Chemical versus physical pressure effects on the structure transition of bilayer nickelates

Introduction
The recent discovery of high-Tc superconductivity (HTSC) in pressurized La3Ni2O7 stands out as a conspicuous breakthrough in the realm of 3d transition-metal oxides, and has thus immediately emerged as a central subject in the community of condensed matter physics1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30. As a member of Ruddlesden-Popper (R-P) phase materials, La3Ni2O7 adopts an orthorhombic Amam structure at ambient pressure, which consists of alternating layers of La-O and Ni-O planes with a bilayer corner-sharing Ni2.5+O6 octahedra structure31,32,33. Recent studies showed that it undergoes an orthorhombic to tetragonal structural transition at high pressure above Pc ≈ 14 GPa, which was believed to play an important role for the emergence of HTSC1. When the higher symmetry I4/mmm structure is induced by pressure, the out-of-plane Ni-O1-Ni bond angle within the bilayer corner-sharing Ni2.5+O6 octahedra is changed to 180°1,6,24. This will cause the dispersion curves originated from the ({3d}_{{{rm{z}}}^{2}}) orbital to intersect with Fermi level, resulting in the emergency of γ band and promoting superconducting pairing1. Such a physical scenario was supported by subsequent theoretical calculations9,11,12,20,34,35, and has been employed to explain the bulk superconductivity in polycrystalline La2PrNi2O7 samples with significantly reduced intergrowth of various R-P phases5.
As an emerging novel class of pressure-induced high-Tc superconductors, it is highly desirable to explore the possibility of achieving superconductivity at ambient pressure. One possible approach is to replace La3+ with either isovalent smaller-size rare-earth R3+ ions or heterovalent cations. While the latter affects both the lattice and charge carriers, the former solely introduces chemical pressure, which is expected to contract the lattice and reduce the physical pressure required to induce structural transition and HTSC. We are thus motivated to investigate the structural evolution of a series of rare-earth substituted La3-xRxNi2O7-δ samples under high pressure, aiming to reveal the relationship between the critical pressure Pc and the average ionic radius of the A-site cations, <rA> ≡ [(3-x) rLa + x rR]/3. Considering the significant challenges in preparing single crystals of this system, we focus on the polycrystalline samples that can be prepared in a controlled manner and are conducive to sample screening before committing to dedicated crystal growth endeavor2,5,31.
In this work, we prepared a series of La3-xRxNi2O7-δ (R = Pr, Nd, Tb, Y) polycrystalline samples with the same procedures and conducted systematic high-pressure (HP) synchrotron X-ray diffraction (SXRD) to determine the critical pressure Pc for the structural transition. The constructed phase diagram of Pc versus <rA> reveals that Pc rises monotonically with the reduction of <rA>, which is attributed to the enhanced orthorhombic distortion upon reducing <rA>. This observation indicates that it is unlikely to reduce Pc through the substitution of smaller R3+ at the La position. In addition, the extrapolation of Pc versus <rA> suggests that it is likely to reduce Pc to ambient pressure via substituting La3+ with larger heterovalent cations, such as alkaline-earth Sr2+ or Ba2+. By unveiling a negative relationship between Pc and <rA>, our results provide valuable insights in achieving superconductivity at ambient pressure in this system.
Results
Impact of chemical pressure
The phase purity of obtained La3-xRxNi2O7-δ samples was first examined with XRD at room temperature. As shown in Fig. S1, all samples are confirmed to be single phase with the orthorhombic Amam structure (No. 63). The substitution of smaller R3+ for La3+ in the perovskite-type La3Ni2O7 is expected to shrink the lattice and enhance local structural distortions. To obtain reliable information about the structural changes upon different R3+ substitutions, we performed NPD measurements at ambient conditions since the oxygen atoms have a large neutron scattering length. Figure 1a and Table S1 display the Rietveld refinement results on the collected NPD data, which further confirm that all samples are single phase with the orthorhombic structure. The obtained lattice parameters are displayed in Fig. 1b, c as a function of <rA>. As can be seen, the lattice parameters exhibit anisotropic shrinkage with increasing <rA>, i.e., a and c are reduced monotonically by 0.6% and 0.9%, respectively, while b remains nearly unchanged, resulting in a net volume reduction of 1.5% from La3Ni2O7-δ to La1.8Nd1.2Ni2O7-δ. Such an evolution confirms that we have successfully introduced some chemical pressure into the La3Ni2O7 lattice by replacing La3+ with smaller R3+.
