Antibody responses against influenza A decline with successive years of annual influenza vaccination

Introduction
Influenza vaccines work by stimulating production of antibodies against the haemagglutinin protein—the principal protein responsible for virus infectivity1. Haemagglutinin is also the influenza protein that mutates most rapidly. As a result, influenza vaccines are reformulated annually to keep pace with virus evolution, and annual re-vaccination with the updated vaccines is recommended to ensure protection against contemporary viruses2.
Although annual revaccination should represent our best option for protection against currently-circulating influenza viruses, it has long been known that repeated vaccination may, in fact, attenuate vaccine effectiveness. This was first reported in the 1970s3, and has been re-visited on a number of occasions in vaccine effectiveness4,5,6 and immunogenicity studies7,8,9,10,11,12. The effects have been noted most often for A(H3N2) viruses, which exhibit greater diversity than other human influenza viruses, making it challenging to identify a candidate vaccine virus (CVV) able to stimulate broad antibody coverage against circulating viruses13. However, A(H1N1)pdm09 has also begun to exhibit increasing diversity and attenuated effectiveness among repeat vaccinees has been observed for these viruses4.
The impact of repeat vaccination on vaccine effectiveness is not consistent across seasons, which may be a consequence of varying antigenic distances between successive vaccine viruses and circulating viruses5. When the vaccine antigen is not updated, but predominant circulating viruses have antigenically drifted away from the CVV, negative interference associated with repeated vaccination appears to be exacerbated14. In such a scenario, vaccination may stimulate a focussed antibody response targeted at epitopes that have since been superseded and these antibodies are therefore incapable of neutralising the antigenically drifted viruses15. Conversely, positive interference may also occur when vaccine antigens are updated, stimulating both recall of prior antibodies as well as generation of new antibodies, the combination of which can provide broader protection against circulating viruses11.
Previous studies that have examined repeated vaccination have been limited to a single season or have often had insufficient sample size to observe clear trends by vaccination history. To better understand the mechanisms underlying observations of reduced immunogenicity and effectiveness in repeatedly-vaccinated persons, we established a multi-year cohort of healthcare workers (HCW) monitored for post-vaccination antibody responses and vaccine failures. HCW are recommended for priority influenza vaccination by the World Health Organization (WHO)16 and are therefore a highly vaccinated group in many countries, including Australia. The cohort was established in April 2020, just as COVID-19 pandemic restrictions were implemented, which impeded recruitment. However, the first two years of the pandemic were also accompanied by local extinction of influenza17. This presented an opportunity to examine the post-vaccination antibody kinetic in a period during which infections were rare. Here, we present the results from the first two years of this cohort. We aimed to compare immunological responses to influenza A vaccination—in terms of geometric mean titre (GMT), seropositivity, mean fold rise (MFR) and seroconversion—by vaccination history.
Methods
Setting and participants
The cohort included healthcare workers (HCW) from six public health services around Australia: the Queensland Children’s Hospital, Brisbane; the John Hunter Hospital, Newcastle; the Children’s Hospital at Westmead, Sydney; Alfred Health, Melbourne; the Women and Children’s Hospital, Adelaide; and the Perth Children’s Hospital, Perth. These hospitals were chosen because they participate in patient influenza surveillance18, and because they provided good geographic coverage, which was hoped to overcome potential variations in seasonality and predominant virus circulation19.
The cohort was an open cohort, permitting new recruitment each year (2020–2023) to reach the target sample size of 250 HCW per site. HCW were eligible to participate if they were employees, students or volunteers at participating health services, were aged 18–64 years with no known contraindications to influenza vaccines and had not yet received the influenza vaccine in their enrolment year. HCWs were excluded if they had received immunosuppressive treatment (e.g. systemic corticosteroid treatment or cancer therapy) within the past 6 months. Enrolment commenced on 2 April 2020. HCWs were asked to complete a brief questionnaire to collect demographic information, employment category, medical history and 5-year influenza vaccination history. Blood was collected by venepuncture from participants immediately prior to vaccination (pre-vaccination), 14–21 days post-vaccination and around the end of the usual Australian influenza season (October-November).
Ethical considerations
This study was approved by the Human Research Ethics Committee of the Royal Melbourne Hospital (HREC Reference Number: HREC/54245/MH-2019). All study staff were trained in Good Clinical Practice and Human Subjects Protection. Written informed consent was obtained from all HCWs upon enrolment.
