Can artificial intelligence be the future solution to the enormous challenges and suffering caused by Schizophrenia?

Introduction
Schizophrenia (SZ) is a severe mental disorder that exerts a devastating impact on the brain and daily life of patients. It can lead to abnormalities in early brain development and induce a range of symptoms, including hallucinations, thought disorders, decreased motivation, and cognitive impairment. The lifetime prevalence of SZ is approximately 0.7%, which makes it the leading cause of disability-adjusted life years1. According to the World Health Organization, approximately 21 million people worldwide are affected by SZ2. The average age of onset is 18 years for women and 25 years for men3,4. SZ has emerged as a significant global public health concern that demands urgent attention5. Currently, the diagnosis of and treatment strategies for SZ primarily rely on symptomatology research. Despite the use of the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) to diagnose SZ, clinicians encounter numerous challenges. For example, SZ poses a major challenge in terms of accurate diagnosis and individualized treatment because of its marked heterogeneity in clinical manifestations, symptom profiles, and disease trajectories. This inherent complexity necessitates a considerable reliance on the clinical expertise and professional discernment of psychiatrists throughout the diagnostic and therapeutic processes. However, the excessive dependence on incomplete clinical impression and noncomprehensive patient evaluation potentially compromises the accuracy of diagnosis and efficacy of treatment interventions. Epidemiological studies have indicated that the misdiagnosis rate of SZ in clinical practice can be as high as 25%6. Approximately 30% of patients with SZ exhibit treatment-resistant characteristics and respond poorly to conventional antipsychotic medications, indicating a major challenge7. Furthermore, patients considered as having treatment-resistant SZ (TRS) typically exhibit significant pharmacological resistance, indicating the inability of the existing medications to adequately alleviate positive or negative symptoms. Despite the lack of efficacy of existing medications, these patients frequently endure severe side effects from pharmacotherapy during treatment8. This situation highlights the limitations of the current diagnostic and therapeutic approaches for SZ. Furthermore, it underscores the urgent need to address the scientific challenge of deconstructing the high heterogeneity of the disorder to achieve individualized and precise diagnostic and treatment strategies. There is a growing consensus that new artificial intelligence (AI) methodologies are essential for the advancement of SZ research. The human brain functions similarly to digital systems, whereas machines operate digitally. AI enables machines to learn and identify patterns and relationships from large, representative datasets. By integrating complex, heterogeneous, and multidimensional data through AI algorithms, AI serves as a revolutionary tool in psychiatric research and precision medicine. Consequently, the development of diagnostic and individualized precision treatment methods based on objective biological markers holds significant clinical importance.
Schizophrenia prompts profound reflection on various issues in contemporary society. To what extent does this illness contribute to the invisible cracks within our social fabric? Is it possible to identify effective solutions or even a cure for this condition? In the face of this challenge, how should humanity navigate the future? Could AI emerge as a potential solution?
AI, particularly machine learning (ML) and deep learning (DL), has significantly advanced the computer-aided diagnosis and treatment of SZ through the detection of subtle biological markers. ML algorithms excel in extracting features from high-dimensional data and making individual-level predictions, primarily through supervised and unsupervised learning9. Supervised learning algorithms (e.g., support vector machines (SVMs)) utilize labeled data to establish classification models; for example, SVMs particularly excel in distinguishing patients with SZ from healthy controls using neuroimaging data10. In contrast, unsupervised learning algorithms (e.g., principal component analysis) can reveal inherent patterns within unlabeled data, which aids in patient subtyping11.
In recent years, semisupervised and reinforcement learning have emerged as promising approaches in SZ research. Semisupervised learning integrates a small amount of labeled data (e.g., features from healthy controls) with a large amount of unlabeled data (e.g., features from patients) to identify meaningful patterns. Thus, this method addresses the challenges of high costs and time-consuming processes associated with data labeling12. Reinforcement learning, which is conducted by simulating interactions between an agent and its environment and leveraging reward–penalty mechanisms, can iteratively optimize treatment strategies, demonstrating a significant potential for personalized treatment and patient behavior modeling13. For example, it can be applied to the development of individualized medication plans or improvement of cognitive–behavioral interventions.
Compared with traditional ML algorithms, which rely on preselected features, DL algorithms can automatically and directly extract features from raw data, thereby preserving more comprehensive information. DL models include convolutional neural, recurrent neural, and graph convolutional networks for image analysis, sequential data analysis, and structural data analysis, respectively. Compared with ML, DL demonstrates superior performance in large-scale data analysis while requiring less domain-specific feature engineering14.
Indeed, the application of DL has advanced personalized psychiatric treatment strategies. However, traditional multilayer perceptron models have certain limitations, including large data requirements and limited interpretability15. The recently developed Kolmogorov–Arnold networks address these challenges through innovative features such as residual activation functions and dynamically updated spline grids, thus enhancing model performance and training efficiency.
Overall, these computational approaches have expanded SZ research from traditional case–control studies to those focused on individualized prediction and analysis. The integration of AI provides an objective and standardized framework for diagnosis and treatment optimization, which overcomes subjectivity in clinical assessments and advances precision medicine across nations, ethnicities, and populations. Figure 1 outlines key components of AI-driven assessment, algorithm selection, and individualized treatment pathways.
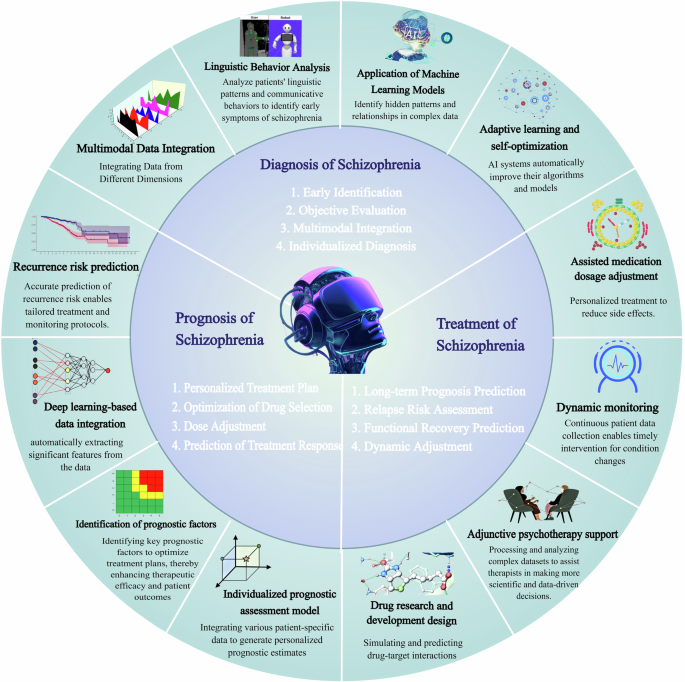
The diagram is divided into three major sections, namely, diagnosis, treatment, and prognosis. The inner circle highlights the advantages of AI in these three clinical domains, whereas the outer circle provides a detailed depiction of the specific applications of these advantages. Taken together, these elements form a comprehensive system that is designed to enhance the management and therapeutic outcomes of SZ through personalized and data-driven AI approaches.