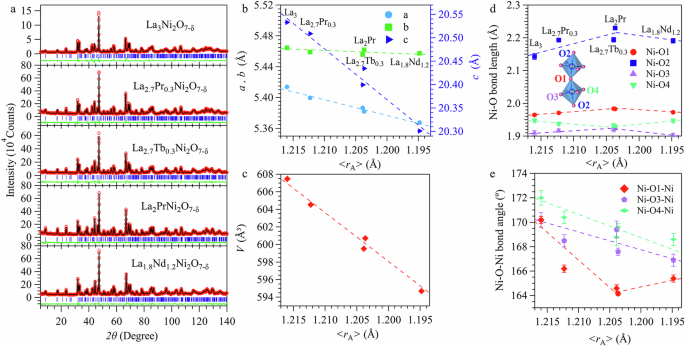
a Rietveld refinements on the NPD data with the space group Amam. b–e The obtained lattice parameters, unit-cell volume, Ni-O bond lengths and Ni-O-Ni bond angles as a function of the average ionic radius of the A-site cations, <rA>, across the series of La3-xRxNi2O7-δ (R = Pr, Nd, Tb) polycrystalline samples. The NPD data for La3-xPrxNi2O7-δ (x = 0, 1.0) samples were adopted from our previous study2,5. The error bars in (b–d) are smaller than symbols.
The observed anisotropic evolutions of in-plane lattice parameters motivated us to inspect the local structure arrangements of the buckled NiO2 planes in the orthorhombic bilayer nickelates. As shown in Fig. S2a, the lattice parameters a and b ≈ 2√2 (Ni-O) are determined by a combination of Ni-O bond lengths, Ni-O-Ni and O-Ni-O bond angles. Since both Ni-O3/Ni-O4 bond lengths and Ni-O3/O4-Ni bond angles with similar magnitudes are involved in determining the a and b axes, their relative magnitudes are mainly determined by the O-Ni-O angles as illustrated in Fig. S2a. It is the obtuse angle O3-Ni-O4 and the acute angles of O3-Ni-O3 and O4-Ni-O4 that results in the experimentally observed b > a. As shown in Fig. S2b, upon reducing <rA>, the obtuse angle O3-Ni-O4 is monotonically enlarged while the average of acute angles O3-Ni-O3 and O4-Ni-O4 is slightly reduced. These factors alone should result in a monotonic elongation of b and shortening of a. Considering the general reduction trends of in-plane bond lengths and angles that should reduce both a and b, the above-mentioned elongation trend of b will be partially compensated while the declining trend of a be further enhanced, resulting in anisotropic variations of a and b as a function of <rA> shown in Fig. 1b.
