Articular cartilage loss is an unmitigated risk of human spaceflight

Introduction
Human spaceflight is a dangerous endeavor1. While in space, astronauts are exposed to hazardous conditions with dramatic impacts on their physiology2 and psychology3. Microgravity (defined as < 1 × 10−6 g) and space radiation are two hazards of human spaceflight consistently shown to have deleterious effects on the musculoskeletal system (MSKS)4. There are multiple lines of evidence indicating that cartilage is at risk in the space environment5,6,7,8. The mechanical unloading of joint cartilage disrupts cellular homeostasis, causing sex-dependent transcriptomic responses, and can result in the loss of viable tissue9. Cell-based studies have shown this disruption occurs rapidly, with chondrocytes, the cells responsible for cartilage homeostasis, adapting to microgravity in less than 24 h10. Though spaceflight data examining the effects of radiation on cartilage is limited, available clinical and animal data document radiation-induced bone and cartilage loss11,12. Additional clinical evidence indicates cartilage loss, characteristic of joint disease, increases fracture risk even in the absence of concomitant bone loss13. Besides increased fracture risk, joint diseases are associated with activity-limiting pain and stiffness that may impede an astronauts ability to perform vigorous exercise which is considered an essential countermeasure for long-duration spaceflight14. Finally, increased intensity and duration of exposures seem to lead to the accumulation of more cartilage damage12. The multifactored effects of cartilage loss must be considered when evaluating risks during long-duration missions. For example, several space agencies, including the National Aeronautics and Space Administration (NASA) and the European Space Agency (ESA), are currently preparing for long-duration missions to Mars with the evaluation of risks to crew health ongoing15,16. Note, the terms “articular cartilage,” “joint cartilage,” and “cartilage” will be used interchangeably throughout this article.
Missions to Mars are projected to be three years in duration with interplanetary travel comprising approximately one year of that time17. This extended transit in microgravity will likely increase the crew’s risk of cartilage dysfunction due to altered loading patterns and increased radiation exposure. Additionally, the physically demanding nature of exploration class missions may increase the risk of MSK injury18. Because of cartilage’s poor regenerative capacity19, injuries sustained during a mission may be more likely to result in joint disease. Moreover, there are currently no efficacious treatments for joint diseases, such as osteoarthritis (OA), for which cartilage loss is a hallmark20. This risk of injury and subsequent disease onset is of serious concern for the long-term health of astronauts as OA is a leading cause of disability world-wide21, impacting both quantity22 and quality23 of life. While various aspects of MSK health are considered at risk, the loss of articular cartilage is not widely recognized as a risk of human-spaceflight17. Subsequently, there is a scarcity of research efforts to better understand and effectively preserve articular cartilage in space. There is an unparalleled opportunity and an urgent need for agencies to accelerate their research efforts and advance our understanding of articular cartilage biology. These advancements could have a tremendous impact on multiple domains of astronaut health through the creation of targeted surveillance programs and dedicated countermeasures.
Articular cartilage biology
Articular cartilage is a dense soft tissue located at the surface of synovial joints (e.g., ankle, knee, wrist). It is composed primarily of water, type II collagen, and proteoglycans. The composition and structure of articular cartilage (Fig. 1) confers many of its biomechanical properties, including a viscoelastic extra cellular matrix (ECM) allowing it to deform and reform under cyclical loading24. Due to its aneural and avascular nature, physiologic loading is essential to the diffusion of nutrients25 into and exchange of waste26 out of the various layers of articular cartilage. The ECM is a hardy network of connective tissue that enables joint cartilage to accept astounding compressive loads with minimal friction27. In-vitro experiments estimate the shock absorption capacity of healthy articular cartilage to be 8 times the bodyweight of its host28,29. The ECM is solely maintained by chondrocytes, highly specialized cells that sense and respond to cues from their environment30. The closely regulated anabolic-catabolic signaling axis of chondrocytes are modulated by mechanical cues from the external environment (i.e. compression, shear) and chemical cues within the local microenvironment (i.e., growth factors)31. The process of converting mechanical forces into biochemical signals which influence cellular behavior is called mechanotransduction32.