Methods
Search strategy
We conducted a comprehensive literature search following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Fig. 2)16,17. This study specifically focused on studies that utilized AI for the classification of SZ. Herein, we first carefully defined the research problem related to SZ, which enables the development of a targeted approach for our review. We conducted a comprehensive search of three databases, namely, PubMed, Web of Science and Cochrane, and included all relevant literature published within the past 5 years up to October 1, 2024. Randomized Controlled Trial (RCT) was conducted to investigate the efficacy of AI-assisted diagnosis and treatment in SZ patients. Additionally, we manually screened the references of reviews, systematic reviews, meta-analyses, and trials to identify further relevant studies, which broadened the scope of the research and enhanced accessibility.
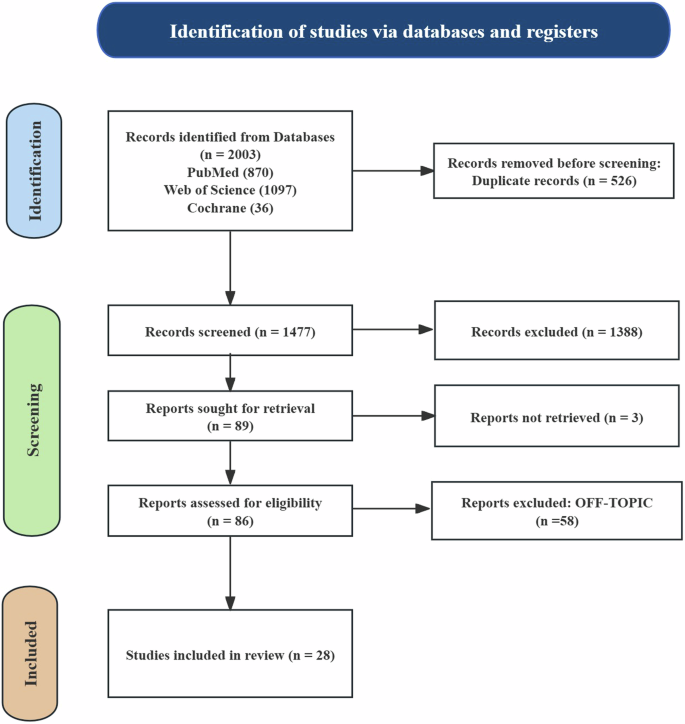
This figure illustrates the flowchart of the literature search and the article inclusion process, detailing the steps for identification, screening, and exclusion.
The search strategy employed the following keywords: (“Artificial intelligence” OR “Machine learning” OR “Deep learning”) AND (“schizophrenia*” OR “schizophrenic disorder*” OR “schizophrenic”). We made the necessary adjustments to accommodate the specific requirements of each database. These terms were selected to precisely capture the research objective, which was to evaluate the effectiveness of AI in the diagnosis, treatment planning, and prognostic assessment of SZ. Table 1 presents the detailed search strategy.
Inclusion and exclusion criteria
Data extraction from the included articles was conducted by two independent reviewers, who screened and evaluated the studies based on the following inclusion and exclusion criteria18. A standardized data extraction form, which was developed based on the Clinical AI Model (CLAIM) Reporting Framework, was used to systematically evaluate the reporting of studies on AI models19. Detailed information regarding data extraction is provided in the online supplementary materials (Appendix S1).
Inclusion criteria
-
(1)
Full-text availability;
-
(2)
The trial method was an RCT;
-
(3)
Scientific studies evaluating the positive role of AI in diagnosis, treatment planning, and therapeutic monitoring;
-
(4)
Studies that meet the Population, Intervention, Comparison and Outcome (PICO) model criteria20.
Exclusion criteria
-
(1)
Non-English publications;
-
(2)
Studies that do not meet the research design requirements or lack data on key outcome measures;
-
(3)
Publications categorized as reviews, commentaries, conference abstracts, case reports, letters, books, or dissertation theses; and
-
(4)
Studies in which the study population is not explicitly defined as patients with SZ and the diagnostic criteria (DSM or International Classification of Diseases (ICD))16,17 are not clearly specified.
Screening and data extraction
Two researchers independently reviewed the titles and abstracts, assessed the full-text publications, selected trials, extracted data, and evaluated the quality of the studies. This process was conducted in accordance with the standards outlined in the Cochrane Handbook for Systematic Reviews. A third researcher resolved disagreements and facilitated consensus through discussion21. The selection of published articles began with an initial screening of titles and abstracts. After excluding irrelevant studies, the remaining articles were reviewed in detail to determine their eligibility based on the inclusion criteria. Data were then extracted using a predesigned form. The following information was extracted from each study: study objective (e.g., prediction, treatment, and prognosis), characteristics, data sources (e.g., participants and databases), and AI models (e.g., SVMs and neural networks). Additionally, we documented the programming languages used for the AI technologies, types of data analyzed (e.g., clinical, laboratory, and biological data), and statistical metrics (e.g., accuracy, specificity, sensitivity, and precision) (Table 2). We adhered to the PRISMA principles and considered various aspects of the Clinical Assessment of Interventional Measurement (CLAIM) when reporting the articles.
AI-guided strategies for addressing SZ
AI-enhanced diagnosis of SZ
AI has been applied in diagnostics across multiple medical fields22,23, such as orthopedics and oncology. However, the application of AI in psychiatry substantially differs from that in other areas. The primary reason is that psychiatry does not typically involve clear organic changes, which makes it difficult to delineate target areas. This poses challenges for AI-based image discrimination techniques, which excel in target delineation. Consequently, the current efforts in psychiatry mainly focus on multimodal technical analysis. The prodromal symptoms of psychosis, such as anxiety and paranoia, are typically subtle, subjective, and difficult to quantify. Thus, these symptoms may not meet the criteria for any specific disorder, which complicates the diagnostic process.