Although the chemical and physical pressures play similar roles in shrinking the overall lattice, their influences on the local structural distortions are distinct. For example, external physical pressure usually compresses the lattice uniformly and drives it to a higher symmetry, while the chemical pressure produces stronger local structural distortions in the perovskite-type structures. As shown in Fig. 1d, e, some interesting features about the local structural modifications induced by smaller R3+ are noteworthy: (1) both the out-of-plane Ni-O2/O1 bonds first experience an elongation, reaching the maximum at <rA> = 1.206 Å, and then decreases upon further reducing <rA> for La1.8Nd1.2Ni2O7; (2) the in-plane Ni-O3 and Ni-O4 bond lengths vary oppositely, resulting in reduction first and then an enhanced splitting of in-plane Ni-O bonds as a function of <rA>; (3) both in-plane and out-of-plane buckling of the corner-shared NiO6 octahedra becomes stronger as the Ni-O-Ni bond angles are reduced. Based on the limited data points in Fig. 1d, e, there seems to exist an anomalous trend for La1.8Nd1.2Ni2O7-δ. However, the presence of four distinct Ni-O bond lengths and three Ni-O-Ni bond angles makes it quite complicated to characterize the overall orthorhombic distortions associated with the tilting and bulking of NiO6 octahedra. To a first-order approximation, here we examine the variations of average <Ni-O> bond length and <Ni-O-Ni> bond angle as a function of <rA>. As shown in Fig. S3, the average <Ni-O> bond length keeps nearly constant around 1.98 ± 0.01 Å, while the average <Ni-O-Ni> bond angle decreases linearly with reducing <rA>. Therefore, the substitutions of smaller R3+ for La3+ in La3-xRxNi2O7-δ not only shrink the overall lattice but also produce stronger local orthorhombic distortions. The former effect is similar as external physical pressure, whereas the latter one should require higher pressure to induce a structural transition to a higher symmetry.
Pressure-induced structural transition of La3-x
R
xNi2O7-δ
To determine the critical pressure Pc for structural transition, we conducted HP-SXRD measurements at room temperature under various pressures up to 40 GPa. The HP-SXRD results on two representative samples, La3Ni2O7-δ and La1.8Nd1.2Ni2O7-δ, are depicted in Fig. 2a, b, respectively. For La3Ni2O7-δ, the SXRD patterns below 4.9 GPa consistently match the orthorhombic Amam structure, as shown by the representative refinement at 3.6 GPa (Fig. 2c). However, upon compression to 7.0 GPa, several adjacent peaks merge, such as the (020) and (200) peaks at ~13.4°, as illustrated in the right panel of Fig. 2a. This observation suggests the occurrence of pressure-induced structural transition towards higher symmetry. Subsequent structure analyses revealed that the SXRD patterns of the HP phase can be better described using the Sr3Ti2O7-type structural model with the tetragonal I4/mmm space group (No. 139), Fig. 2d, consistent with previously reported results24. For La1.8Nd1.2Ni2O7-δ, the same structural transition was also observed, with the critical pressure Pc ≈ 17 GPa (Fig. 2b, e, f). The HP-SXRD results for the other three samples of the La3-xRxNi2O7-δ (R = Pr, Tb, Y) series were shown in Figs. S4–S6. They all undergo the same structural phase transition under high pressure with the Pc ≈ 8, 10.5, and 8.7 GPa for La2.7Pr0.3Ni2O7-δ, La2.7Tb0.3Ni2O7-δ, and La2.87Y0.13Ni2O7-δ, respectively.
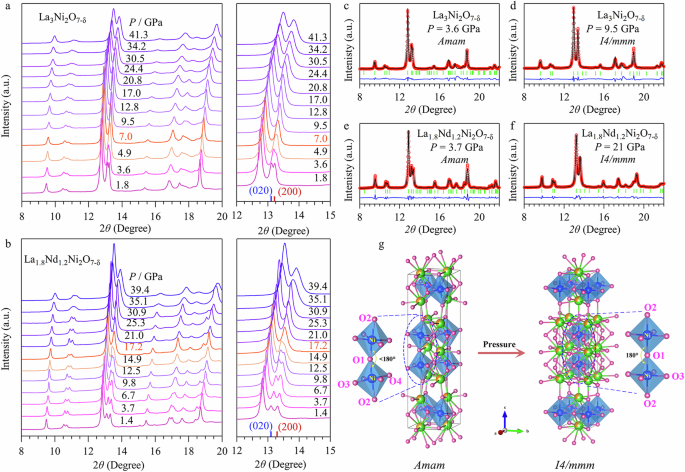
a, b SXRD patterns of La3Ni2O7 -δ and La1.8Nd1.2Ni2O7-δ polycrystalline samples under various pressures up to 41.3 and 39.4 GPa, respectively. The enlarged view of SXRD around the representative 2θ ranges highlight the gradual merging of the diffraction peaks upon compression. c–f Refinement results of the SXRD patterns at pressures before and after the structural phase transition for La3Ni2O7-δ and La1.8Nd1.2Ni2O7-δ. g Crystal structure transformation of La3-xRxNi2O7-δ under high pressure.