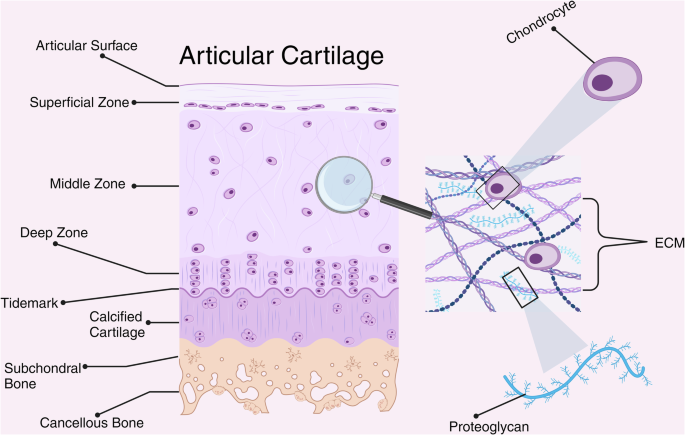
Cross-sectional schematic of healthy human articular cartilage. Cartilage is zonally organized with distinct compositions and functions of each zone. Chondrocytes are embedded within and responsible for maintaining the extra-cellular matrix. Figure created with BioRender.com.
At the cellular level, one mechanism of mechanotransduction occurs when mechanical forces generated by bodily movement temporarily deforms the pericellular matrix (PCM), a network of collagen adhered to and immediately surrounding chondrocytes32. In general, the deformation of the PCM from mechanical loading results in an increase in the production of type II collagen by chondrocytes. Additionally, chondrocytes can be influenced biochemically, through direct cell-receptor interactions including growth factors (i.e., FGF-18)33, inflammatory cytokines (i.e., IL-1β)34, and catabolic enzymes (i.e., MMP-13)34. If these mechanical and chemical signals are of sufficient intensity, they can result in adaptations to cellular structure and function through a process called mechanoadaptation35. These two biological principles, mechanotransduction and mechanoadaptation, underpin scientist’s understanding of how cartilage responds in the space environment and has direct implications for long-duration missions.
This astonishing ability of our tissues to attune to our environment has both beneficial and detrimental consequences. Being that we have evolved over millions of years with a relatively stable degree of radiation exposure and gravitational load, perturbations to these conditions, as seen during spaceflight, disrupt many key physiologic processes35,36 and can lead to the degeneration of cartilage. The health of this weightbearing tissue is essential for astronauts to perform mission-critical tasks including exercise countermeasures, post-landing procedures, and surface operations17,37. As a result, the compromise of cartilage could jeopardize mission success and crew safety during deep-space exploration6,18. There is a dire need to deepen our understanding of articular cartilage biology to better mitigate the effects of spaceflight on crew health and performance.
Microgravity – unloading
The gravitation field strength acting on our bodies directly influences joint homeostasis due to the mechanosensing capacity of cartilage. The proportional relationship between gravitational conditions and loading patterns is one potential mechanism of microgravity induced cartilage loss. Broadly, as gravitational strength decreases so too does the mechanical load acting on cartilage. Though astronauts engaging in deep-space exploration will experience a variety of gravitational conditions, the mechanical unloading experienced in microgravity (i.e. 1 × 10−6 g or one millionth Earth’s gravity)36 will shift the homeostatic balance of bone, muscle, and cartilage towards a catabolic phenotype38.
A systematic review published in 2020 calculated the average bone loss of the lower limb to be −0.8% per month totaling an estimated −4.8% for a 6-month International Space Station (ISS)39. During the same duration aboard the ISS, the loss of skeletal muscle mass is more severe, ranging from −6% at the quadriceps to −10% at muscles of the lumbar spine4. Data on cartilage loss during spaceflight is sparse, though analog studies show the rate and magnitude of cartilage loss to be greater than or equal to the loss of bone and muscle40,41. In addition to structural changes, biomarkers of cartilage metabolism, such as cartilage oligomeric matrix protein (COMP), appear sensitive to spaceflight analogs and immobilization40,42,43,44.
Though flight data examining the effects of microgravity on cartilage is extremely limited, available data demonstrates changes in biomarkers of cartilage metabolism indicative of a catabolic shift in the joint microenvironment45. A technical report published in 2021 identified significant metabolic alterations of 12 astronauts who spent 4–6 months on the United States Orbital Segment (USOS) of the ISS46. The investigators assessed pre, intra, and post-flight levels of urinary cross-linked C-telopeptide of type II collagen (uCTX-II). CTX-II is a mechanosensitive biomarker of type II collagen degradation that is elevated in joint diseases like OA47. The preliminary results showed in-flight measurements sharply increased from pre-flight levels and peaked around 4 months on station. Crucially, uCTX-II levels remained elevated one-year post-flight in over 40% of astronauts, indicating the continued degradation of cartilage. The investigators also noted significant variability between astronauts though the reason for these differences is unknown.