Unlike other diseases where diagnosis can rely on objective biological markers, schizophrenia diagnosis, based on DSM and ICD criteria, remains entirely dependent on clinical symptomatology24. This symptom-oriented diagnostic approach presents significant limitations. Primarily, the heterogeneous definitions between DSM and ICD regarding diagnostic thresholds, symptom duration, and functional impairment severity may lead to diagnostic heterogeneity, subsequently affecting disease identification and classification. Furthermore, the prodromal symptoms of psychotic disorders, such as anxiety and paranoia, often manifest insidiously and resist quantitative assessment. More critically, the natural progression pattern of these prodromal symptoms remains incompletely elucidated, posing substantial challenges in prognostic prediction. Longitudinal data demonstrate that in a two-year follow-up period, only approximately 20% of young individuals presenting with high-risk symptoms ultimately developed psychosis, while 41% experienced symptom remission25. Although untreated psychosis significantly correlates with poor prognosis, the current lack of effective tools for identifying high-risk individuals necessitates waiting for explicit psychotic symptoms and excluding alternative diagnoses before confirmation, thereby potentially delaying optimal therapeutic intervention.
In response to these challenges, artificial intelligence technologies, utilizing computational methods such as deep learning algorithms and natural language processing, can integrate multidimensional data including clinical symptomatology characteristics, neurocognitive functional assessments, and social functioning scales to provide standardized diagnostic decision support. Notably, in the comprehensive analysis of prodromal symptoms, the development of more reliable AI-based diagnostic systems may facilitate early identification of high-risk youth.
Early identification of high-risk populations
A prospective study involving a high-risk young population revealed that individuals with a high genetic risk for schizophrenia exhibited greater SZ spectrum symptoms than the general population26. These high-risk individuals also exhibited abnormalities in brain structure and function, which contribute to the prediction of whether they will eventually develop SZ or related psychopathological symptoms27. These findings provide valuable evidence for predicting the onset of SZ and highlight the importance of early identification among high-risk populations.
To enhance the accuracy of prediction, advanced neuroimaging techniques, such as magnetic resonance imaging (MRI) combined with ML algorithms, have been employed. For instance, in a prospective cohort study, researchers utilized structural MRI data to construct an SVM model based on gray matter volume (GMV)28. This model effectively classified healthy controls, clinical converters (i.e., individuals who developed SZ during follow-up), and clinical nonconverters and achieved high accuracy rates. Additionally, other studies further identified the key features contributing to the classification model, which primarily involved changes in GMV in specific brain regions29. These findings underscore the potential of combining neuroimaging with ML techniques to improve early detection and intervention strategies for SZ.
Building on this, other researchers have developed a DL model based on MRI data to monitor mental health risks. This model was trained and tested on an MRI dataset containing data from 14,915 patients with severe mental disorders and 4538 healthy controls. The results demonstrated that the model achieved high accuracy and sensitivity in distinguishing individuals with mental disorders from healthy controls30. Similarly, functional MRI (fMRI) studies have identified enhanced functional connectivity within the cerebellar–thalamo–cortical circuit as a neurofunctional characteristic of patients with SZ31. This enhanced connectivity has also been demonstrated to support individualized predictions of SZ onset32. These findings further underscore the significant potential of combining ML algorithms with MRI for predicting SZ and identifying high-risk populations.
However, despite the promising performance of MRI-based analyses in the early prediction of SZ, the limitations of relying on a single data source cannot be overlooked. To achieve higher predictive and diagnostic accuracy, a multimodal and multidimensional integrative approach is warranted in clinical practice. This approach should incorporate diverse data sources, including genetic information, environmental factors, psychological assessments, behavioral observations, and other biomarkers. For instance, Chen et al. demonstrated that33 combining imaging features with behavioral characteristics derived from cognitive assessments within a multitask ML framework effectively distinguished individuals with early psychosis from healthy controls, achieving high classification accuracy. Similarly, Ebdrup et al.34 evaluated the diagnostic potential of multimodal data, including cognitive, electrophysiological, structural MRI, and diffusion tensor imaging data, combined with ML algorithms in First episode drug naive (FEDN) patients with SZ.
In addition, a previous study35 based on resting-state electroencephalography (EEG) microstate segmentation revealed that variations in microstate measurements can effectively distinguish patients with SZ from healthy individuals. By integrating genetic data with neuroimaging findings, researchers36 revealed that the unaffected siblings of patients with SZ exhibited significant differences in specific functional network connectivity patterns compared with healthy controls. The changes in these functional connections are associated with polygenic risk scores, suggesting that such network abnormalities may serve as early neurobiological markers of genetic susceptibility to schizophrenia. This finding supports previous research indicating that bioinformatics analyses can identify potential biomarkers37. This finding provides new insights into the early identification of and intervention strategies for the disorder. Collectively, these studies highlight the critical importance of multimodal data integration in improving diagnostic precision.
AI has demonstrated significant advantages in the prediction of SZ. First, AI is capable of processing and analyzing complex, high-dimensional data. By leveraging ML, AI can identify intricate patterns and relationships through high-performance computing in tasks that are highly challenging for clinicians to perform manually, thus greatly enhancing clinical efficiency. Second, AI systems possess the ability to continuously learn and self-optimize. As new data become available, algorithms can be refined to improve predictive accuracy, which enables AI to effectively address the challenges posed by highly heterogeneous diseases such as SZ. Furthermore, AI can integrate diverse data sources, including linguistic and behavioral data from patients, providing more comprehensive insights for disease prediction and diagnosis.
Nevertheless, relying solely on disease prediction models is insufficient for fully demonstrating the unique advantages of AI in SZ diagnosis. Achieving precise and individualized diagnostic capability requires a more comprehensive approach that not only predicts the likelihood of disease onset but also evaluates diverse symptoms, identifies disease subtypes, assesses disease severity, and predicts disease progression trajectories. By integrating these complex dimensions, the potential of AI in psychiatry can be fully realized, providing patients with more personalized and accurate diagnoses.
Language behavior analysis and SZ diagnosis
Recent research published in prestigious journals, such as Nature and Science, has revealed the intricate connections between language and the brain from a neuroscience perspective22,38,39. According to the DSM-5, SZ diagnosis can be made in the presence of at least two of the five characteristic symptoms, one of which must be a positive symptom, such as hallucinations, delusions, or disorganized thinking and speech40. These positive symptoms are typically reflected in the patient’s language. In addition, other symptoms, including severely disorganized or catatonic behavior (positive symptoms) and negative symptoms such as affective flattening, can be typically identified through the prosody of speech behavior41,42.
