Cellular response in three-dimensional spheroids and tissues exposed to real and simulated microgravity: a narrative review

Introduction
The 21st century has been a period of unprecedented advancement in the space sector. More and more countries have commenced their own space agencies and commercial spaceflight companies are changing the entire industry, increasing access to space for humankind. The primary goal of this increased space exploration is to find innovative solutions for the biggest problems on Earth and improve our scientific knowledge about the universe. Some studies even suggest the possibility of creating an inter- and multiplanetary humanity, which in theory, would significantly increase long-term population survival1,2. However, the expansion of space exploration missions leads to an increase in human exposure to the extreme environment of space. NASA defines the five major hazards as altered gravity, increased radiation exposure, isolation and confinement, distance from Earth and the hostile and closed environment of the spacecraft itself3.
The speed with which the International Space Station flies around the Earth in Low Earth Orbit results in a balance between the gravitational pull and the orbital motion, resulting in a state of infinite free-fall. This altered gravitational state is defined as microgravity. Objects and people appear weightless, while in fact they still experience 10−6 times the force of gravity at the Earth’s surface4. Under this condition, it is well documented that human physiology undergoes significant and drastic changes, including bone5 and muscle loss6, neurovestibular dysregulation7, cardiovascular deconditioning8, Spaceflight-associated neuro-ocular syndrome9, and immunological10 and hematological alterations11. Many of these conditions directly mimic diseases and physiological degradation as found on Earth including osteoporosis, physical inactivity and anemia12. Thus, one of the key advantages of conducting research in microgravity is studying the onset of these diseases but at an accelerated rate. As such, microgravity provides a unique environment in which we can study and develop technologies for human space exploration whilst supporting and translating these lessons for human healthcare here on Earth.
Tissue engineering is one of the technologies potentially synergizing with space exploration13,14,15,16. Although microgravity is described as one of the five major hazards of human space exploration, it also presents new opportunities for technological developments within the medical field. Microgravity potentially helps to overcome major challenges within the field of tissue engineering by facilitating scaffold-free three-dimensional (3D) tissue formation and altering cell proliferation and differentiation. Several reviews have focused on the effects of the microgravity environment on tissue formation, stem cell differentiation and tumor growth17,18,19,20,21,22. This paper will shed light on the cellular responses related to tissue engineering within microgravity. An overview is provided of the research related to 3D multi-cellular structure formation in simulated (s-µG) and real microgravity (r-µG), elaborating on the observed alterations in cellular mechanisms. The two techniques by which multi-cellular structures are formed are bioassembly and bioprinting. The effects of microgravity on both techniques are discussed separately. Finally, future perspectives and important knowledge gaps are presented.
Space and tissue engineering
Mutual benefits of tissue engineering and space exploration
Our world is confronted with a significantly increasing population aging, and as such, tissue engineering and regenerative medicine development will be pivotal to ensure the health and well-being of humanity. However, we currently lack a fundamental understanding of the underlying mechanisms of cellular regeneration and homeostasis to allow control and development of personalized tissue engineering. The microgravity environment provides novel opportunities to enhance our comprehension of the underlying cellular mechanisms. Additionally, tissue engineering could play a key role in finding solutions for several problems on Earth and enabling safe long-term human space exploration. Here, we introduce the benefits of tissue engineering for space exploration and we discuss how microgravity could be a tool for the advancement of tissue engineering.
Tissue engineering and biofabrication for space exploration
The foundation principles of tissue engineering involve the in vitro formation of functional tissues and organs with the use of scaffolds, cells and biologically active molecules with the aim of restoring, maintaining or enhancing damaged tissues or organs23. A promising facet within the field of tissue engineering is biofabrication. Biofabrication is defined as a process that creates functional multicellular tissues or constructs. This facet can be separated into two distinct approaches: bioassembly and bioprinting. Both methods can be used to create tissues, organoids and organs, but the techniques and methodologies in these two approaches differ. Bioassembly is the cell-driven self-assembly of pre-formed cell-containing fabrication units24. This also includes the creation of multicellular spheroids, stacking of spheroid building blocks and guided self-assembly by magnetic levitation. Bioprinting, on the contrary, is the computer-aided direct three-dimensional (3D) organization of cells, materials and biological factors24. This method is more automated and fabrication is faster and simpler compared to bioassembly, with scaffolds and cells being seeded simultaneously. Bioprinting also facilitates better control of the spatial structure. The most common methods for bioprinting include micro-extrusion, inkjet/drop-on-demand and laser-induced forward transfer25.
Biofabrication techniques could enable more accurate and efficient methods to study human physiology in space without the need for actual human or animal models to be exposed to the hazardous environment of space. Furthermore, engineered tissues would enable new opportunities for effective treatment of astronauts in remote situations with the use of bioprinting and ameliorating the wound healing process25.
Microgravity as a tool to advance tissue engineering
The space environment could enhance developments within the field of tissue engineering and biofabrication. The final goal of tissue engineering is to create whole organs in vitro, solving major problems on Earth like donor organ shortage and decreasing the need for animal studies. However, before whole organs can be engineered a lot of challenges have to be overcome26. One of the major issues is the lack of vascular-like structures providing nutrition and disposing of waste, which limits the creation of more complex structures with larger cell numbers23. Another important issue is the delicate balance between the porosity of the scaffolds and the cell viability within those scaffolds. A denser scaffold is stronger and enables more complicated structure formation in 1G. Nevertheless, when the hydrogel-based scaffold density increases, cell viability significantly drops, as there is less capacity for nutrients to diffuse into the denser hydrogel mesh27. The microgravity environment might help to overcome some of these key challenges.