Vaccines
At all sites, state governments provide influenza vaccine to all public hospitals for staff vaccination. At the time of the study, influenza vaccination was not mandatory for HCW. However, there was a strong influenza campaign in 2020 to avoid the risk of a dual influenza and SARS-CoV-2 epidemic. The state of Victoria set very high targets for vaccine coverage among its HCWs ( > 90%)20, and New South Wales also introduced vaccination mandates for certain clinical staff in 201921. Given the strong recommendation for vaccination among HCW, it was expected that a majority of HCW willing to participate would have received multiple prior vaccinations. Therefore, extra effort was made to recruit vaccine-naïve participants with a target of at least 10 per site per year.
In 2020, HCW received quadrivalent influenza vaccines containing egg-grown inactivated viruses that were A/Brisbane/02/2018 (H1N1pdm09)-like, A/South Australia/34/2019 (H3N2)-like, B/Washington/02/2019 (B/Victoria)-like virus and B/Phuket/3073/2013 (B/Yamagata)-like virus. In 2021, the formulation was updated to include 2 new influenza A viruses, an A/Victoria/2570/2019 (H1N1pdm09)-like virus and an A/Hong Kong/2671/2019 (H3N2)-like virus. Only responses against the influenza A antigens are reported here.
Serological assays
Sera were tested for the presence of antibodies against each of the vaccine influenza A antigens using the haemagglutination inhibition (HI) assay as previously described22. Both egg- and cell-grown influenza antigens were used, where the egg-grown antigen provides an indication of response to the vaccine, while cell-grown antigen provides an indication of the level of protection a person might have against circulating viruses. Cell-grown A(H1N1)pdm09 viruses were grown in Madin-Darby canine kidney (MDCK) cells while A(H3N2) were grown in MDCK-a-2,6-sialyltransferase (SIAT) cells. Sera were treated with receptor destroying enzyme (Denka Sieken) to remove non-specific haemagglutination inhibitors and were adsorbed with a mixture of erythrocytes from turkeys (H1N1pdm09) and guinea pigs (H3N2) to remove non-specific haemagglutination. Sera were diluted 2-fold starting at 1:10 to a maximum dilution of 1:10240. HI antibody titres were read using a CypherOne automated reader (InDevR, Colorado, USA) as the reciprocal of the highest serum dilution causing complete inhibition of agglutination.
Statistical analysis
Where relevant, HI titres of <10 were assigned the value 5. HI titres of 10240 could potentially be >10240 but none exceeded this. Titres were log2 transformed for analyses, and later back-transformed to titre values for interpretation. We initially assessed crude geometric mean titres (GMT), seroconversion (proportion exhibiting at least a 4-fold rise in titre post-vaccination) and seropositivity (proportion with titres ≥40), by prior vaccination status. The association between post-vaccination GMT and the number of prior vaccinations was assessed by linear regression and the Jonckheere-Terpstra test for trend.
GMTs and geometric mean ratios (GMRs) were also estimated using a log-linear regression model where the outcome was the day 14 log-post-vaccination titre or geometric mean fold rise. In univariable models, prior vaccination status was modelled as a linear term to estimate the incremental association of each successive prior vaccination. In multivariable analysis, prior vaccination was modelled as an ordinal term to allow for non-monotonicity. Potential covariates that were explored included pre-vaccination titre (centred at a titre of 5), age (centred to 18 years), sex, body mass index (BMI), the presence of any health conditions and vaccine brand. The full model including all covariates was compared with more parsimonious models based on Akaike Information Criterion (AIC). For each post-vaccination outcome explored, the same model parameters were used for each virus examined. Estimated GMTs and GMRs were plotted for visual assessment and compared with crude (observed) values. The same approach was used to estimate seroconversion and seropositivity in logistic regression models. All statistical analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
In 2020, 637 HCW were recruited across 6 sites, of whom 24 opted not to be vaccinated in 2020 and were therefore not considered in this analysis. Pre-vaccination and post-vaccination blood samples were available for 595 vaccinated HCWs (586 with both visits), and end-of-season blood samples were available for 564. The median time between vaccination and post-vaccination blood draw was 15 days (inter-quartile range (IQR): 14, 18) and the median time between vaccination and end-of-year blood draw was 174 days (IQR: 165, 188) (Supplementary Figure 1). In 2021, the number newly recruited was 759, while 339 recruited in 2020 (including 2 who were unvaccinated in 2020), were vaccinated in 2021 and continued follow up. Eighteen of the HCWs newly recruited in 2021 were unvaccinated and not considered further. Pre-vaccination blood samples were available for 1070 vaccinated HCWs, post-vaccination samples for 1031 and end-of-season samples for 1002. The median time between vaccination and post-vaccination blood draw was 15 days (IQR: 14, 19) and the median time between vaccination and end-of-year blood draw was 165 days (IQR: 154,184). See Fig. 1 for the STROBE flowchart detailing patient recruitment and follow-up and Supplementary Figure 1 for a summary of follow-up times.