Though flight data is limited, analog studies have consistently documented the deleterious effects of simulated microgravity on joint cartilage. An early 6˚ head-down tilt (HDT) bedrest study showed a −14.8% reduction in serum COMP levels and an −8.3% average and −14.6% maximum tibial cartilage loss via MR imaging following a 14-day campaign40. A late 21-day bedrest study, titled “MNX” (medium duration nutrition and exercise study), showed similar catabolic effects using serum and urinary measures of cartilage metabolism48. Urinary CTX-II showed a marked response and stayed elevated one week after re-ambulation. Additionally, the investigators assessed the anabolic-catabolic signaling axis by measuring Procollagen II C-Propeptide (CPII) and Collagen type II Cleavage (C2C). These measures showed a pronounced increase in catabolic activity through C2C and reductions in anabolic activity through CPII. Notably, the exercise and nutritional countermeasures were unable to mitigate the catabolic effects of bed rest on cartilage.
Consistent with these findings, a recent five-day bed rest study evaluated the ability of submaximal weightbearing exercise to mitigating the catabolic effects of immobilization42. Researchers repeatedly measured COMP and matrix metalloproteinase-3 (MMP-3) to assess changes in cartilage metabolism. MMP-3 is a mechanosensitive protease capable of cleaving proteoglycans and type II collagen. The results indicate the exercise intervention was ineffective at counteracting the negative effects of immobilization on cartilage with reductions in serum COMP levels reaching –11.7% and MMP-3 levels reaching –17% in the first 24 hours of bed rest. Taken together, these studies reinforce the need to accelerate research efforts, in real and simulated microgravity, to design efficacious countermeasures intended to mitigate the structural and metabolic effects of spaceflight on articular cartilage.
Microgravity – Fluid shifts
Earth’s gravity not only influences the external mechanical forces generated during movement, it also influences the internal osmotic forces generated by the flow of fluids within our body, namely fluid-shear stress and hydrostatic pressure49. Though it’s well-established that microgravity degrades focal adhesions and pressure gradients within our body, fluid mechanics are complex and not fully uderstood49. The limited data available on the effects of fluid shifts on the MSKS is concerning. Changes in fluid behavior in microgravity have been documented to produce a 99% reduction in fluid shear stress, decreased fluid velocity, and cause the near complete dissolution of hydrostatic pressure within bone43,44. Alterations in these critical properties result in multi-cellular spheroids, altered stem cell gene expression, cell-cycle arrest, and increased apoptosis49,50,51. Though these biophysical changes are believed to be mechanisms of microgravity induced bone loss, they have not been rigorously examined in the context of articular cartilage.
While on Earth (1 g), the high-water content and negatively charged proteoglycans of cartilage impart an electrostatic force that controls the flow of fluid out of and into the ECM52. During joint loading, proteoglycans are compressed together, creating a repulsive force which pushes water out of the ECM and into the interstitial space26. This interstitial fluid, unable to return into the ECM, generates a hydrostatic pressure which resists the compressive forces being exerted on the joint26,27. Once the load is removed, proteoglycans spread out, their electrostatic repulsion diminishes, and fluid flows back into the ECM26. This sponge-like behavior of the ECM is critical for the protection of cartilage under load and is the primary route of nutrient delivery to chondrocytes (Fig. 2)26. Furthermore, gravity-induced hydrostatic pressure is the mechanism for focal adhesion formation. Focal adhesions are the adherence of cells like chondrocytes to surrounding material like the PCM. Without these adhesions, chondrocytes may lose their ability to sense changes within their mechanical environment. Changes in fluid behavior may result in reduced force transmission, weightbearing capacity, and inadequate delivery of nutrients to cartilage.
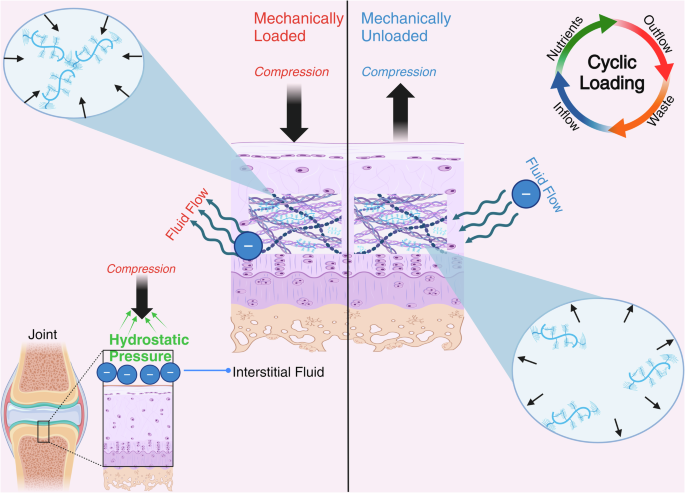
Under 1 g conditions, the repulsive nature of negatively charged proteoglycans creates a hydrostatic force that resists compressive forces acting on the joint during mechanical loading. Cyclic loading, typical of human movement, produces fluid inflow and outflow enabling waste removal and nutrient delivery to articular cartilage. Figure created with BioRender.com.