Figure 3 displays the lattice parameters as a function of pressure for these two samples extracted from their HP-SXRD patterns after Rietveld refinements. As seen from Fig. 3a, b, their lattice parameters decrease continuously with increasing pressure, but exhibit anisotropic compressions. In the lower pressure range, lattice parameter b decreases faster than a and they converge at Pc, where the structural transition takes place. As the crystal structure transforms into a higher-symmetry tetragonal structure, the lattice parameter a contracts by a factor of (frac{1}{sqrt{2}}), leading to a 0.5 times reduction in the unit-cell volume, V. The collected pressure-volume P(V) data in the whole pressure range can be fitted to the third-order Birch-Murnaghan equation36, yielding a bulk modulus of B0orth = 137.1 GPa and B0tetra = 208.4 GPa for La3Ni2O7-δ and B0orth = 179.3 GPa and B0tetra = 221.7 GPa for La1.8Nd1.2Ni2O7-δ, respectively, with B0′ fixed at 3.5, as shown by the dashed lines in Fig. 3c, d.
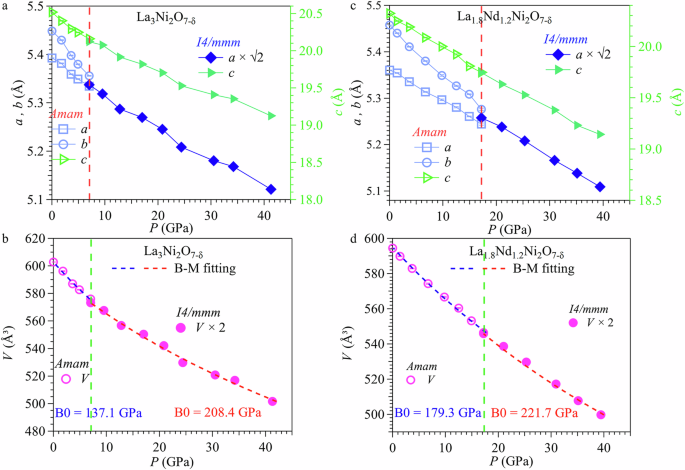
Lattice parameters and cell volume as a function of pressure for a, b La3Ni2O7-δ and c, d La1.8Nd1.2Ni2O7-δ samples under various pressures up to 41.3 and 39.4 GPa, respectively. The critical pressure Pc for the structural transition is marked by the vertical broken line in (b, d).
DFT insights on structural transition
From the above experimental results, we can see that Pc increases with reducing <rA>. To understand the structural transition of bilayer nickelates as a function of chemical and physical pressures, we employed the DFT calculations to study the structural evolution of the R3Ni2O7-δ (R = La, Pr, Nd) samples under high pressure. Figure 4 shows the calculated results of lattice parameters for R3Ni2O7-δ (R = La, Pr, Nd) as a function of external pressure. As can be seen, all R3Ni2O7 compounds undergo an orthorhombic to tetragonal structural transition under high pressure, consistent with the experimental observations mentioned above. Additionally, our DFT calculations show that the calculated Pc at U = 3 eV for La3Ni2O7 is ~9 GPa, which is close to the HP-SXRD observations, indicating the reliability and accuracy of our theoretical methods. It should be noted that the critical pressure Pc from DFT calculations depends sensitively on the choice of Hubbard U values, and a higher U value results in a lower calculated Pc. As shown in Fig. S7, the calculated Pc of La3Ni2O7 decreases monotonically from ~ 12 GPa for U = 1 eV to ~ 5 GPa for U = 6 eV. The U value of 3 eV applied in this work is favored by many theoretical studies of this system7,9,15,19,37 and is close to the value of ~3.5 eV obtained from angle-resolved photoelectron spectroscopy experiments21.