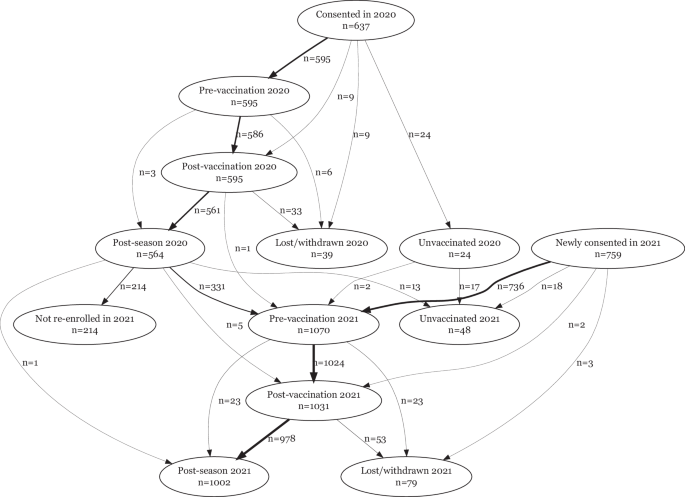
Strobe flowchart showing participants enroled in the cohort for whom samples were available for serology at pre-vaccination, post-vaccination and post-season visits.
Selected demographic and workplace characteristics are presented in Table 1 by study year and the number of prior vaccinations. HCW were median 39 years at recruitment (39 y in 2020; 40 y in 2021) and predominantly female (81% in 2020; 85% in 2021). In both study years, most HCW were full-time employed (57% in 2020; 62% in 2021) and around half were in clinical roles (53% in 2020; 46% in 2021). Fourteen percent had at least one health condition (13% in 2020; 15% in 2021).
A(H1N1)pdm09 antibody titres over the course of vaccination
The A(H1N1)pdm09 vaccine antigens included in egg-based vaccines in 2020 and 2021 were from genetically distinct subgroups, with A/Brisbane/02/2018 in 6B.1A.1 subgroup, and A/Victoria/2570/2019 in the 6B.1A.5a.2 subgroup. Vaccine antigens for the 5 years prior to 2020 were also genetically distinct, being an A/Michigan/45/2015 6B.1 virus in 2017–19 and A/California/7/2009 in 2015–16 (Supplementary Figure 2).
In both 2020 and 2021, pre-vaccination GMTs against both cell and egg-grown antigens were lowest among HCWs with 0-prior vaccinations at around 10–20, and the group with 5+ prior vaccinations had the next lowest pre-vaccination GMTs (Supplementary Table 1, Supplementary Fig. 3A). In contrast, day 14 post-vaccination GMTs were higher in the 0-prior group compared with the 5-prior group. The linear trend of decreasing post-vaccination GMTs with increasing numbers of prior vaccinations was statistically significant and suggested an average 0.79 to 0.85-fold decrease in titre with each additional prior vaccination (Table 2). Post-vaccination titres were higher in 2021 compared with 2020 against both cell and egg and for all vaccination groups. Post-vaccination titres were also dependent on the pre-vaccination titre (Fig. 2A; Supplementary Fig. 4A). Estimated post-vaccination GMTs from the model were adjusted for pre-vaccination titre (centred at 5), vaccine brand, age in decades (centred at 18 years), sex, BMI, and presence of any pre-existing health conditions and continued to show an inverse association with both number of prior vaccinations as well as a positive association with pre-vaccination titre (Fig. 2B; Supplementary Table 2; Supplementary Fig. 5). Age and vaccine brand were also important predictors against egg-, but not cell-grown, antigens (Supplementary Table 2).