However, relying solely on psychological scales cannot fully capture the complexity of human discourse, particularly the influence of cultural and emotional factors on psychopathology. Conversely, AI offers robust tools to analyze language behavior by detecting subtle acoustic parameters in speech and calculating semantic coherence from transcribed text. This enables a more comprehensive evaluation of social cognition expressed through language, facilitating precise diagnosis of mental disorders. Therefore, this section focuses on the specific applications of AI in the assessment of speech behavior symptoms to assist in SZ diagnosis.
Recent research has shown that individuals with schizophrenia exhibit significant deficits in emotional prosody processing (EPP)43. Specifically, they demonstrate a relatively diminished ability to convey emotions through voice characteristics such as tone, pitch, speech rate, and rhythm during recognition tasks. Furthermore, these EPP deficits are closely associated with abnormal activity in certain brain regions, underscoring the importance of emotional prosody recognition in the early diagnosis of schizophrenia. By integrating natural language processing (NLP) techniques with neurophysiological data, such as magnetoencephalography, the analysis of language fluency in patients with schizophrenia, particularly focusing on EPP, can significantly enhance diagnostic accuracy. Another study evaluated the effectiveness of an NLP model in detecting cognitive distortions in text messages exchanged between patients with severe mental illnesses and their clinicians. The results illustrated that the model’s performance (F1 = 0.62) was comparable to that of human annotation (F1 = 0.63)44. These findings highlight the significant role of NLP technology in analyzing language behavior and identifying symptoms of mental disorders.
Further neuroscience research has combined speech analysis with neuroimaging data, revealing a strong correlation between abnormalities in syntax and semantics and disruptions in neural connectivity in patients with mental disorders45. For instance, fMRI studies have shown that patients with SZ exhibit significantly reduced activation in the left primary auditory cortex during sentence and reversed speech tasks regardless of the presence of hallucinations. This phenomenon indicates that such reduced activation reflects early deficits in information processing and is not a consequence of hallucinations46.
Neuroscience research has also integrated speech analysis with neuroimaging data, revealing that abnormalities in syntax and semantics among patients with mental disorders are highly correlated with disruptions in neural connectivity47. This finding provides a neurobiological basis for AI-assisted diagnosis of SZ. By combining speech analysis with neuroimaging, this approach not only enhances the efficiency and accuracy of diagnosis but also offers new quantitative metrics for understanding patients’ language impairments It aids clinicians in the identification and comprehension of cognitive deficits in conceptual organization and language coherence, thus leading to more comprehensive and accurate diagnostic assessments of mental health conditions. These findings provide a neurobiological basis for AI-assisted diagnosis of SZ and offer new perspectives for understanding the language impairments observed in these patients.
In addition to syntax and coherence analysis, semantic density has emerged as a key research area in understanding the language deficits associated with SZ. A study conducted by a research team from Harvard University utilized vector unpacking techniques to quantify semantic differences by measuring the distances between words within a “semantic space”48. The researchers developed a baseline algorithm that analyzed over 30,000 posts by Reddit users and compared these linguistic data with clinical experimental data to detect semantic density. The results indicated that by automatically analyzing just two linguistic variables—semantic density and sound-associated vocabulary—it is possible to predict whether high-risk individuals will develop mental disorders. This approach highlights the extensive potential of AI in processing large-scale linguistic data and identifying individuals at risk of developing mental illnesses.
In summary, the use of AI in analyzing patients’ language behavior symptoms has been demonstrated to significantly improve the accuracy and efficiency of SZ diagnosis. However, SZ is characterized by high individual heterogeneity, with substantial differences in initial symptoms among patients and considerable fluctuations in the disease course. For instance, depressive symptoms may emerge during SZ treatment; however, antidepressant treatment is typically overlooked because of the difficulty in recognizing changes in disease progression. This can lead to the exacerbation of symptoms or even suicidal behavior. Therefore, accurate diagnosis should focus not only on the initial prediagnostic stage but also the timely detection of heterogeneous changes throughout the disease course. For example, Xu et al.49 demonstrated that by integrating multimodal behavioral features with ML techniques, it is possible to classify individuals into healthy controls, patients with SZ, and those with major depressive disorder. This approach provides scientific evidence and technical support for the timely identification of dynamic changes in patients with SZ.
This dynamic and individualized diagnostic capability is a critical factor influencing the precision of clinical treatment and is essential for the improvement of therapeutic outcomes and patient prognosis. In the following sections, we will further outline the specific applications of AI technology in assisting with symptom recognition and predicting the progression trajectories of patients with SZ.
Critical role and challenges of dynamic diagnostic capabilities in symptom recognition
A study demonstrated the unprecedented potential of integrating large-scale neuroimaging data with AI algorithms to map the progression trajectory of SZ50. Through the implementation of disease progression models, researchers have identified two distinct origins of cerebral atrophy: language region and hippocampal subtypes. In a subsequent investigation involving over 500 patients, the study found51 that the efficacy of antipsychotic medications was significantly correlated with different phenotypic subtypes. Furthermore, the research indicated that transcranial magnetic stimulation exhibited enhanced therapeutic effects during the pre-atrophy phase. These findings underscore the immense potential of artificial intelligence in tailoring therapeutic interventions based on the stages and subtypes of the disease.
As complementary approaches for dynamic disease monitoring, researchers have developed various ML methods that enable the continuous assessment of SZ progression. For example, Khare et al.52 engineered a hybrid decision support system based on EEG signals and achieved real-time monitoring of neural activity patterns through the integration of robust variational mode decomposition with optimized extreme learning machine classification. Additionally, cognitive function and electrophysiological assessments successfully distinguished between two different subtypes of schizophrenia patients24. Wearable devices demonstrated a particular utility in the continuous monitoring of negative symptoms53. Moreover, significant neurocognitive distinctions have been reported between deficit SZ and amnestic mild cognitive impairment54. Structural MRI analyzing gray matter density serves as a valuable auxiliary diagnostic tool for differentiating SZ from bipolar disorder55. A computer-adaptive diagnostic system56 further enhanced dynamic monitoring capabilities by evaluating only 11 key parameters, achieving remarkable accuracy in distinguishing disease states (93% for initial classification and 86% for subtype differentiation). These diverse methodologies collectively illustrate the potential of AI to capture the dynamic characteristics of SZ progression, facilitating timely and targeted interventions based on real-time disease monitoring.
Despite these advancements, it remains uncertain whether high-throughput cross-sectional data can accurately represent the natural progression of SZ. Although data-driven models have successfully stratified patients and identified pathological subtypes, their reliance on static datasets limits their ability to capture dynamic disease trajectories. Prospective trials or flexible dimensional approaches could address this limitation by validating subtypes and considering interindividual variability. These strategies would enhance precision in diagnosis and treatment, enabling clinicians to better understand the heterogeneous progression of SZ.