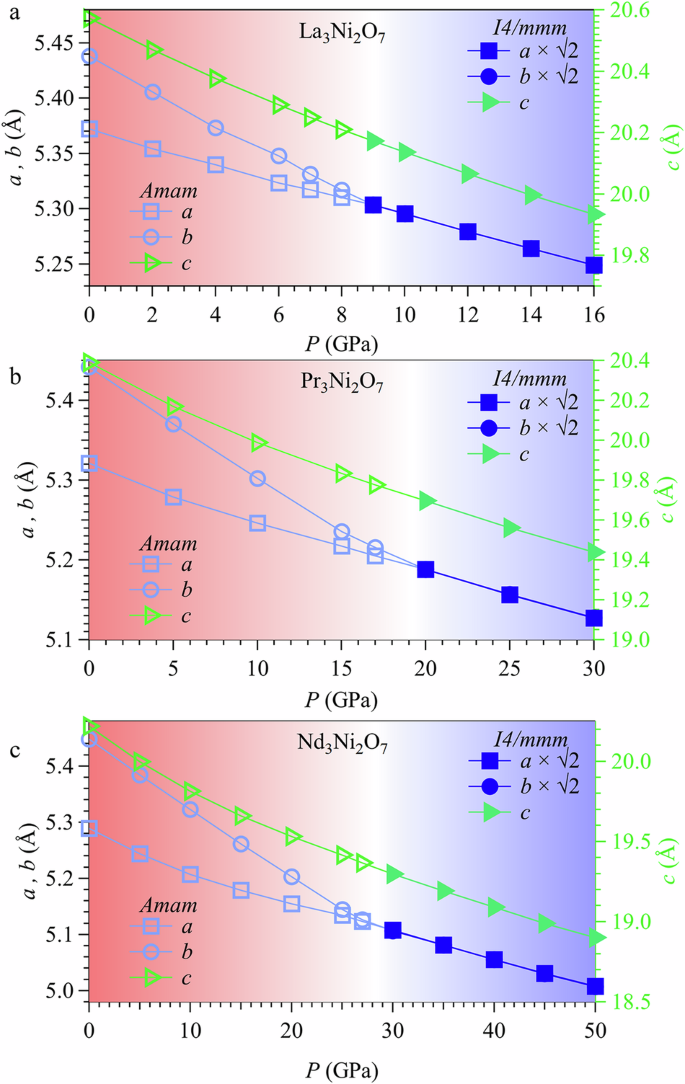
a–c Lattice parameters of R3Ni2O7 (R = La, Pr, Nd) as a function of the external pressure from DFT calculations. As can be seen, the lattice parameters a and b of all R3Ni2O7 compounds gradually converge under high pressure, but with different critical pressures.
To better clarify the impact of physical pressure on the structural evolution of the system, we extracted the bond lengths and bond angles of La3Ni2O7 under various pressures from DFT calculations. As shown in Fig. S8a, all bond lengths decrease progressively with applied pressure but exhibit anisotropic compressions. For the two out-of-plane bonds, Ni-O2 shortens more rapidly than Ni-O1 with increasing pressure, leading to a reduction in the length difference between these bonds. For the in-plane bonds, Ni-O4 decreases in length faster than Ni-O3, and they converge at the critical pressure Pc. In addition, as the Ni–O–Ni bond angle gradually increases with pressure and reaches 180° at Pc, both in-plane and out-of-plane buckling of the corner-shared NiO6 octahedra are diminished (Fig. S8b). It is noteworthy that these calculated changes of bond lengths and angles under physical pressure are different from those induced by reducing <rA> shown in Fig. 1b, d, e, further highlighting the distinct effects of physical pressure versus chemical pressure on the structural evolution as discussed above.