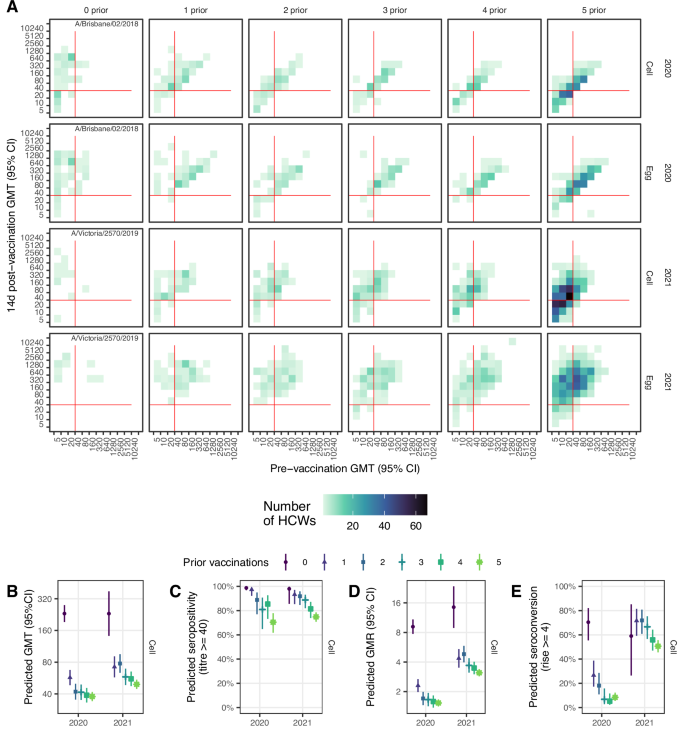
A Observed post vaccination titres by pre-vaccination titres for both cell and egg-grown antigens. Data convergence on the diagonal is indicative of minimal titre rise. Red lines indicate the seropositivity threshold titre of 40. B Estimated GMT 14 d post-vaccination from the linear regression model adjusting for baseline titre, vaccine brand, age, sex, BMI and presence of any health conditions. C Estimated seropositivity 14 d post-vaccination for cell-grown antigen from the logistic regression model adjusting for baseline titre. D Estimated geometric mean titre ratios (GMR) 14 d post-vaccination from the linear regression model adjusting for baseline titre, vaccine brand, age. E Estimated proportion of HCW who seroconverted 14 d post-vaccination from the logistic regression model adjusting for baseline titre, vaccine brand, age. Panels D-E show results for cell-grown antigens only.
Pre-vaccination seropositivity was lowest for the 0-prior vaccination group, but those with 5-prior vaccinations had the next lowest seropositivity and this group remained lowest post-vaccination (Supplementary Table 1). For all vaccination groups seropositivity increased to above 50% post-vaccination for cell-grown antigens and was even higher for egg-grown antigens (above 85%), and seropositivity was sustained above pre-vaccination levels 6 months post-vaccination. There was a clear trend of decreasing seropositivity with increasing numbers of prior vaccinations in 2021 but not 2020 (Supplementary Fig. 3B); however, a clearer trend emerged after adjustment for pre-vaccination titre (Fig. 2B; Supplementary Fig. 6, Supplementary Table 3).
Post-vaccination geometric mean titre rises (GMRs) were highest for the vaccine-naïve group but were not very different among the 4 vaccine-experienced groups, all with a mean GMR of ~2 in 2020 and were higher in 2021 ranging from 3–4 against cell antigens and from 6–8 against egg antigens 2021 (Supplementary Figure 3C; Supplementary Table 1). GMRs decreased with increasing pre-vaccination titre and fell below 4-fold for all prior vaccination groups with pre-vaccination titres exceeding 80 (Supplementary Fig. 4C). Prior vaccination continued to be associated with reduced titres after adjusting for pre-vaccination titre, age at enrolment and vaccine brand (Supplementary Table 4, Supplementary Fig. 7). Similarly, seroconversion was higher among the vaccine-naïve, but not very different among vaccine-experienced groups (Supplementary Table 1; Supplementary Fig. 3D). Adjustment for pre-vaccination titre, age and brand reduced the differences in seroconverted proportions between the vaccine-naïve and vaccine-experienced, and, in 2021, revealed no apparent trend of declining seroconversion from 0 to 5-prior vaccinations (Supplementary Table 5, Supplementary Figure 8). For all vaccination groups seroconversion was higher in 2021 compared with 2020.