To address these challenges, an international collaborative team proposed a novel approach known as individualized structural covariance network analysis57. This method was used to analyze T1 images from 114 patients with various mental disorders, including schizophrenia, compared to 110 healthy controls. The study revealed significant individual differences in structural covariance changes, despite the marked heterogeneity among patients, which were associated with the severity of hallucinations. Notably, certain structural covariance edges, such as connections between the left hippocampus and bilateral putamen/pallidum, consistently classified patients into subgroups with distinct anxiety and depression profiles. These subgroup differences remained stable across disease stages, underscoring their biological relevance and potential for guiding personalized interventions.
The individualized predictive capabilities of AI represent a paradigm shift in SZ diagnosis. By constructing structural covariance networks at the individual level, researchers can delineate patient-specific variations with greater precision than conventional group-level approaches58.
Recent neuroimaging studies have revealed distinct brain connectivity patterns associated with various symptom dimensions. In the domain of thought disorders, a study demonstrated that the resting-state functional connectivity between the frontal and temporal lobes serves as a predictor of the severity of formal thought disorders59. Notably, another study revealed that functional connectivity patterns within the frontotemporal–thalamic network are significantly correlated with the severity of positive symptoms of formal thought disorders60. Moreover, in the cognitive domain, research has established significant associations of socioemotional and default mode networks with overall cognitive dimensions61. Furthermore, a study62 conducted dynamic functional connectivity analyses and revealed that the frontoparietal and somatomotor networks were negatively correlated with cognitive performance, whereas visual and default mode networks were positively correlated with response latency.
In summary, AI has demonstrated significant potential in advancing SZ diagnosis and management. By integrating multidimensional biomarkers such as symptomatology, genetic markers, and imaging features, AI-driven models can predict disease progression and symptom evolution with increased accuracy. This capability not only enhances diagnostic precision but also provides a basis for developing individualized treatment strategies. Future research should focus on leveraging AI to monitor diverse biomarkers, reveal structural network heterogeneity, and link the findings to clinical characteristics. Such efforts can improve the specificity and effectiveness of clinical interventions, ultimately leading to better outcomes for patients.
AI-enhanced treatment of SZ
The clinical manifestations of SZ include positive symptoms (e.g., delusion, hallucination, and formal thought disorder) and negative symptoms (e.g., avolition, poverty of speech, and flat affect). In recent years, cognitive dysfunction has been recognized as a core feature of the disease, with patients often exhibiting poor performance in executive function, long-term memory, and sustained attention as well as a potential overall decline in intelligence. These cognitive deficits severely impact the social and occupational functioning of patients, which presents a major challenge in current treatment approaches63. To date, the only class of medications that has been proven effective for SZ is antipsychotics, which act by blocking postsynaptic dopamine D2 receptors. However, these drugs are often associated with significant side effects, including sedation, weight gain, and extrapyramidal symptoms. Tardive dyskinesia, an irreversible but rarely life-threatening syndrome of involuntary movements, significantly affects the quality of life (QoL) of patients. Based on these findings, we recognize that SZ treatment continues to face major challenges. The primary issue is the individualized dosing strategy for antipsychotics. Clinicians need to strike a balance between maximizing therapeutic effects while minimizing side effects, which requires precise dose adjustments. Additionally, a significant heterogeneity exists in the responses of patients to treatment, which necessitates personalized treatment plans. Clinicians often undergo a systematic trial-and-error process to determine the optimal treatment strategy, which is time-consuming and may delay the treatment opportunity.
Identification of biomarkers and prediction of treatment outcomes
In recent years, AI technologies have achieved significant progress in identifying biomarkers and predicting treatment outcomes for SZ. A study employing ML algorithms combined with functional connectivity of the superior temporal cortex successfully predicted treatment responses in FEDN patients with SZ, achieving an accuracy of 82.5%64. The findings demonstrated that FC features based on mutual information and correlation play a crucial role in detecting individual FEDN cases and predicting their treatment outcomes. This breakthrough provides essential objective biomarkers to support precision medicine approaches for SZ.
Furthermore, a study applied ML techniques to the analysis of brain imaging data derived from 2803 patients with SZ and 2598 healthy controls to assess the discrepancy between brain age and chronological age of patients with SZ. The results revealed that the predicted brain age of patients with SZ was, on average, 3.55 years older than that of healthy controls, which indicates accelerated structural brain aging65. This is the first study to propose brain age prediction as a potential biomarker for SZ, highlighting its value in evaluating the efficacy of therapeutic interventions.
Recent investigations have leveraged exosome proteomics and XGBoost ML algorithms to develop a personalized differentiation score (PDS) for predicting therapeutic responses in patients with SZ. Analysis of plasma-derived exosomes from 343 participants demonstrated that elevated PDS values were significantly correlated with enhanced symptom improvement after antipsychotic treatment, as evaluated using the Positive and Negative Syndrome Scale (PANSS)6. In other parallel developments, researchers have developed advanced machine learning tools utilizing data from various first episode schizophrenia trials66. This tool demonstrated robust accuracy (approximately 75%) in predicting treatment outcomes and successfully identified patients at risk of symptom persistence, poor treatment adherence, and rehospitalization67. Collectively, these findings suggest that AI not only provides crucial objective biomarkers for SZ but also serves as a reliable predictor of treatment response, thus establishing a solid foundation for personalized therapeutic strategies.
The abovementioned studies indicate that the precision of AI in SZ treatment primarily lies in identifying objective biomarkers to predict the effectiveness of therapeutic interventions. This process lays the groundwork for subsequent individualized treatment, particularly for patients with poor response (e.g., those with TRS). However, although the identification of biomarkers provides critical insights into treatment outcomes, translating these findings into actionable, patient-centered interventions remains a significant challenge. Addressing this gap requires the integration of these biomarkers into comprehensive treatment frameworks that consider the biological, psychological, and social dimensions of SZ.
Development of personalized treatment plans based on the biopsychosocial model
Statistical data have revealed that patients with TRS have higher rates of substance abuse, and 20–50% of them do not experience improvement in symptoms68. In addition, these patients exhibit higher incidences of early cognitive decline and suicidal ideation69. For such patients, based on a comprehensive body of evidence, the current study recommends the implementation of more holistic strategies during the early phase of treatment, such as combining psychotherapy with transcranial magnetic stimulation, to guide personalized treatment plans70.