P
c -<r
A> phase diagram
We plotted in Fig. 5 the obtained Pc of La3-xRxNi2O7-δ from both experiments and DFT calculations as a function of <rA>. As seen clearly, Pc increases with the reduction of <rA>. This result reveals that the structural transition is dictated mainly by the local orthorhombic distortions in chemically pre-compressed La3-xRxNi2O7-δ samples, i.e., the stronger local structural distortion, the higher Pc for structural transition. The observed evolution of Pc versus <rA> implies that it is not feasible to reduce Pc to ambient pressure via substituting La3+ with smaller-size R3+ ions. A linear extrapolation of Pc versus <rA> to zero would yield an <rA>c ≈ 1.232 Å for the stabilization of the tetragonal phase at ambient pressure, as shown by the black dashed line in Fig. 5. If this assumption holds, alkaline-earth-metal substituted La2.5Sr0.5Ni2O7 and La2.8Ba0.2Ni2O7 can satisfy such a requirement of <rA> ≈ 1.232 Å. In addition, extra charge carriers will be introduced into this system by doping alkaline-earth cations. Although the replacement of La3+ with heterovalent alkaline-earth cations could be a viable approach to stabilize the tetragonal phase at ambient pressure, it remains quite challenging to synthesize these samples.
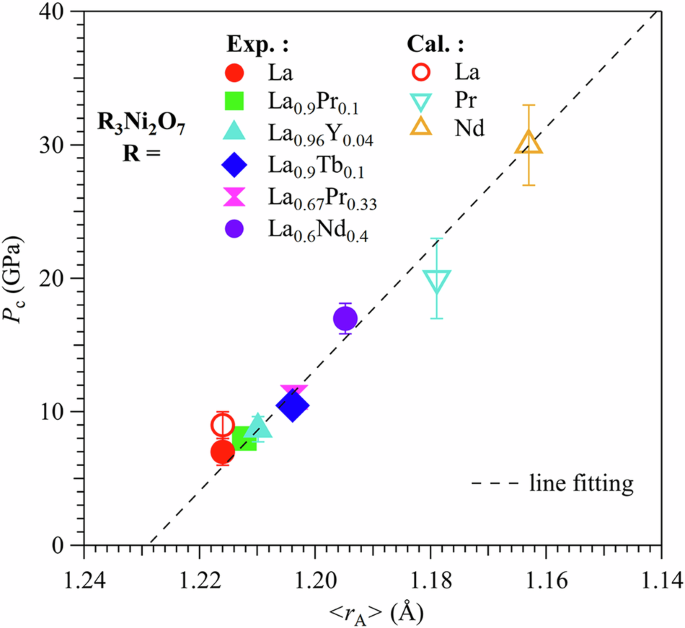
The critical pressure Pc for the orthorhombic-tetragonal structural transition as a function of the average ionic radius of the A-site cations, <rA>, across the series of La3-xRxNi2O7-δ (R = Pr, Nd, Tb, Y) polycrystalline samples. As can be seen, the data derived from theoretical calculations and experiments can be well described by linear fitting. The error bars are mainly determined by the pressure intervals in either SXRD experiments or theoretical calculations.