Microgravity enables scaffold-free, nozzle-free and label-free formation of 3D tissues13. Cells exposed to microgravity spontaneously assemble into 3D multicellular structures. The direction of growth can be influenced by magnets or low-density hydrogel scaffolds, since there is no need for high-density strong scaffolds when the gravitational pull of Earth is negated28. Moreover, it has been shown that microgravity improves nutrient and waste exchange between cells and surrounding medium29,30. Microgravity could therefore facilitate an ideal environment for cells to form tissues.
Currently, there are two classifications of microgravity exposure, real and simulated. Research and development in real microgravity (r-µG) has traditionally been conducted on the International Space Station. Transporting cells to and sustaining cell-cultures in space is challenging and requires unique hardware. Therefore, several different set-ups have been developed. Table 1 summarizes the different hardware used in prior research. With the introduction of the private space industry, more options are becoming available, including, but not limited to: nanosatellites called CubeSats from ICECUBES, manned and un-manned space shuttles from AXIOM space, Blue Origin, Virgin Galactic, SpaceX and Astra Space. Studies in r-µG are expensive, have strict mass and time constraints and require extensive preparation and automation of the research set-up. Additionally, short-term r-µG exposure can be reached by using platforms like parabolic flights, drop-towers and sounding rockets. However, these platforms remain complicated and expensive31.
Therefore, alternative methods have been developed to simulate microgravity on Earth32. Platforms for simulated microgravity (s-µG) exposure on cell and tissue level include: random positioning machines (RPM), rotating wall vessels (RWV), the NASA-developed high aspect ratio vessel (HARV), 1D and 3D clinostats and magnetic levitation33. Clinical analogs to simulate effects of microgravity on the human body include head-down tilt and dry-immersion34. It is important to note that each platform has its merits and limitations and is dependent on the applications, cell type used and the outcome that is intended. We refer to previous studies for a thorough description of the mentioned platforms and their inherent differences35,36,37. Furthermore, s-µG only approximates r-µG, rendering it challenging to make a direct one-to-one comparison between the two situations. Clinostats and other rotating systems are able to average the gravitational vectors, but they never eliminate gravity. The further from the center, the more the rotation exerts shear stress on the cell and tissue samples31,38.
Studies using the prior stated s-µG and r-µG platforms have demonstrated sustained viability of cells in microgravity and observed increased cell proliferation, differentiation and growth behavior in various cell types including cartilage, fat, myocardium and skin39,40,41,42,43,44,45. On the contrary, bone and muscle proliferation and differentiation decrease in microgravity44,46,47,48,49,50, which is also observed as a physiological effect in astronauts during their stay on the ISS. Later in this review, we elaborate on the specific effects on different cell- and tissue types.
The potential of bioassembly in microgravity
Formation of 3D cell structures in microgravity
The formation of 3D cell culturing systems knows numerous advantages over 2D cell culture, allowing cells to aggregate and facilitating unrestricted interaction between cells and their environment51. In r-µG and s-µG sedimentation and buoyancy are negligible and cells are mechanically unloaded. In this environment cells tend to cluster together, forming 3D structures within short time frames. It has been shown that the microgravity environment fosters mechanical, structural and chemical interactions between these clusters of cells and their extracellular matrix (ECM) in any direction44. It is suggested that cells sense the alterations in gravity via interactions with the ECM, cell adhesion and connection to the cytoskeleton52. These changes in biological processes and cells cannot be achieved under 1G conditions. Microgravity could provide a scaffold-free and label-free approach for the generation of tissues from (stem) cells and 3D multicellular spheroids.
Stem cells in microgravity
Here, we only briefly touch on the subject of stem cells in microgravity, as this has been thoroughly described in several reviews19,20,22,40,53. For the purpose of tissue formation in microgravity, both stem cells and specialized cells can be used. However, more extensive research has been done on stem cells. Stem cells used in tissue engineering are divided into somatic and cancer stem cells. These cells are much alike. However, cancer stem cells additionally possess the ability to replicate the parental tumor after transplantation53. When talking about stem cells, we refer to both somatic and cancer stem cells.
Stem cells are of great interest in regenerative processes as they naturally form the basis of growth for all bodily tissues. The aim is to precisely preserve, promote and steer stem cell development in vitro to grow tissue structures. They form the basis of many bioassembly and bioprinting methods. Microgravity seems to influence the growth and differentiation behavior of these cells. How and to what extent microgravity alters these parameters depends both on the cell type and the duration of exposure to microgravity. At the moment of writing, several stem cell types have been studied in microgravity, with some contradictory results, as visualized in Table 2. Mainly cartilage and bone tissue have been investigated, since physiological and pathological changes have been observed in astronauts related to these tissues. Cancer stem cells that have been studied so far include: gastrointestinal, lung, prostate, skin and bone tumors and are described thoroughly53.
The study of Xue et al. illustrated that duration of exposure to µG of stem cells steers differentiation and cytoskeleton construction. When rat MSCs were exposed to µG for a short period, this promoted epithelial, neural and adipogenic differentiation. Extended µG promoted differentiation into osteoblasts. It turned out that RhoA expression correlated with differentiation and probably regulates this osteogenic pathway. In short µG exposure, RhoA was decreased, while it was increased during long µG exposure. Moreover, a disruption of the cytoskeleton was observed in short µG exposure (72 h). On the other hand, during longer µG exposure (10 days) the tension increased in the cytoskeleton and tubulin polymerization activity was maintained54.
Thus, alterations in stem cell parameters have been studied extensively, proving the potential of using µG as a novel means to steer their differentiation. The alterations in stem cells due to the mechanical unloading depend on cell type and exposure duration. Still, we are far away from controlling these alterations precisely, emphasizing the need for thorough research.