AI has demonstrated a significant potential in psychological interventions for SZ. In a pioneering study involving 76 patients who underwent remote social cognitive training for 8–12 weeks, latent class growth analysis identified five distinct response trajectories71. Notably, the first subgroup, which comprised 29% of the participants, exhibited significant improvements in social cognitive abilities. Subsequent analyses revealed that random forest modeling demonstrated superior performance in predicting membership in this high-response group. In addition, computerized cognitive remediation therapy (CCRT) has shown remarkable therapeutic efficacy. An 8-week comparative study demonstrated that compared with the treatment-as-usual group, the CCRT group exhibited significantly enhanced resting-state brain activity in the dorsolateral prefrontal cortex and anterior cingulate cortex, accompanied with marked improvements in processing speed and problem-solving capabilities72. These findings underscore AI’s capacity to effectively predict therapeutic outcomes and optimize treatment protocols and intervention durations.
For patients exhibiting inadequate response to antipsychotic medications or conventional psychosocial interventions, repetitive transcranial magnetic stimulation (rTMS) has emerged as a viable alternative therapeutic approach. The integration of AI has substantially enhanced the treatment efficiency in this domain. A recent comprehensive study51, which incorporated structural MRI data, clinical parameters, sociodemographic information, and polygenic risk scores, developed a sequential prediction workflow that achieved a balanced accuracy rate of 94% in an active treatment cohort. The investigation identified gray matter density in the default mode network and limbic system, along with education-related polygenic risk scores, as crucial predictors of rTMS treatment response. Furthermore, a study73 utilized AI algorithms in conjunction with MRI and successfully predicted individual responses to rTMS therapy in patients with SZ with 85% accuracy. These findings have revealed essential biomarkers for personalized rTMS treatment in patients with SZ, which potentially enhances therapeutic outcomes while significantly reducing the use of ineffective treatments.
The integration of AI extends beyond treatment optimization to facilitate a comprehensive understanding of the biopsychosocial factors that influence individual treatment outcomes.
AI has also been used to explore the complex relationships between psychological symptoms and metabolic characteristics in patients with SZ. Recent studies have constructed psychometabolic interaction networks and optimized graph attention networks (GAT) using graph neural networks for the first time to reveal complex data patterns that cannot be captured by traditional statistical methods74. The research revealed significant correlations between a history of psychiatric symptoms and physical health indicators (e.g., triglycerides and low-density lipoprotein cholesterol), which further support the importance of the biopsychosocial model.
Patient adherence is another critical factor that influences treatment outcomes. Rebecca et al.75 analyzed adherence to exercise interventions in patients with SZ and found that patients with higher baseline daily functioning were more likely to adhere to the interventions. In contrast, symptom severity, cognitive performance, QoL, and physical fitness exerted less impact on treatment adherence. This finding implies that when developing personalized intervention plans, baseline functional levels should be fully considered to improve the practical effectiveness of and adherence to treatment.
In summary, AI-assisted treatment plays a critical role in developing personalized treatment plans based on the biopsychosocial model. However, several challenges continue to exist, such as decreased adherence to psychological interventions and limited reproducibility of the findings. Therefore, future research should focus on improving adherence and validating results to ensure the clinical feasibility of personalized interventions, ultimately enhancing the precision and effectiveness of treatments.
AI-assisted research in antipsychotic medication management
In recent years, research on AI-assisted antipsychotic treatment has attracted significant attention. A study76 developed and validated individualized treatment rules (ITRs) for patients with first-episode SZ. By integrating ML methods, the ITR achieved a treatment success rate of 51.7% in the training sample, which was significantly higher than that observed for the general sample (44.5%). Notably, the ITR recommended medications such as aripiprazole and amisulpride, which markedly differed from the commonly prescribed drugs in observational studies, such as risperidone and sulpiride. This finding implies that the current prescription practices should reduce the use of nonrecommended medications to further optimize treatment outcomes.
In addition to ITR research, Mellem et al.77 proposed an interpretable AI model by combining the personalized advantage index and Bayesian rule lists. This model was designed to assist in the evaluation of the therapeutic efficacy of paliperidone and selection of patients suitable for treatment. The approach effectively identified patients who were more likely to benefit from therapy, thereby improving the efficiency of clinical trials and treatment outcomes. This approach represents a significant advancement in patient selection and personalized medicine for antipsychotic treatment. Furthermore, a recent study used the PANSS Score Matrix (UPSM) method to transform PANSS scores, identifying five distinct patient subtypes, each characterized by specific severity levels across five symptom domains78. This classification provides a scientific basis for personalized treatment, which enables more targeted pharmacological interventions while reducing false specificity across symptom domains.
AI technology has also demonstrated a significant potential in predicting treatment responses. For instance, a number of studies have utilized AI to identify EEG features associated with treatment response, which enables clinicians to determine whether a patient is suitable for clozapine treatment before the initiation of therapy79. This approach optimizes treatment plans and minimizes trial-and-error processes. Additionally, by integrating genetic and epigenetic data into ML techniques, researchers have developed and validated a predictive model for estimating treatment responses to various antipsychotic drugs (APDs) in patients with SZ. This model identified six genes associated with treatment response, providing critical biological insights for personalized therapy80.
Adherence to medication is another key factor in the success of treatment81. Chen et al.82 developed and validated a smartphone application, known as MedAdhere, which uses smart reminders, medication identification, and camera monitoring to track patient adherence. The intervention group achieved a medication adherence rate of 94.72%, which was significantly higher than that observed for the control group (64.43%). Importantly, patients in the intervention group demonstrated significant improvements in cognitive domains such as memory, language, and executive function. These findings indicate that AI technology not only enhances adherence to medication but may also positively impacts overall cognitive functioning in patients.
In summary, although numerous studies have leveraged high-throughput data to enhance personalized APD selection, high-quality research that focuses on dose adjustment for patients with SZ is lacking. Specifically, AI models can integrate genetic data, clinical characteristics, and drug response information to predict optimal medication dosages. These multimodal AI systems can assist clinicians in fine-tuning dosages to maximize therapeutic efficacy while minimizing side effects.
Apart from dose adjustment, AI technology can identify key factors that influence drug metabolism and response, providing deeper insights into the responses of patients to different medications. For example, AI can analyze biomarkers or symptom patterns to recommend appropriate drug combinations and dosages, thereby optimizing treatment strategies. These advancements hold great promise for improving the precision and effectiveness of SZ treatment. However, rigorous clinical trials and validation studies are still required to ensure the safety and efficacy of these technologies in real-world applications.