Discussion
Recently, Jiao et al.18 synthesized a series of Sr-doped La3-xSrxNi2O7-δ (0 ≤ x ≤ 0.1) polycrystalline samples via conventional solid-state-reaction method. They found that upon increasing x, the lattice constants a and b expand while the c-axis contracts, with a simultaneous increase of Ni-O-Ni bond angles. In addition, the resistivity decreases monotonically in the entire temperature range due to the introduction of hole carriers and the reduced structural distortions. Xu et al.38 successfully obtained Sr-doped La2.8Sr0.2Ni2O6.95 single crystals with the orthorhombic bilayer structure by treating the precursors at 20 GPa and 1400 °C in a Walker-type multianvil press. For such samples, both out-of-plane Ni–O–Ni bond angles of 173.4(2)° and in-plane Ni-O-Ni bond angles of 175.0(2)° and 176.7(2)° are indeed larger than those of pristine La3Ni2O7. The HP-SXRD was not performed on those La3-xSrxNi2O7-δ polycrystalline samples and La2.8Sr0.2Ni2O6.95 single crystal. According to the present study, it is expected that the structural transition to the tetragonal phase should take place at a lower Pc than La3Ni2O7. Thus, HP structural studies on these samples are highly desirable. Intriguingly, it was found that the La2.8Sr0.2Ni2O6.95 single crystal exhibits an insulating behavior at ambient pressure and displays pressure-driven insulator-metal-insulator crossovers up to 19 GPa. These transport properties are dramatically different from those of the parent compound La3Ni2O7-δ and warrant further investigation. Nevertheless, the successful synthesis of Sr-doped La2.8Sr0.2Ni2O6.95 single crystals would open an avenue for exploring possible ambient-pressure HTSC via further enhancing the substitution levels of Sr2+ for La3+ in the bilayer nickelates.
It is noteworthy that the critical pressure Pc ≈ 7 GPa for our polycrystalline La3Ni2O7-δ sample is lower than that of ~14 GPa for the single-crystal samples reported by Sun et al. in ref. 1. At present, the origin of this discrepancy remains unclear, which might arise from the distinct grain sizes or diverse levels of structure defects/disorders for the different forms of samples. Nonetheless, our HP-SXRD results on the polycrystalline La3-xRxNi2O7-δ samples prepared at the same conditions should provide self-consistent information about the effect of substitution of smaller R3+ ions at the La sites.
Based on our combined HP-SXRD and resistivity measurements on the polycrystalline La3-xPrxNi2O7-δ (x = 0 and 1) samples, we found that the emergence of superconductivity is in general concomitant with the structural transition in this system. For the La3Ni2O7-δ sample, the structural transition occurs at Pc ≈ 7 GPa, while the resistance begins to decrease around 6–8 GPa and reaches zero at roughly 9 GPa2. Similarly, the structure transition takes place at Pc ≈ 11 GPa while the resistance drop appears at ~ 8 GPa and approaches zero at about 11 GPa for La2PrNi2O75. Some discrepancies between the critical pressures for structural transition and the emergence of superconductivity should be partially ascribed to the pressure distribution/variation in different HP techniques. This effect should be magnified for the pressure cells upon cooling down. It is noted that the structural transition was monitored via HP-SXRD measurements at room temperature whereas the emergence of superconductivity was evidenced by HP-resistance measurements at low temperatures. We have shown in our previous study that the pressure values in palm-type cubic anvil cell (CAC) for resistance measurements will change upon cooling down due to the thermal contraction mismatch of different parts of pressure cells39. For example, the CAC locked at 8 GPa at room temperature increases to ~10.5 GPa at low temperatures. Considering these extrinsic factors, the critical pressures for the orthorhombic-tetragonal structural transition are in general coincidence with the emergence of superconductivity in the bilayer nickelates.
The series of La3-xRxNi2O7-δ samples prepared in the present study offer an excellent material platform for comprehensive investigations on structure-property correlations. As the Pc ≈ 17 GPa of La1.8Nd1.2Ni2O7-δ is over the pressure limit of our CAC apparatus and is also close to the limit of two-stage multianvil pressure cell, we have to employ the diamond anvil cell (DAC) to measure its HP resistance. Some preliminary results (not shown here) indeed evidenced the onset of superconductivity at an elevated pressure ~16 GPa, further reinforcing the intimated correlation between structural transition and the emergence of superconductivity in these bilayer nickelates. However, due to the non-hydrostatic pressure conditions in DAC filled with solid pressure transmitting medium, we can only observe the drop of resistance without reaching zero resistance. For these bilayer nickelates, it is highly desirable for resistance measurements under hydrostatic pressures above 20 GPa.