Spheroid formation in microgravity
A spheroid is a 3D assembly of cells of the same cell type. These structures closely mimic the in vivo environment, creating a more optimal cellular study model. Moreover, novel methods enable the fusion of spheroids to assemble macroscopic tissue structures for the purpose of transplantation55,56,57. Spheroids can be formed using several techniques, but the challenge remains to find a technique that enables controlled bulk production. For cells to self-assemble, an environment has to be created that promotes differentiation, viability and cell-cell attachment. The mechanical unloading of cells in µG appears to create an environment that stimulates cells to form spheroids. In µG cells tend to cluster together, naturally forming spheroids. Therefore, µG could play a key role in the development of techniques for spheroid production. Inhibition of specific genes or proteins results in inhibition or acceleration of the spheroid formation. However, the exact mechanism inducing spheroid formation in microgravity has not been clarified yet. This chapter will summarize research on the mechanisms involved in spheroid formation in µG.
Several research groups have set up studies to culture cells in µG. The formation of 3D spheroids has been observed as early as 1997 in a study with the NASA rotary cell culture system58. Interestingly, cells cultured in the same vessel respond in two distinctly different ways: part of the cells detach from the vessel walls and cluster together into 3D spheroids, while the other part remains an adherent 2D culture attached to the walls. This difference has been observed in both simulated and real µG and occurs both in healthy cells and tumor cells. However, numerous differences are observed between the formed spheroids from healthy and tumor cells. Thyroid tumor cell spheroids grew more voluminous with a higher cell count over multiple weeks, while healthy cells created small spheroids in the first week with an increase in size during the following weeks but no further multiplication. Many differences in the expression of genes and proteins were observed between healthy and tumor thyroid cells, including: NGAL, VEGFA, OPN, IL6 and IL17, and secretion of VEGFA, IL-17 and IL-659. It needs to be noted that angiogenic factors and growth factors are also upregulated in tumor cells compared to healthy cells regardless of altered gravity. The upregulated angiogenesis and abundance of growth factors in the tumor cells might explain the observed difference in cell dimensions. However, it is not clear whether gravity influences tumor cells directly or indirectly60.
Therefore, research has been performed to determine the mechanism behind this scaffold-free formation of spheroids. This proves to be a challenging endeavor, as cells exposed to µG show a great variety of differentially expressed genes and secreted proteins. Some of these are similar in all cell types; others are cell-type-specific. To find the specific genes and proteins involved in the spheroid formation is to look for a needle in a haystack. Some genes and proteins seem to be more closely related than others. The inhibition of proto-oncogene tyrosine-protein kinase Src (C-Src) prevented spheroid formation, while antibody-mediated blocking of e-cadherin promoted spheroid formation61. Sahana et al. confirmed the central role of e-cadherin and additionally indicated that fibronectin, β-catenin and vinculin are key mediators in 3D growth and adhesion in µG62.
Krüger et al. observed a similar 3D growth of spheroids on RPM and on ISS, although the protein expression was different63. This study also demonstrated the influence of the material used for the cell containers on protein secretion. Warnke et al. observed similar growth of thyroid cancer spheroids in RPM and 2D-clinostat compared with the Shenzhou-8 spaceflight. This group also observed device-specific alterations in cytokine production64.
Table 3 gives an overview of alterations in cellular mechanisms that have been studied. It can be deducted that many cytoskeletal proteins are altered when exposed to µG. This is supported by research showing the morphological shape changes. Specifically, altering cell-cell connections and cell shape regulation appear important. The non-equilibrium theory might explain how the cytoskeleton can sense gravitational changes and induce the transfer of mechano-signals into biochemical pathways60. One study observed that the shape of actin filaments of cells in µG became more spherical. The actin skeleton maintains cell stability and mediates a variety of cell-matrix and cell-cell interactions. Thus, it was theorized that actin could act as a gravity sensor, transducing external forces into the inner cell, which likely affects cell adhesion during spheroid formation65. Kopp et al. observed a similar response of the cytoskeleton structure in chondrocytes, thyroid, endothelial and osteoblasts, possibly mediated by differential expression of IL-8 and osteopontin. This suggests that the cytoskeleton remodeling process may be key to 3D growth, supporting the gravity sensor theory. This same study observed an alteration in the distribution of F-actin with perinuclear clustering within s-µG (RPM) compared to equal distributions and accumulation towards the cell membrane in 1G controls. β-actin was also upregulated in both the adherent and spheroid cells in RPM59.
Stem cells have also been used to form spheroids, showing a promising platform for high-yield production. Jha et al. used human pluripotent stem cells and exposed them to s-µG (RPM) for three days during differentiation to cardiomyocytes. This led to the production of highly enriched cardiomyocytes (99% purity) with high viability and expected functional properties. Yields were increased 1.5- to 4-fold from each stem cell compared to 3D standard gravity culture. This demonstrated that culturing stem cells as spheroids in µG increased induction, proliferation and viability. Moreover, genes associated with proliferation and survival in the early stage of differentiation were upregulated39.
Spheroid formation in microgravity has been used in some studies as a possible treatment solution. Tanaka et al. created pancreas beta-cell spheroids in a 3D clinostat, producing 1000 spheroids per ml with sizes of 100/250 micrometers on average. This size would be optimal for graft survival after islet transplantation. Transplantation of these spheroids in diabetic mice improved their hyperglycemia with better outcomes than the 2D cultured beta-cells. The next step would be to use human stem cells or mature pancreatic beta-cells66.