AI-driven innovation in psychiatric drug development
AI technology has demonstrated remarkable potential in revolutionizing the drug development process. Research has indicated that AI can decrease the time required for drug development by approximately 50%, potentially saving >$70 billion in the drug discovery process by 202883. Given the current limitations of antipsychotic medications, AI-assisted drug development offers a transformative opportunity by significantly decreasing research and development costs as well as the risk of failure.
By leveraging large datasets, AI can simulate and analyze preclinical data, which increases the success rate of candidate drugs in clinical trials. For instance, the AI technology platform of BioXcel played a pivotal role in the discovery of Igalmi, a sublingual film formulation of the selective α2-adrenergic receptor agonist dexmedetomidine. This platform sets search parameters for specific indications and screens millions of scientific papers to identify potential therapies. Using this approach, dexmedetomidine was identified as possessing the characteristics necessary to treat agitation. Consequently, BioXcel initiated the development of Igalmi, which was approved by the United States Food and Drug Administration (US FDA) in 2022 for the acute treatment of agitation associated with SZ or bipolar I/II disorder84.
The application of AI to drug development demonstrates its unprecedented potential and value. Compared with traditional methods, AI can accelerate the discovery of new molecular compounds and therapeutic targets, increase success rates at various stages of drug development, and optimize the design of clinical trials by analyzing real-world data and patient characteristics. This revolutionary integration of AI into pharmaceutical research is opening new avenues for clinical drug development, which promises to expedite the emergence of breakthrough therapies.
Despite its promising potential, AI in drug development faces several challenges. These include the integration of AI into biotechnology; issues related to the quality, availability, and standardization of datasets, and establishment of clear rules and guidelines for AI-driven drug development to ensure safety and efficacy. These challenges highlight the need for further innovation and collaboration within the industry to fully realize the potential of AI in psychiatric drug development.
In conclusion, AI demonstrates extensive potential in the field of SZ treatment. First, AI can identify objective biomarkers and effectively predict treatment outcomes. Second, based on the biopsychosocial model, AI can develop personalized treatment plans for patients, which optimizes medication selection and dosage adjustment using big data. Additionally, AI demonstrates remarkable potential in new drug development. The current study proposes that AI can be used to conduct comprehensive biopsychosocial assessments in the future and provide optimal treatment strategies for patients through big data analysis. This involves precise drug selection, dosage regulation, psychotherapy method recommendation, and real-time monitoring of patient emotions and disease progression, thus assisting clinicians to achieve more effective treatments for SZ.
However, despite the promising prospects of AI, it is crucial to adhere to the core principle that AI should serve as an auxiliary tool rather than replacing the decision-making role of clinicians. Owing to the complexity and individual variability of SZ, doctors should have professional clinical judgment, extensive experience, and compassionate care, which are key elements that AI cannot fully replicate at present. Therefore, the organic integration of AI into clinical expertise will be a critical direction for future development in the treatment of SZ.
AI-enhanced prognostic assessment of SZ
SZ is one of the most severe mental disorders, with a significant challenge of achieving full recovery. The recurrence of psychotic symptoms not only leads to substantial distress to patients but also severely impairs their social functioning. A meta-analysis revealed that the proportion of patients who achieved full recovery was only 13.5%. However, the average follow-up period in these studies was only 1 year; thus, the long-term prognosis of patients remains unclear85.
A major challenge in current clinical practice lies in the lack of reliable tools for predicting individual relapse risk, which directly limits the development of personalized treatment plans, particularly in decisions regarding adjustments to antipsychotic medications86. Although identifying the predictors of relapse is essential for recognizing high-risk patients, traditional statistical methods struggle to capture the complex interactions among the clinical, social, and behavioral factors. In this context, the integration of AI technologies offers a promising solution to address these limitations.
Advances in ML and DL for relapse prediction
In recent years, ML techniques have made significant progress in the prediction of relapse risk in SZ. A study involving 700 patients analyzed 36 baseline variables and successfully identified key predictors of relapse87. The common risk factors included positive urine drug tests, specific subtypes of SZ, poor social functioning, and younger age. Additionally, elevated prolactin levels, frequent hospitalizations, and smoking were identified as specific predictors of relapse following the discontinuation of antipsychotic medications. Meanwhile, an analysis of neuroanatomical features further advanced research in the prediction of relapse risk. Chilla et al. used a combination of multiple neuroanatomical features and ensemble methods and achieved high accuracy rates (83–87%) in distinguishing patients with SZ from healthy controls. They also revealed potential associations between these neuroanatomical features and relapse risk88.
Other studies have also accurately predicted QoL in patients with SZ by identifying key factors such as socioemotional symptoms, neurocognition, treatment attitudes, and physical health89,90.
Furthermore, DL techniques have enhanced prediction accuracy. For instance, an evaluation tool integrating the Bidirectional Encoder Representations from Transformers (BERT) and Tabular Neural Network (TabNet) models demonstrated excellent performance, achieving a high prediction accuracy (>0.78) and an AUC value of 0.7091.
Innovative applications of digital phenotyping
The rapid development of digital technologies has opened new avenues for the prediction of relapse risk through digital phenotyping. By leveraging real-time behavioral data collected via smartphones and wearable devices, researchers have comprehensively assessed the clinical, cognitive, and functional states of patients with SZ92. This dynamic monitoring approach not only complements traditional clinical assessments but also facilitates early intervention.
The AiCure application is particularly notable, which integrates ML models to enable real-time monitoring of adherence to medication. This tool effectively predicts the risk of poor adherence and supports the early identification of relapse risk by capturing behavioral patterns93. These technological innovations provide novel solutions for the long-term management of patients with SZ.
In summary, the application of AI technologies has significantly improved the accuracy and utility of the prediction of relapse risk in SZ. From baseline variable analysis to neuroimaging feature analysis and from digital phenotyping to DL models, the integration of multidimensional data offers novel insights into the prediction of relapse. These advancements not only provide clinicians with more precise predictive tools but also lay a scientific foundation for planning long-term recovery strategies for patients.
Nevertheless, the clinical application of AI technologies requires rigorous scientific validation to ensure their reliability and effectiveness. Future research should focus on three key areas: (1) optimizing large-scale data analyses by stratifying patients into clinically meaningful subgroups to address the challenges and opportunities posed by data heterogeneity; (2) precisely selecting and controlling key variables, such as social stressors and life events, to manage potential confounding factors; and (3) continuously exploring the novel predictors of relapse to further enhance the accuracy and clinical applicability of predictive models. Through these efforts, we aim to develop more personalized and effective treatment strategies, ultimately improving the long-term outcomes of patients with SZ.
