths and limitations. A survey-based, quasi-experimental study design without prospective group assignment was necessary given restrictions prohibiting the use of commercially-available products in clinical trials in the US. In order to conduct a clinical trial of a CBD-containing product, the product must be studied under an Investigational New Drug (IND) application; this means it would be illegal for a product to simultaneously be for sale in the marketplace while being studied under an IND, as it would be considered an unapproved drug54. Importantly, within the US, hemp-derived products, defined as those containing ≤0.3% THC by weight, are federally legal55, which has resulted in thousands of commercially-available “low THC” or “THC-free” products flooding the marketplace without any ability to study their impact using standard clinical trial models. As there is a paucity of data assessing the efficacy of these commercially-available, “real world” products, investigations like the current study provide critical information that is otherwise unavailable.
While randomized, controlled clinical trials have the highest level of scientific rigor for assessment of causality, when randomization of the intervention is not possible (i.e., due to federal restrictions) or unethical, non-randomized, quasi-experimental studies are typically the most rigorous option56. Accordingly, the current study assessed the efficacy of individuals’ naturalistic (i.e., PRN) use of a commercially-available CBD suppository for menstrual-related symptoms, with participants providing information regarding suppository use. Correlation analyses further strengthened study findings, providing evidence for a potential dose-dependent response to suppository use. Additionally, since the CBD suppositories are commercially-available in the US and cannot be used as part of a clinical trial, participants were required to deal directly with the manufacturer/study sponsor regarding all issues related to the receipt and use of the product; the research team could not have any clinical interaction with study participants. As a result, individuals were instructed to direct concerns regarding suppository use directly to the sponsor, and no information regarding side effects or adverse events was collected by the research team. Information about the safety profile and potential side effects (i.e., lowered blood pressure, light-headedness, drowsiness, dry mouth) of these suppositories was readily available in the Health, Safety, and Ingredients section of the sponsor’s website38, which also includes links to Certificate of Analyses for all of their products.
Notably, the CBD group reported significantly greater menstrual-related symptomatology at baseline relative to the TAU group, which aligns with previous work demonstrating that poorer health and inadequate symptom relief with conventional treatment are associated with utilizing alternative treatment strategies such as MC57. To account for significant between-group differences at baseline, autoregressive LMM were utilized; interestingly, post hoc analyses revealed no significant between-group differences at either follow-up, suggesting the more severe baseline symptom presentation was eliminated by the use of the CBD suppositories.
Additionally, previous research has also demonstrated differential efficacy of over-the-counter (OTC) analgesics for dysmenorrhea. A recent meta-analysis identified ibuprofen as the optimal OTC analgesic for dysmenorrhea with the best efficacy and safety profile; whereas aspirin (acetylsalicylic acid) was no more effective than placebo58. While information regarding analgesic use and effectiveness was collected for this study via well-validated clinical scales (e.g., VMS, RSS), comprehensive information regarding participants’ individual analgesic regimens was not collected. Therefore, baseline between-group differences may have been related to greater prevalence of ineffective analgesic use within the CBD group. However, even if the CBD group had a higher prevalence of ineffective analgesic use at baseline, the significant improvement of symptoms within this group is still notable. Future research should investigate the potential impact of CBD suppositories on the efficacy of conventional analgesics.
Further, observational studies of MC use for gynecological symptoms have demonstrated that treatment expectancies can significantly mediate self-reported outcomes32,59. Unfortunately, treatment expectancies were not directly assessed in the current study. However, while well-validated metrics exist to assess expectancies related to recreational cannabis use (e.g., Marijuana Effect Expectancy Questionnaire60), no validated metrics were available to asses MC treatment expectancies. Future work should include measures of treatment expectancy specifically designed for MC use.
The racial distribution of the current study is similar to the most recent US census61, suggesting good sampling and high generalizability. Notably, participants were not required to be cannabis-naïve at baseline. While additional cannabis use likely impacted results, rates of current cannabis use were not significantly different between treatment groups. Further, given increased prevalence of MC use for gynecological symptoms33,35 and evidence that individuals who have previously tried/used cannabis are more likely to use MC to address gynecological symptoms62, requiring participants to be cannabis-naïve would not accurately reflect this clinical population. Future studies should use a comprehensive metric (e.g., CannaCount63) to assess and control for additional cannabis use.
In conclusion, although dysmenorrhea is quite common, the current study is the first preliminary study to assess a “real world”, high-CBD vaginal suppository for menstrual-related pain and discomfort. Findings suggest these suppositories alleviated a range of menstrual-related symptoms, improved daily functioning, and reduced use of analgesics. Increased suppository use was significantly associated with greater reduction of symptoms, suggesting a potential dose-dependent response. Current study results expand previous preclinical and observational findings; future studies should replicate and expand current findings and further examine the pharmacokinetics and pharmacodynamics, mechanism(s) of action, efficacy for other gynecological indications, and potential for adverse events (e.g., drug-drug interactions), ultimately using clinical trial models.
Responses