Although the previous statements give an auspicious perspective on the use of µG to efficiently grow spheroids, there is an abundance of problems that need solving to optimize this tissue engineering method. Technical issues like air bubble formation and long-term construct viability for actual clinical applications needs attention. Phelan et al. presented a method to extract air bubbles from a rotating wall vessel, increasing the size of the formed spheroids and increasing experimental reproducibility67.
In conclusion, the µG environment is a promising platform to trigger the formation of spheroids in many different cell types. It provides a scaffold-free method that creates spheroids with increased yield, greater size and improved viability. The consequently formed spheroids could have a multitude of Earthly and in-orbit applications, including models in cancer research by simulating tumor growth, metastasis and testing drug efficacy. Also, stem cell spheroids can be used to produce differentiated cells with increased yield. Although much has to be discovered about the mechanisms of spheroid formation, different gene and protein targets have been identified that influence spheroid formation and mechanisms explaining the cellular sensing of gravity have been theorized. We suggest that control over these cellular processes could facilitate control of spheroid formation in µG.
Tissues and organoids in microgravity
After having discussed the effects of µG on stem cells and the formation of 3D spheroids, this section will delve into the formation of small-scale tissues and organoids. A tissue is defined as an assembly of different cell types working together in one system and an organ is defined as an assembly of different tissues integrated in one system68. When different tissues are assembled and mimic organ functions in vitro we talk about organoids. Although the field of tissue engineering in microgravity is an emerging field, the formation of some tissue types and organoids have already been studied in r-µG and s-µG. The developments are discussed per tissue type. Figure 1 and Table 4 provide an overview of the formed multicellular structures.
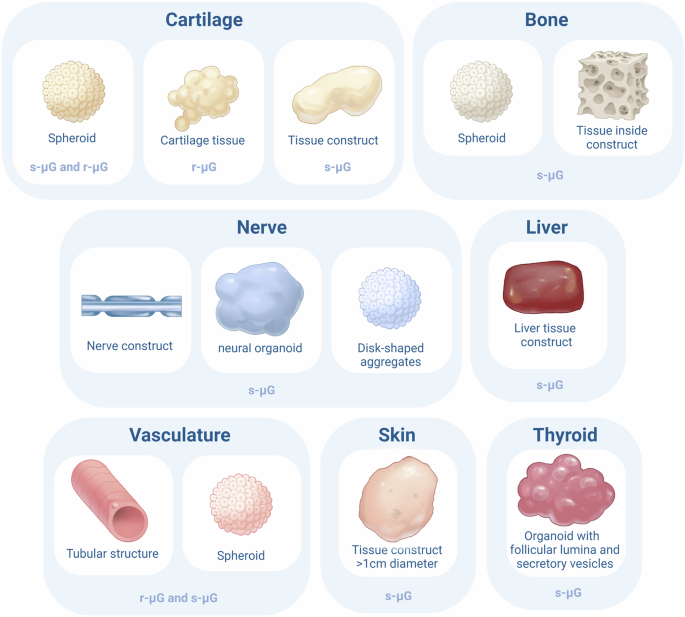
This figure summarizes the different types of cellular structures formed in microgravity for each cell type. For each formed structure, it is described whether this structure was formed in real microgravity (r-μG) or simulated microgravity (s-μG).
Cartilage tissue
Cartilage tissue-engineering could play a key role in the treatment of several joint related pathologies, including rheumatic arthritis and arthrosis. Wehland et al. succeeded in creating cartilage transplants by exposing human articular chondrocytes to RPM. Scaffold free spheroid formation started after 5 days of exposure and tissues reached a diameter of 2 mm after 28 days. These spheroids expressed genes involved in cartilage formation and showed typical cartilage morphology with aggrecan positivity. However, the spheroids reshaped to confluent chondrocyte monolayers after repositioning to 1G43.
Successful formation of 3D cartilage tissue from human chondrocyte spheroids was also achieved by using magnetic fields for magnetic levitation bioassembly. This enabled scaffold-free, label-free and nozzle-free formation of tissue structures in r-µG. The same article concluded that thermos-responsive hydrogels enable the delivery of viable tissue spheroids to the ISS and prevented undesirable preliminary tissue fusion and spread28.
Another study used porcine chondrocytes and cultured them on the ISS, in an RPM, and in 1G. After 16 days the tissues were analyzed. The results showed a weaker ECM staining of neocartilage tissue formation on the samples on the ISS compared to both the 1G and RPM samples. The tissues on ISS showed a higher expression of collagen type II/ collagen type I ratios. Higher aggrecan-versican gene expression profiles were seen in 1G samples compared to microgravity samples. In microgravity, the cell density was significantly lower. It was concluded that simulated and real microgravity environments differently influence cartilage tissue formation and can, therefore, not be seen as exchangeable techniques69.
Finally, a Rotating wall vessel has been used for the scaffold-free creation of cylindrical-shaped homogeneous cartilage tissue from rabbit bone marrow cells with a size of 1.25 (CI: 0.06) × 0.6 (CI: 0.08) cm (height × diameter). The structures formed without significant necrosis. The cells were cultured for four weeks within the vessel with a chondrogenic medium, after which cartilage formation was confirmed by expression of mRNA markers aggrecan, collagen type I and II, and GAG/DNA ratio. Moreover, cartilage formation was confirmed by toluidine blue and safranin-O staining. The created structures in the RWV were superior to the structures created in a centrifuge70.
In conclusion, cartilage tissue formation in microgravity is feasible with matured cells and bone marrow derived cells in r-µG and s-µG. However, the transition from µg to 1G and vice versa still needs optimization. Moreover, the s-µG environment might provide a more optimal environment for chondrogenic tissue formation compared to r-µG. Future r-µG research could therefore use both 1G and s-µG controls and build upon the performed experiments to increase complexity and size of the tissues.