AI-enhanced novel approaches for SZ treatment
Over the past decade, AI research in SZ has achieved remarkable breakthroughs, which highlights the immense potential of AI in this field. The contributions of AI extend beyond its robust computational capabilities, particularly in the advancement of innovative applications of human–machine collaboration that expand the boundaries of diagnosis and treatment.
Brain–computer interface (BCI) technology has demonstrated significant advancement over the past two decades, which enables healthy individuals and those with disabilities to control devices through cognitive processes94,95. By decoding the neurophysiological signals associated with brain activity, these technologies have achieved diverse applications, which transcends the conventional boundaries of human–computer interaction.
Recent investigations have highlighted the innovative applications of BCI technology in psychiatric disorders. A groundbreaking study developed a multimodal neural prosthetic interface designed for the recording, modulation, and classification of electrophysiological biomarkers associated with neuropsychiatric disorders. Utilizing 3D printing technology, a biocompatible implanted device was created, which successfully recorded event-related potentials from the mouse prefrontal cortex. Moreover, machine learning algorithms were applied to accurately classify the effects of various treatments on neural activity. Experimental results demonstrated that this neural prosthetic could effectively monitor and modulate both healthy and impaired brain states, providing a promising new tool for personalized treatment of neuropsychiatric disorders96. In clinical applications, computer-assisted broad learning EEG system has enhanced the detection accuracy of brain activity patterns related to SZ through real-time monitoring and signal decoding97. Additionally, researchers have developed a cost-effective wireless BCI system that is specifically designed to detect auditory hallucinations in patients with SZ, providing a reliable diagnostic auxiliary tool98.
These innovative studies have emphasized the significance of enhancing human-to-AI data transmission rates, which not only improves the efficiency of human–machine collaboration but also facilitates transformative experiential advances. Through a “human–machine synergy,” clinicians can leverage AI to perform parallel analyses of multimodal data, ultimately enabling more personalized and flexible intervention strategies. At the same time, the physician’s expertise and empathetic support offer an indispensable dimension of patient care. This complementary relationship between humans and AI holds promising potential for significantly enhancing treatment outcomes. In the future, it is essential to further explore optimization and customization pathways for human-computer interaction interfaces, particularly focusing on the design of interaction workflows for clinicians utilizing AI decision support systems. This is crucial to ensure that the model outputs can meet the diagnostic and intervention needs across various clinical scenarios.
Limitations and challenges
AI has created unprecedented opportunities for the diagnosis and treatment of schizophrenia, yet its development still faces notable limitations. Currently, most AI-based systems exhibit deficiencies in generalizability and clinical applicability, especially when dealing with schizophrenia medical data that have significant differences in diagnostic criteria99. Consequently, it is often necessary to retrain these systems using data from specific populations or regions to accommodate a wide range of needs. For example, in medical imaging classification tasks, integrating and managing large-scale, heterogeneous, and multi-center datasets can partly address these challenges.
Moreover, many existing studies focus predominantly on retrospective analyses, relying on historical labeled data for algorithm training and testing. This approach often fails to reflect real-world clinical performance. Prospective studies, therefore, offer a more accurate means of evaluating AI’s effectiveness in actual practice. Meanwhile, although AI demonstrates strong performance when handling complex, large-scale datasets, it is prone to overfitting when sample sizes are small, thereby limiting its generalizability. Randomized controlled trials can help improve data quality and external validation in AI research, especially in assessing clinical efficacy and reducing bias100. Future studies should incorporate clinical outcomes as key endpoints to confirm whether AI systems can deliver long-term benefits. Simultaneously, it is essential to recognize that algorithms might influence sociocultural contexts or healthcare pathways, necessitating more sophisticated evaluation methods.
To accurately gauge AI’s real-world clinical performance and generalizability, rigorous external validation studies should be conducted using multi-center, independently sourced datasets101. This approach ensures that variations in patient demographics and disease states are sufficiently represented in the target clinical setting.
In today’s healthcare landscape, the issue of “inequality” is a significant concern. The improper use of AI may exacerbate existing disparities, as many algorithms have inherent biases stemming from underrepresented minority groups in training data, which could lead to widened gaps in health outcomes.
Additionally, there is a notable lack of transparency in how AI in healthcare arrives at its decisions, posing a serious challenge because physicians and patients alike require sufficient trust to accept AI-based diagnoses and treatment recommendations. Consequently, AI should not fully replace clinical decision-making but instead promote collaborative interactions among healthcare professionals and patients. In the future, efforts should be focused on integrating research into multidimensional data visualization, explainable AI algorithms, and human-machine trust metrics to enhance the transparency and interpretability of AI models.
Moreover, the widespread adoption of AI in healthcare faces ongoing privacy and data security challenges. Owing to the highly sensitive nature of medical data, patient privacy is vulnerable to breaches or cyberattacks. The current model of health data management requires reform to ensure patients have rightful ownership and control over their personal data.
Conclusion
This study thoroughly explores the potential applications of AI in the diagnosis, treatment, prognostic assessment of SZ as well as innovative human–machine collaborations, highlighting the critical role of AI in addressing the complex challenges posed by this condition. With advancements in AI technology, the early identification, personalized treatment, and prognostic evaluation of SZ are evolving from traditional symptomatology to more precise and objective biomarker analysis. AI demonstrates significant advantages in diagnostics. In particular, it can effectively identify high-risk groups and precisely classify and track the speech and behavioral symptoms of patients. Moreover, AI demonstrates immense potential in the development of personalized treatment plans, especially in assessing treatment responses, which provides clinicians with essential decision-making support. By integrating biological, psychological, and sociological data, AI is poised to enhance SZ management efficiency and patient outcomes. In the future, the efficient collaboration between clinicians and AI will result in the practical application of personalized precision medicine for SZ. During this process, the bidirectional development of the human–machine interaction is crucial to achieving deeper integration through innovative collaboration mechanisms. In particular, in psychiatry, combining the analytical capabilities of AI with the insights of clinicians can enable the construction of comprehensive and dynamic patient models, which facilitates early intervention and continuous monitoring. Despite the vast potential of AI, it currently serves as an auxiliary tool for clinicians in diagnosis and treatment rather than a replacement. This is because, in addition to the need for continued iteration of the decision-making processes of AI, the human aspect of healthcare remains paramount.
In the future, as more scientific validation and large-scale clinical trials proceed, AI is likely to become a crucial force in tackling the significant challenges and burdens posed by schizophrenia, opening new avenues for improving patients’ quality of life and overall prognosis.





Responses