A(H3N2) antibody titres over the course of vaccination
The vaccine administered in 2020 contained an A/South Australia/34/2019-like virus, which fell in the 3C.2a1b.2 genetic subgroup (Supplementary Fig. 9). This virus was genetically distinct from the 2021 vaccine virus, A/Hong Kong/2671/2019, which fell in the 3C.2a1b.1b subgroup, and both were distinct from the vaccine viruses used in the 5 years prior to 2020. However, there were some shared epitopes, including the T160K substitution in the egg antigens of 2016–2021 vaccine strains, which is a known egg-acquired adaptation associated with a loss of glycosylation23. All vaccine strains apart from A/Hong Kong /2671/2019 contained several other glycosylation sites within antigenic sites A and B that were retained in egg-grown strains (Supplementary Table 6).
As with A(H1N1)pdm09, responses to A(H3N2) antigens exhibited a pattern of declining GMTs by number of prior vaccinations (Supplementary Table 7; Supplementary Fig. 10), and increasing GMTs with higher pre-vaccination titres (Fig. 3A; Supplementary Fig. 11). The trend was more apparent in 2021 than in 2020, with an expected reduction in GMT of 0.93 in 2020 and 0.87 in 2021 (Table 2), which was maintained after adjustment for pre-vaccination titre, vaccine brand, age, sex, BMI and pre-existing health conditions (Fig. 3B; Supplementary Table 8, Supplementary Figure 12).
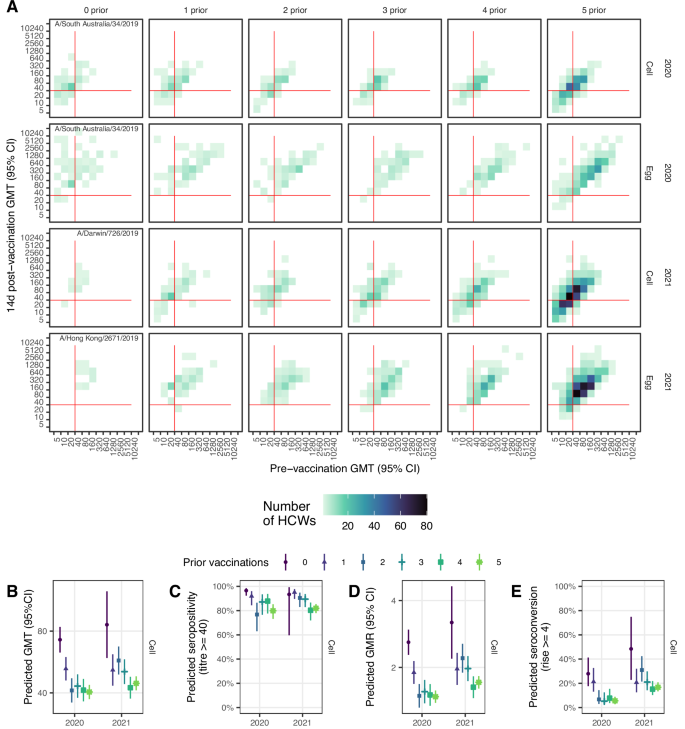
A Observed post vaccination geometric mean titres (GMTs) by pre-vaccination GMT for both cell and egg-grown antigens. Data convergence on the diagonal is indicative of minimal titre rise. Red lines indicate the seropositivity threshold titre of 40. B Estimated GMT 14 d post-vaccination from the linear regression model adjusting for baseline titre, vaccine brand, age, sex, BMI and presence of any health conditions. C Estimated seropositivity 14 d post-vaccination for cell-grown antigen from the logistic regression model adjusting for baseline titre. D Estimated geometric mean titre ratios (GMR) 14 d post-vaccination from the linear regression model adjusting for baseline titre, vaccine brand, age. E Estimated proportion of HCW who seroconverted 14 d post-vaccination from the logistic regression model adjusting for baseline titre, vaccine brand, age. Panels D,E show results for cell-grown antigens only.
Post-vaccination seropositivity was high and above 65% for cell-grown antigens and above 90% for egg-grown antigens, consistent with higher post-vaccination HI titres against egg compared with cell-grown antigens (Supplementary Table 7; Supplementary Fig. 10B). In 2020, the raw data suggested increasing seropositivity from 0 to 4 prior vaccinations; however, after adjustment for pre-vaccination titre, this trend reversed, albeit not monotonically (Fig. 3C; Supplementary Table 9, Supplementary Fig. 13).