Bone tissue
Bone tissue from astronauts is known to degenerate in microgravity. However, several studies indicate the possible counter intuitive use of microgravity for the generation of bone tissue. A rotating wall vessel in combination with bio-ceramic hollow microspheres was used to produce 3D aggregates of bone marrow stromal cells with ECM production and mineralization, supporting 3D bone like tissue formation71. Human fetal osteoblasts exposed to RPM showed morphological changes and assembly into 3D structures after seven days. Several genes involved in growth and structural protein formation were altered and release of cytokines and bone biomarkers were significantly altered. After two weeks of RPM exposure spheroids with bone-specific morphology were present46. These data clearly suggest the possibility of bone tissue-engineering within microgravity.
One other study used bone marrow mesenchymal stem cells cultured in a rotating vessel on ceramic bovine bone scaffolds to form bone constructs. After 15 days of culturing, the biomarkers for alkaline phosphatase were increased in the rotating cultures compared to the static cultures. The formed constructs were used for transplantation into cranial bone defects of rats. Remarkably, the constructs produced in the RWV showed better repair of the defect and better histological bone connection. This suggested that the microgravity environment can modulate the composition, morphology and function of engineered bone. Not only rotation enhanced bone formation. Steady hydrodynamic shear led to enhanced osteogenesis, intermittent motion of medium in a roller bottle stimulated the BMSC to form osseous nodules and mixing during cell seeding and cultivation of cells led to increased fractions of tissue components in 3D bone constructs. The study thus theorized that the hydro-dynamic forces applied to the freely settling constructs in rotating flow mimic to some extent the mechanical loading in in-situ osteogenesis, thus enhancing the in vitro bone construct formation49.
Not all studies show positive effects of µg on bone formation. Rat marrow mesenchymal cells were cultures in a 3D clinostat within pores of calcium hydroxyapatite for 2 weeks in the presence of dexamethasone. This resulted in a decrease in alkaline phosphatase activity and less extensive ECM formation. The formed structures were implanted subcutaneously in rats and showed a significantly lower volume of bone formed in the µg cultured group48.
In summary, although results on the effect of microgravity on bone tissue engineering seem inconsistent, it is possible to form bone-like tissues in s-µG models. The unique environment might even improve osteogenic differentiation of progenitor cells due to the continuous forces exerted on the cells. Although several studies have investigated cellular and physiological mechanisms of bone formation in r-µG, no study has yet investigated the feasibility of growing bone spheroids or tissues in r-µG. This would be an important next step.
Nerve tissue
Nerves are known as one of the most difficult tissues to regenerate. Earth-based facilities mainly use neural progenitor cells for this purpose. In space, the differentiation into nerve tissue by other cell types might be facilitated. In 1G situations, mesenchymal stem cells show low proliferation and migration when aiming to engineer nerves. In a s-µG model, however, one study showed improved differentiation of adipose-derived mesenchymal stem cells in a rotating wall vessel within acellular nerve grafts. Within this cell culture, genes associated with neural differentiation were promoted and the constructed nerves showed improved regenerative characteristics for nerve repair in sciatic nerve transplantation in rats. It is theorized that this effect is achieved by the improved mass-nutrient transport due to the rotation of medium29.
Moreover, Mattei et al. have succeeded in creating neural organoids by culturing hESCs in a rotary cell culture system (RCCS). This system induced lower sheer stress on the cells and promoted nutrient flow. Both neural and glial differentiation was observed in the created organoids. However, changes were observed in the expression of rostral-caudal neural patterning genes compared to organoids cultured as 1G controls. The created organoids were used to investigate the potential effects of microgravity on the development of the forebrain. The RCCS was demonstrated to be a suitable platform for creating neural organoids. The organoids generated with the RCCS were significantly larger than the ones created in static controls. Organoids cultured in the RCCS from day 1 showed a significant decrease in expression of forebrain cortical markers with a corresponding increase in caudal markers, suggesting brain function alterations in microgravity30.
Another study used the HARV to create tissue structures from Rat Sertoli cells (SC) and NTerra-2 immortalized human neuron precursor cells (NT2). This coculture provided an environment suitable for the formation of disc-shaped aggregates with physiological cellular organization inside the aggregate. The coculture of these cells provided a new transplantable tissue source with treatment potential for Parkinson’s disease72.
In brief, neural tissue engineering is generally considered difficult, yet the s-µG platforms provide a promising environment for the formation of nerve tissue and brain organoids by differentiating stem cells and precursor cells. The low shear stress and increased nutrient flow might be crucial for this process.
Liver tissue
Liver cells, on the other hand, are known for their regenerative capabilities in vivo. Liver tissue engineering is useful as an alternative to whole liver transplantation. However, the engraftment efficiency of hepatocytes is very poor. This is where microgravity could come to use. Zhang et al. used a µG bioreactor to grow neonatal mouse liver cells within a biodegradable scaffold within growth-factor reduced Matrigel73. The cells showed stable viability and reorganized to form tissue-like structures. Increased albumin secretion, urea production and cytochrome P450 activity indicated significantly upregulated hepatic function. The formed tissue structures were implanted and showed higher engraftment efficiency and higher levels of albumin than in static cultures. Moreover, blood vessel density was improved in the µG cultured tissues and lower initial apoptosis was observed. The survival from the acute liver failure was significantly better in mice implanted with the µG-cultured tissues73. It was theorized that this extraordinarily positive effect of µG on hepatic tissue formation and engraftment was caused by the prevention of hepatocyte cell and function loss due to lower shear stress in this environment. Moreover, it improved nutrient supply and waste disposal.