There are differences between upper extremity and lower extremity fluid shifts in microgravity49 which may provide unique conditions researchers can use to distinguish between the effects of mechanical unloading from the effects of fluid shifts. There are also considerable discrepancies between the rate of articular cartilage loss during HDT bedrest studies (−8% to −14% in 14 days)40 and the rate of articular cartilage loss observed during immobilization or spinal cord injury studies (−6.6% in 7 weeks to −13% in 12 months)41,53,54. Considering HDT bedrest studies are designed to recreate both the mechanical unloading and cephalic fluid shifts of microgravity, the accelerated cartilage loss may indicate an additive effect of these two conditions. Indeed, evidence suggests the magnitude of tissue loss and rate of recovery are dependent upon the tissue type and body region. For example, muscle loss of the lower extremity often exceeds that of the upper-extremity55,56,57, while recovery of spinal cartilaginous tissue generally recovers slower than spinal muscle volume following spaceflight56,58. Furthermore, a reduction in bone mineral density of the spine is associated with decreased vertebral strength and a resulting increase in the risk of vertebral fracture59. This differential loss and recovery of MSK tissues has direct health and performance implications that must be considered during the design of long-duration missions, countermeasures, and reconditioning protocols.
Space radiation
Earth’s magnetosphere is a magnetic field that forms a semi-permeable shield around the planet, deflecting radiation from the sun and other cosmic sources. During voyages to the Moon and Mars, astronauts will travel beyond Earth’s magnetosphere potentially exposing themselves to higher doses of space radiation. Models predict, during a 3-year Mars mission, astronauts will absorb 450 mGy (~150 mGy/yr)17 or nearly 5 times the average yearly exposure on Earth. Possibly more concerning than the level of exposure is the volatile type of radiation astronauts will be exposed to, including: galactic cosmic radiation (GCR) from supernovas and solar particle events (SPE) from the sun60. These highly energetic forms of radiation are substantially more biologically damaging than ionizing radiation experienced on Earth (Fig. 3)61.
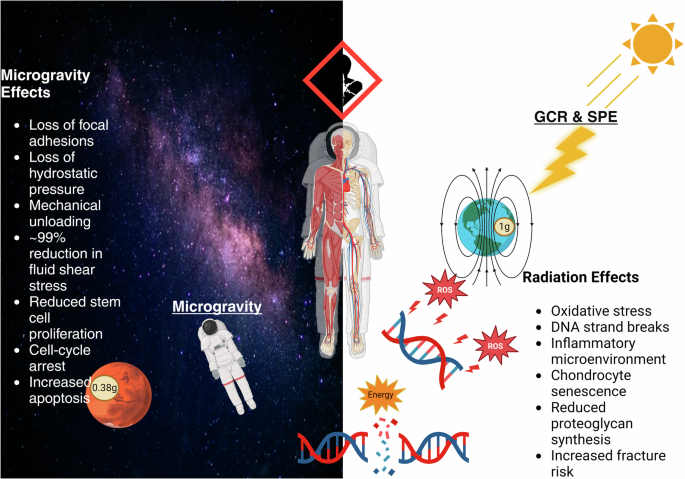
Microgravity and space radiation alter the mechanical, chemical, and osmotic environments of articular cartilage resulting in detrimental changes to its cellular structure and function. Figure created with BioRender.com.
Animal and human models show both bone and cartilage degradation following radiation exposure62. Sclerostin, a glycoprotein primarily produced by osteocytes, induces a catabolic shift in bone by inhibiting the Wnt signaling pathway63. Sclerostin increases following bone irradiation while sclerostin antibodies mitigate radiation-induced bone loss64. Due to the well-established role sclerostin plays in spaceflight induced bone loss, there is increased interest in the use of recombinant parathyroid hormone and other therapeutic agents to lower sclerostin levels65. Interestingly, due to shared biochemical pathways, sclerostin inhibition may be protective for bone but destructive for cartilage64,66. Specifically, sclerostin inhibition increases subchondral bone hypertrophy and osteophyte formation in joints, both of which are characteristic of OA66. The extensive biologic cross-talk between bone and cartilage is one reason why bone research alone is not sufficient to progress our understanding of cartilage radiobiology.