Post-vaccination GMRs were highest for the vaccine-naïve group with a mean rise of 3.3 against cell-grown antigens (compared to mean rises ranging from 1.4 to 2.3-fold for the vaccine-experienced groups (Supplementary Table 6; Supplementary Fig. 10C). Correspondingly, around half the vaccine-naïve HCWs seroconverted to cell-grown antigen (56% in 2020 and 47% in 2021), but fewer than half of the vaccine-experienced groups seroconverted, with seroconversion as low as 7.7% for those receiving 3-prior vaccinations in 2020 (Supplementary Table 7; Supplementary Fig. 10D). Seroconversions were higher against egg- compared with cell-grown antigens, and for HCWs with lower pre-vaccination titres (Supplementary Fig. 11). GMR and seroconversion trends were maintained after adjustment for pre-vaccination titre, age and vaccine brand (Fig. 3C,D; Supplementary Tables 10 & 11; Supplementary Figs. 14 & 15).
Discussion
We observed decreasing post-vaccination antibody titres with increasing numbers of prior vaccinations in a cohort of Australian HCWs vaccinated with southern hemisphere quadrivalent vaccines in 2020 and 2021. Trends were not monotonic but were statistically significant for both influenza A subtypes. The magnitude of response to vaccination, whether measured as the absolute titre or the rise in titre, was consistently highest for the group with 0-prior than the group with at least 5 prior vaccinations. Pre-vaccination titres and seropositivity for the 5-prior group were second lowest to the 0-prior group, suggesting that attenuation associated with prior vaccination is incremental and sustained. These observations were consistent across antigens. When adjusted for pre-vaccination titre and other covariates, the magnitude of differences between the vaccination groups sometimes diminished, but the trend of decreasing post-vaccination responses with increasing numbers of prior vaccinations was generally preserved or became clearer. The magnitude also varied between years with the change in vaccine antigens, suggesting a common mechanism that may be modulated by factors such as antigenic distance24. While studies that only consider prior year vaccination report conflicting impacts on vaccine immunogenicity8,12 the results presented here confirm previous observations from our group9,10 and others8,12 that repeated influenza vaccination over multiple years attenuates immunogenicity for both influenza A subtypes.
Our study revealed that a range of factors other than prior vaccination influence antibody responses to influenza vaccination, which may explain why relationships with number of prior vaccinations are not monotonic. Most importantly, pre-vaccination titre strongly predicted post-vaccination responses consistent with studies elsewhere including studies of post-infection titre rise25. It is plausible that if titres are high pre-vaccination, it may be difficult to observe post-vaccination titre rises and seroconversions. This has been referred to as the ceiling effect25,26,27. To avoid false ceiling effects associated with the limit of detection, we used an extended titration series where the highest dilution was 1:10240. Only three titrations reached 10240; by comparison, most ( > 90%) post-vaccination titres against cell-grown antigens were 160 or below. The negative influence of pre-vaccination antibody on post-vaccination antibody responses may account for the differences observed between subtypes. For example, the estimated reduction in GMT with each subsequent prior vaccination was greater for A(H1N1)pdm09 than for A(H3N2), and pre-vaccination titres were generally higher for the latter.
In 2020, the A/Brisbane/02/2018 virus was included in the vaccine for the first time, as an update to the previous A(H1N1)pdm09 CVV, A/Michigan/45/2015, which had been used for 3 years. The CVV was again updated in 2021 to A/Victoria/2570/2019, which was a large antigenic change from the Brisbane and Michigan viruses, associated with a N156K substitution. This appears to have led to a marked improvement in the antibody boost from vaccination in 2021, with higher GMRs and proportion seroconverted in 2021 compared with 2020, even for the vaccine-experienced groups. Indeed, vaccine effectiveness in Europe was higher in the 2021/2022 season, when A/Victoria/2570/2019 was included in the vaccine, than in any other season since the A(H1N1)pdm09 pandemic28. Previous studies have identified age cohort effects of reduced vaccine effectiveness attributable to recall of epitopes shared with older A(H1N1) viruses in some age groups29, which can be exacerbated by repeated vaccination30. Further work is underway to better understand the underlying mechanism via which antigenic change impacts immunogenicity, including whether antigen recall and birth cohort play a role.