A second study described the construction of functional hepatic tissue from mouse E15.5 fetal liver cells using a rotating wall vessel bioreactor. The created hepatic structures included mature hepatocytes, blood/vessel-like structures, and bile duct-like structures. The created tissue was able to produce albumin and store glycogen. Between the hepatocytes, bile canaliculi were formed as well. The bile duct structures secreted mucin and formed complicated tubular branches74.
In conclusion, liver tissue formation in s-µG delivers superior tissues compared to 1G controls and even enables production of functional liver organoids capable of protein production and mucus secretion. These results are particularly promising, although we are only starting to grasp the potential that microgravity has for tissue formation. Future research should focus on exposing liver tissue to r-µG, an environment with even lower shear stress, potentially resulting in even lower liver cell and function loss.
Other tissues
The inner layer of the heart, blood- and lymphatic vessels is made up of endothelial tissue which enables nutrient exchange and creates a barrier for several pathogens to enter other tissues. Endothelial cells are an unmissable building block for the creation of functional vascular structures. Endothelial cells have been shown to survive spaceflights and formed 3D spheroids on the ISS within a cell incubator. One of the greatest challenges in tissue engineering is the formation of vasculature to enable nutrient transport in larger tissues. It is challenging to form tube-like structures with endothelial cells on Earth. Surprisingly, endothelial cells formed tubular structures without the need of scaffolds during the exposure to s-µG75 and r-µG41,63. This brings the field a step closer to the formation of vascular structures and demonstrates the potential role of microgravity tissue engineering.
During long-term space-flight, the organ that is most directly exposed to the space environment and endures the biggest risk of injury is skin. Investigating ways to replace or regenerate skin lesions, including tissue engineering of skin, is of great importance. Despite the relevance of the subject, only one study has described the engineering of skin tissue in microgravity. This study described the formation of free-floating tissue structures of more than one centimeter by using the HARV to aggregate keratinocytes which were used as scaffolding for melanoma cells76.
As discussed earlier, several studies have shown that thyroid cancer cells can be used to form spheroids in microgravity. This is relevant to investigate tumor growth or to test pharmaceutical agents. However, it would also be interesting to study healthy thyroid tissue in general and the effect of space on this tissue. Utilizing organoids could facilitate this research. Martin et al. created thyroid organoids in s-µG with the HARV and keratinocyte growth factor facilitating thyrocyte aggregation and 3D differentiation. Growth was substantially increased in organoids cultured in s-µG compared to 1G. The organoids closely resembled the natural thyroid tissue and formed follicular lumina and secretory vesicles77.
Thus, it is possible to form tubular tissues with endothelial cells in r-µG and s-µG, skin tissue and thyroid organoids in s-µG. The first steps have been set towards microgravity tissue engineering and several tissue types have successfully been formed in either r-µG or s-µG. Mainly, the formation of liver, vascular, and neural tissue demonstrates probable added value compared to 1G tissue engineering.
The potential of bioprinting in microgravity
Additive manufacturing has proven to be a useful tool for fast and easy fabrication of required objects during a space flight, increasing the autonomy of the crew. In recent years, tissue engineering developments have led to the creation of bioprinters, enabling computer-controlled tissue formation. This could theoretically enable in situ fabrication of medical treatments or food for long-term space flight. The microgravity environment could also have positive effects on bioprinting, as the lack of sedimentation enables low-viscosity bioinks, and scaffold collapse is prevented. Moreover, specific techniques like melt electrowriting and exothermic photo-crosslinking reactions might be more optimal in microgravity, resulting in higher-resolution prints15. It is theorized that microgravity could stabilize the electrical potential which stretches the jet in melt electrowriting, allowing for printing in different orientations. In printing techniques with exothermic photocrosslinking, problems arise with localized temperature gradients as they result in flows of convection, resulting in impaired resolution due to dislocation of the resin from the original cross-linking location. The convective flow is almost completely absent in microgravity, resulting in higher resolution prints.
Currently, three bioprinters are in Low Earth Orbit. The first is the Magnetic levitation bioprinter Organ.Aut, which is on board of the International Space Station since 2020 and will stay there at least until 202528. The second is the 3D BioFabrication Facility (BFF) developed by the company Techshot, part of Redwire78. This printer is part of the U.S. National Laboratory. The third printer is the Bioprint FirstAid device, which has recently been delivered to the ISS in collaboration with ESA. This is a hand-held bioprinter developed by German company OHB for the German Aeroscpace Center (DLR). The aim of this device is to print dressings consisting of autologous cells within a bioink to cover superficial wounds of the skin79.
To our knowledge, only one publication has yet described the creation of tissue structures with a bioprinter in r-μG. Parfenov et al. used a magnetic levitation bioprinter on the ISS to create tissues composed of human chondrocyte spheroids. The created structure showed incomplete fusion of the spheroids, but no signs of apoptosis were present. The chondrocytes maintained their typical viability and physiological activity within r-μG. Moreover, due to the μG, a low practically non-toxic dose of Gd3+-salt could be used for the magnetic field28. In s-μG, a study used a synchronized multi-material bioprinter to print droplets containing hepatocytes and endothelial cells that were subsequently exposed to a rotary cell culture system for 48 h. This resulted in increased hepatocyte cytoplasm diameter, 10-fold increased metabolic rate and decreased drug half-life compared to static culture80.
Additionally, ESA reported on up-side-down (-1G) skin and bone tissue 3D bioprinting. This novel bioprinting method serves as a model to assess the transferability of the bioprinting technique to space. This development is part of the 3D Printing of Living Tissue for Space Exploration project81.