To date, there are no published human spaceflight studies examining the effects space radiation has on articular cartilage though there is pertinent clinical data. Radiation therapy is often used to treat focal cancers of the prostate and cervix using external beam radiation therapy. One retrospective analysis of 346 men treated with radiation therapy for prostate cancer identified a dose-response relationship between hip OA and radiation delivered to the femoral head11. Similarly, in a retrospective sample of over 500 women who underwent radiation therapy for advanced cervical cancer found a significant increase in pelvic fractures especially with radiation therapy involving high dose rates67. Although the clinical data is limited, findings seem to indicate that the higher the rate and dose of radiation exposure, the higher the risk of bone and cartilage loss. It is important to note, these clinical studies utilized significantly higher radiation doses (30–50 Gy) than what is expected in the space environment (0.1 Gy–1 Gy) and because of this the generalizability to spaceflight is limited. Human spaceflight research is needed to determine if these dose-response relationships identified in clinic data are also present at doses typical of spaceflight.
Animal experiments are congruent with clinical data showing radiation exposure at a spaceflight relevant dose (0.1 Gy–1 Gy) creates an inflammatory microenvironment with increased MMP-13 expression, reduced proteoglycan synthesis, and chondrocyte senescence68. Two animal model studies have investigated the separate and combined effects of radiation and hind-limb unloading on the cartilage of mice and rats (Fig. 4)12,68. Hindlimb unloading is used to simulate microgravity by eliminating weightbearing forces acting on the limbs. The radiation doses used by Kwok et al. were 0.1 Gy, 0.5 Gy, and 1.0 Gy while Willey et al. used 1 Gy for all groups. These radiation doses were specifically selected to simulate spaceflight conditions.
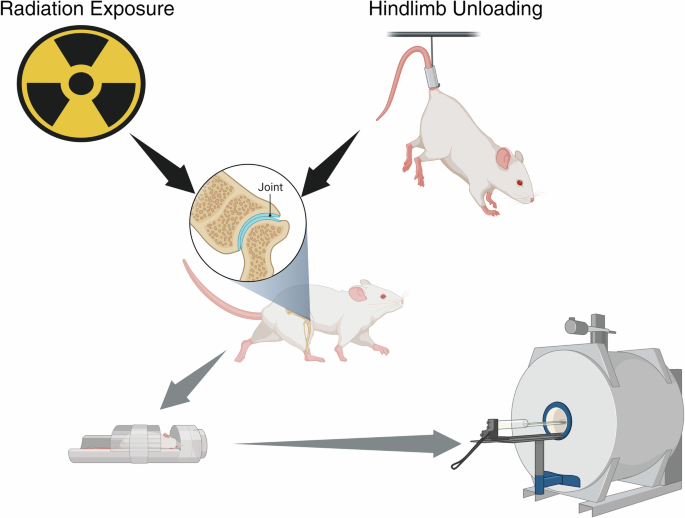
Schematic of animal experiments modeling the individual and combined effects of microgravity and radiation on joint cartilage. Figure created with BioRender.com.
Kwok et al. utilized contrast-enhanced computerized tomography (CECT) following the exposure protocol and identified a maximal cartilage loss of −27% in the unloading plus 0.5 Gy radiation group68. Notably, the severity of cartilage loss was located at the areas of maximal weightbearing68. Willey et al. also examined the rate of cartilage loss induced by mechanical unloading or radiation exposure with joint cartilage showing signs of degeneration ~40% sooner following irradiation than mechanical unloading alone (8 days vs. 13 days)12. Furthermore, combined mechanical unloading and radiation exposure (1.0 Gy) demonstrated an additive effect, resulting in increased T2 relaxation times on magnetic resonance imaging (MRI), a quantitative index of collagen disorganization12.
These studies12,68 provide compelling evidence that a spaceflight relevant dose of radiation independently leads to cartilage loss and can pathologically synergize with unloading to produce accelerated damage to cartilage. Though the mechanisms of radiation-induced cartilage loss are ambiguous, the relevant animal and clinical studies clearly define cartilage as at-risk in the space environment, illustrating the need for more consistent inclusion of cartilage in radiobiologic research.
Conclusion
Preliminary evidence seems to indicate microgravity and space radiation have individual and additive effects on joint cartilage. It is though unclear how cartilage will adapt to long-duration spaceflight and how these adaptations will impact the health and performance of astronauts. This represents an existential blind-spot in aerospace research that is of critical concern for future exploration- class missions. There is an urgent need for more high-quality analog and spaceflight research examining the effects of microgravity and radiation on articular cartilage. Considering the robust research infrastructure of agencies like NASA, there’s tremendous potential for scientists to advance our knowledge of cartilage biology and develop efficacious countermeasures that could revolutionize joint health practices of both aerospace and terrestrial medicine.

Responses