A(H3N2) seropositivity was comparable with and sometimes higher than for A(H1N1)pdm09. This is somewhat at odds with our understanding of antibody titre as a correlate of protection, since vaccine effectiveness is usually higher for A(H1N1)pdm09 than for A(H3N2), irrespective of prior vaccination4. We could not corroborate these titre values with vaccine effectiveness in our study because there were no infections in 2020 or 2021. However, the threshold for seropositivity may differ for the two influenza A subtypes. Indeed, in a longitudinal household cohort study in Vietnam, the protective titre for A(H1N1)pdm09 was around half the threshold for A(H3N2)31. Studies which have combined immunogenicity and vaccine effectiveness data from the same cohort have suggested that the proportion of the vaccine’s effect that is mediated by antibody titre may be low32,33, indicating the need for alternative or additional correlates of protection. Measuring the breadth of protection, such as with antibody landscapes that measure responses against several antigens11, may provide a more robust measure of seroprotection.
In all measures of antibody response explored values for egg-grown antigens were higher than the corresponding cell-grown antigens, even among vaccine-naïve HCWs. This may not be surprising given that all HCWs received egg-based vaccines, both during the study and in prior years. Egg-acquired adaptions that direct the antibody response towards certain epitopes not necessarily shared by circulating viruses (and thus not providing protection) are thought to exacerbate the negative interference from repeated vaccination23,34. We assessed both cell- and egg-grown antigens since the egg-grown antigens can acquire changes that affect immunogenicity and effectiveness23,35, while cell-grown antigens are more closely represent circulating viruses. Strong responses to egg-grown antigens may not provide a reliable correlate of protection against circulating viruses and we therefore recommend caution be applied when interpreting immunogenicity studies that report egg-grown antigens, only.
Our study was hampered by its sample size, particularly for the vaccine-naïve group in 2021 when just 15 vaccine-naive HCW were recruited, despite targeted recruitment efforts. The 2021 vaccine-naïve participants were younger and more of them were male than in 2020, which could have contributed to conflicting observations about their antibody response between years. Sex differences have previously been reported to influence immunogenicity36; however our regression analysis did not indicate any meaningful difference between sexes. Model fit and precision were also poorer in 2021, despite the overall greater availability of data in that year. It is possible that some of this imprecision results from an actual increase in variability of antibody responses to the new vaccine antigens received compared with 2020. Unfortunately, we had limited opportunity to explore sources of this heterogeneity and whether some of it may have arisen through effect modification. The sample size also limited out ability to consider using a causal approach to the analysis, since we would have been unable to control for all important confounders. Notably, we did not collect the prior infection history of participants, which is expected to be a strong confounder and effect modifier, but is impractical to collect. Attempts have been made to infer infection history using mathematical models of serological data37. However, such models tend to poorly discriminate prior vaccination from prior infection.
Our ascertainment of prior vaccination history was largely by self-report and it is possible that in some cases it was misreported and the vaccination histories for some HCWs may have been misclassified. This probably affects the vaccine-naïve group the least as many of them were people who do not usually get vaccinated against influenza but did so because of strong hospital and government campaigns to get vaccinated in 2020 to avoid a dual epidemic of COVID-19 and influenza.
Our outcomes may also be imperfectly measured as observed antibody titres for the same serum can vary when repeated. To avoid inter assay variation, all sera were titrated with a single batch of red blood cells across two days for each virus. Although egg-grown and cell-grown equivalents for each antigen were run as separate batches, titres were well correlated (0.5–0.84) and concordance for seroconversion ranged from 71–94% suggesting that assay variation was low (data not shown).
Our use of a single antigen to measure vaccine-induced antibody responses could also be considered a source of measurement error. A single antigen is unlikely to adequately approximate protection against all circulating antigens and does not capture the potential for repeat vaccination to limit the breadth of the antibody responses11. The clinical consequences of repeated vaccination may vary depending upon which viruses circulate, their diversity and frequency15,29. Further work on a subset of participants is underway to examine responses to a range of old and contemporary antigens using antibody landscapes 11,38 to assess whether repeat vaccination limits the breadth of the antibody response.
In conclusion, we observed diminishing antibody responses with successive years of vaccination that was modified by the pre-vaccination titre and to some extent age and vaccine brand. While we did not note any important differences by sex or health status, our findings may not generalise to less healthy populations, nor to older adults who were not represented in our study. Further analyses on a subset of these HCWs are underway to understand the relative stimulation of de novo and recalled B cells that underlie these observations. Expanded cohorts are needed to better understand how attenuated immunogenicity among repeat vaccinees translates to vaccine effectiveness.





Responses