Finally, bioprinting might be a solution for food production on long-term space missions. The load that can be taken on a spaceship is limited as the increase in weight complicates the launch and increases the fuel that is burned. Therefore, the crew can only take limited amounts of food with them on long-term missions. The ability to grow or print food in situ would solve this problem. Studies have demonstrated the successful printing of different foods using extrusion printing. A wide range of foods can be produced with this type of printing using a low number of food inks within a confined space82,83,84. Although culturing and printing foods has proven feasible on Earth, the process is still inefficient and production comes with high costs. The real and simulated microgravity environments could facilitate a more optimal environment to increase the yield and efficiency of the food production. Primarily, this could be a game-changer in global food shortages. Moreover, enabling the in situ printing or culturing of food in long-term space missions would prevent the undernutrition of astronauts.
Bioprinting in microgravity is still in its infancy, with many challenges to overcome. This includes liquid handling in space, as liquids tend to form spherical objects in microgravity due to their surface tension. This could disrupt the printing or final shape of the hydrogel bioinks. Liquids also have more difficulty streaming out of the print nozzles and bubbles formed within the inks are harder to remove. Moreover, for long-term space flight the shelf-life of the bioinks needs to be long enough15,85. Long-term space mission could last up to years, so durable storage is of utmost importance. One study specifically looked at a possible long-term storage method of Alginate-methylcellulose based bioinks and found that after four weeks the gel remained printable and microalgae were able to survive at 4 °C85. This could serve as a starting point to investigate possible storage techniques, but more extensive research is required.
The prior described data on the formation of spheroids and tissue structures in s-μG and r-μG, and the fast-paced developments within biofabrication promise a future increase in related research. The interest of international space agencies in this subject has grown tremendously in the past years. Several projects and funding opportunities have recently been set up by space agencies and foundations to support initiatives to perform bioprinting studies on the ISS86,87,88. Moreover, two projects will be executed on the BFF focusing on the creation of meniscal and cardiac tissue89,90.
Although bioprinting in microgravity shows a great promise, several practical challenges need to be overcome. This includes the minimization of expenses for shipment and optimization of printer performance in microgravity. Important considerations in light of these challenges are discussed by Rezapour Sarabi et al.91.
Future perspectives and knowledge gaps
The future of tissue engineering in microgravity
The previous sections described the current knowledge on the effects of microgravity on the cellular processes involved in the formation of multi-cellular spheroids, tissues and organoids by means of bioassembly and bioprinting. This section sheds light on the possible future improvements and applications of tissue engineering in microgravity. The development of tissue engineering techniques and the rapid growth of the space sector could become an unexpected synergy, accelerating the advancement in both fields. On one hand, tissue engineering might provide a way to grow or restore tissues in space. It could even enable the production of medication and food. This would be a game-changer for astronaut health in long-term space flight. On the other hand, microgravity provides a unique environment for cells, by mechanically unloading and consequently altering cell-cell and cell-matrix interactions, genetics and protein synthesis. Surprisingly, research in µG shows that these alterations might improve cell viability and proliferation, and stimulate 3D structure formation in stem cells and several tissue types. The µG environment provides scaffold-free, nozzle-free tissue engineering, as cells spontaneously self-assemble into multi-cellular structures. Figure 2 provides an overview of the possible advantages of tissue engineering in microgravity. However, microgravity tissue engineering is still in its infancy, with only a few studies performed and some contradictory results on the effects of µG on certain tissue types like cartilage and bone.
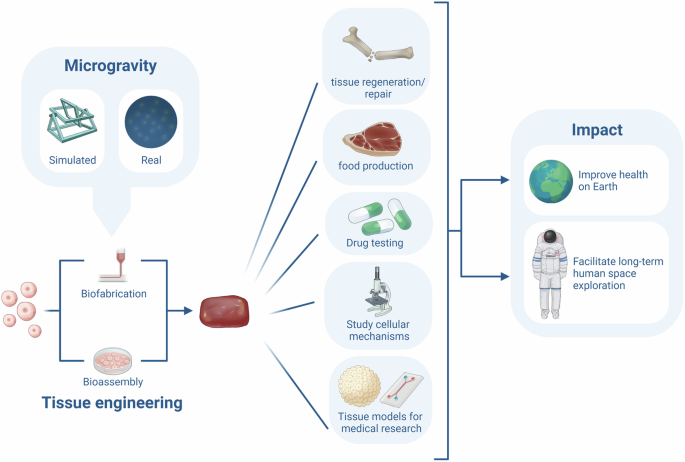
From left to right this figure depicts the formation of cellular structures in real- or simulated microgravity with tissue engineering (including biofabrication and bioassembly). The formed cellular structures can aid in tissue regeneration, food production, drug testing, studying cellular mechanisms and creating tissue models. This could have implications for health on earth and long-term human space exploration.
Exploration of altered cellular and molecular processes
The self-assembly of multicellular structures in µG is initialized by mechanical unloading, resulting in the clustering of cells. It is theorized that cells sense this mechanical unloading via mechano-sensing of the cytoskeleton following the non-equilibrium theory60. This process is accompanied by an altered cellular response, including changed gene and protein expression. Specifically, E-cadherin, c-Src, fibronectin, β-catenin, vinculin, IL-8 and osteopontin are important in mechanical alterations and tissue assembly. This results in the rearrangement of cytoskeleton and extracellular matrix molecules and changes in cell-cell and cell-matrix interactions. Consequently, the cell morphology changes and surprisingly, these changes often improve differentiation, growth and proliferation. Most spheroids formed within 24 hours, demonstrating the fast cellular response. The prior listed processes are not the only ones affected by microgravity. Bradbury et al. provided a thorough review of all the studied alterations on the cellular level92. In microgravity, cells are exposed to new degrees of freedom, which could unveil molecular mechanisms that would not be detected in normal gravity conditions.