Finally, we would like to highlight that the experimentally observed Pc for structural transition can be excellently simulated by DFT calculations, which demonstrated the power and accuracy of structural modeling using ab initio calculations. Thus, incorporating simulations into future research may provide valuable insights before conducting specific experiments. This can potentially accelerate material discovery and lead to more favorable outcomes while reducing experimental workload.
In summary, we conducted systematic HP-SXRD measurements on a series of La3-xRxNi2O7-δ (R = Pr, Nd, Tb, Y) samples and established a quantitative relationship between the critical pressure Pc for orthorhombic-tetragonal structural transition and the average size of A-site cations, <rA>. By unveiling the inverse relationship between Pc and <rA>, our results provide useful guidelines for achieving the tetragonal phase at ambient pressure in this system. Replacing La with alkaline-earth metals can increase <rA> and introduce additional charge carriers, making it a promising approach to achieve HTSC at ambient pressure.
Methods
Sample synthesis
A series of La3-xRxNi2O7-δ (R = Pr, Nd, Tb, Y) polycrystalline samples were synthesized by the sol-gel method as described in refs. 2,31. Stoichiometric mixtures of rare-earth oxides and Ni(NO3)2·6H2O (99.99%, Alfa Aesar) were used as the starting materials. All samples were synthesized using the identical procedures and sintered at the same temperature conditions. Each dopant has its own solubility, which we determined by experimenting with different compositions. In this work, the highest experimentally obtained doping level of La3-xRxNi2O7-δ (R = Pr, Nd, Tb, Y) samples with a pure phase are La2PrNi2O7-δ, La1.8Nd1.2Ni2O7-δ, La2.7Tb0.3Ni2O7-δ, and La2.87Y0.13Ni2O7-δ. Further increasing the substitution content results in the formation of (La, R)2NiO4 or other oxide impurities.
Structural characterizations
The phase purity and crystal structure of La3-xRxNi2O7-δ at ambient conditions were determined by powder X-ray diffraction (XRD) collected via a Huber diffractometer with Cu-Kα radiation. Neutron powder diffraction (NPD) measurements were carried out using the HB-2A diffractometer at the High Flux Isotope Reactor (HFIR) of Oak Ridge National Laboratory (ORNL)40,41. Powder samples of La3-xRxNi2O7-δ were placed inside a 6 mm-diameter vanadium container and then inserted into a closed cycle refrigerator. NPD data was collected at 295 K, utilizing a constant wavelength of λ = 1.5365 Å, derived from the Ge (115) monochromator. The NPD pattern was collected by scanning a 120° bank of 44 ³He detectors in 0.05° steps to give 2θ coverage from 5° to 150°. Rietveld refinements were performed with the FULLPROF program42. HP-SXRD measurements were performed at BL15U1 station of Shanghai Synchrotron Radiation Facility (SSRF) with a wavelength of λ = 0.6199 Å. Rietveld analysis was performed with GSAS-II program43.
DFT structure calculations
Density functional theory (DFT) calculations were conducted using the projector-augmented wave (PAW) method, implemented in the Vienna ab initio simulation package (VASP)44,45. All calculations utilized the Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation (GGA) exchange-correlation functional46. To correct the self-interaction error on the Ni species, a rotationally averaged Hubbard U correction of 3 eV was applied47. This value is commonly used in theoretical studies of this system7,9,15,19,37 and is close to the value of ~3.5 eV obtained from the angle-resolved photoelectron spectroscopy experiments21. For all calculations, a plane wave energy cutoff of 520 eV, an electronic minimization threshold of 10−6 eV, and a k-point grid of nkpoints × natoms > 1000 were adopted. For structural relaxation, all degrees of freedom of atoms and lattices were allowed to relax until the atomic forces were less than 0.001 eV Å−1. Given that La3Ni2O7 displays no long-range magnetic ordering in previous experiments1, all DFT calculations in this study were performed without spin-polarization. The PSTRESS parameter was employed to control the external hydrostatic pressure.


Responses