Interestingly, the cellular response to microgravity within one cell type is not homogenous. Instead, it could be divided into two groups: one part detaches from the flasks to form 3D multicellular spheroids, while the other part remains attached to the flasks and grows two-dimensionally. Riwaldt et al. describe this as the bifurcation phenomenon42. Changes between the two groups go back to the genetic level. When cells are exposed to microgravity it is hypothesized that they enter a state in which tiny secondary effects like gene variations in single-cell transcriptomes could trigger altered development of single cells within an ostensibly homogenous population. It is important to take this division into account within the tissue engineering process to enable selection of the cells that are prone to form 3D structures.
Tissue and organ formation
The formation of tissues and organoids follows after the initial assembly of cells. The formation of larger tissue structures in microgravity is mainly facilitated by the increased nutrient and waste flow provided by the movement of the s-µG platforms. Moreover, the decreased shear stress and mechanical unloading lowers cellular stress and avoids collapsing of structures under their own weight. Larger structure formation has been demonstrated for cartilage, bone, nerves, liver, vasculature and thyroid tissue. Exposure of nerve, liver and vascular tissues to microgravity resulted in exceptionally commendable functional tissues and organoids. Most of the tissues have been formed in s-µG until now. Future research should initiate more tissue engineering attempts in r-µG. Moreover, bioassembly and bioprinting were discussed separately in this review, as both techniques are influenced differently by the space environment. To reach the goal of creating clinically relevant large-sized tissues or functional organs, it will be key to combine both techniques into hybrid biofabrication methods93,94.
Models of physiology and pathology in space
Engineered tissues and organoids can be used to study the effects of the space environment on physiological and pathological processes. As thoroughly discussed in this review, spheroids and organoids can be used as models for both physiology and pathology. In addition to these micro-tissues and micro-organs, novel developments have led to the combination of some of these structures into microphysiological systems. In these systems, different natural or engineered tissues and organoids are connected inside a microfluidic chip. Tissue chip technology, or organ-on-a-chip, aims to mimic the interactions of different tissue and organ systems to create a simplified system resembling the human body95. This could accelerate research on space-related pathology, like radiation disease, osteoporosis and aging96,97. Several chips have been sent to space and numerous projects are planning to perform research with this new system97,98. Further development of these techniques into reliable, robust, automated hardware promises to accelerate our understanding of space-related physiological and pathological processes. In turn this could have significant benefits for human health on Earth.
µG platform optimization and study reproducibility
The platforms for µG exposure of cells still need optimization. Several aspects require improvement, including transportation methods, r-µG and s-µG platform comparability and methodological reproducibility. It has been shown that cells and spheroids can be transported from and to space within hydrogels to avoid preliminary fusion or cell interactions. However, it might be necessary to slowly acclimatize the created tissues or organoids to an increasing degree of gravity to avoid collapsing the structure upon returning to Earth. When microgravity is simulated on Earth, this difficulty is surpassed. Although these s-µG platforms all aim to mimic the environment in space, they appear non-interchangeable, as the degree of shear on the cells differs. This was demonstrated by Stamenković et al. who compared porcine chondrocytes cultured on ISS with control samples in 1G and RPM. The resultant ISS tissues showed evident differences in structural protein and gene expression69. Each platform might thus affect each cell type differently. Therefore, it is interesting to investigate which method is most suitable for each cell type to assist in forming larger tissues.
Moreover, study reproducibility and replicability are of crucial importance to optimize tissue engineering techniques in space. We define reproducibility as obtaining consistent results with the same input data and described methods. Replicability is defined as obtaining consistent results across different studies with similar scientific questions99. Most studies assessed in this review describe their methods in a reproducible way, as described in Table S3. However, the methodology for biomedical research in r-µG and s-µG across different studies is heterogeneous and could benefit from universally standardized methods to improve replicability. Especially in r-µG, the experiments are exposed to extreme circumstances that can differ tremendously for each launch and return.
Concluding remarks
In conclusion, this review shed light on the potential synergy between tissue engineering and microgravity, offering novel prospects for advancements in both fields. Microgravity provides a unique environment that triggers a cellular response that facilitates self-assembly of several stem cells and specialized cells into spheroids, tissues and even organoids. Disentangling the intricate mechanisms behind spontaneous self-assembly could facilitate control over these processes via bioassembly and bioprinting methods. This could lead to innovations that benefit the Earth and long-term space travel, including tissue and organ fabrication, spheroid and organoid production for pharmaceutical, toxicological, radiation, and cancer research, and studying the effects of the space environment on different physiological systems. Finally, astronauts could benefit from new treatment options and food production, enabling a safe exploration of space.
Despite this promising outlook, many challenges and opportunities remain, underscoring the need for further investigation. Firstly, microgravity tissue engineering remains in its infancy, with limited studies and conflicting results for specific tissue types such as cartilage and bone. The platforms for microgravity exposure also require optimization, with variations in shear stress among simulated and real microgravity environments. Additionally, acclimatizing tissues to Earth’s gravity upon return poses a challenge, necessitating careful consideration for the structural integrity of the structures. Furthermore, the heterogeneity in cellular responses within the same cell type, illustrated by the bifurcation phenomenon, emphasizes the importance of tailoring tissue engineering processes to select cells predisposed to form 3D structures. Future research endeavors should address these challenges, exploring optimal methods for each cell type and advancing towards reliable and reproducible microgravity tissue engineering techniques.

Responses