Clinical characteristics and outcomes of BCMA-targeted CAR-T cell recipients with COVID-19 during the Omicron wave: a retrospective study
Introduction
Coronavirus disease 2019 (COVID-19) is an epidemic disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). It was first identified at the end of 2019, after which it quickly spread to become a global pandemic in 2020 [1, 2]. Infected patients often experience no or mild respiratory symptoms, but the virus may also lead to acute respiratory failure and even death in severe cases. In November 2021, a new SARS-CoV-2 variant, dubbed “Omicron”, began to spread and soon became the dominant strain worldwide, thus significantly altering the epidemiological status of COVID-19 [3]. Though the more highly mutated Omicron variant generally caused lower severity illness and mortality compared to the earlier Delta variant, its higher transmissibility and resistance to existing vaccines should not be ignored [4,5,6,7,8].
Multiple myeloma (MM) is a common hematologic malignancy, the global incidence of which has been increasing in recent years—possibly owing to worldwide population aging [9]. Previous studies have demonstrated that patients with MM were often more susceptible to infect with SARS-CoV-2, and develop severe COVID-19 illnesses and adverse outcomes. This may be attributable to the immune dysfunction caused by the disease or the associated anti-tumor therapy typically used to treat it [10,11,12,13,14]. The overall mortality of this patient group to COVID-19 reached 33–37% before the Omicron infection wave [11, 12].
As a novel therapy, B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR)-T cells have resulted in very favorable response rates in patients with relapsed or refractory MM (R/R-MM) [15,16,17,18,19]. However, immune deficiency represents one of the most significant complications following BCMA-targeted CAR-T cell therapy, which may further increase the risk of infection [20, 21]. In addition, patients who have received BCMA-targeted CAR-T cell therapy before SARS-CoV-2 vaccination present impaired vaccination responses, implying a lack of immunity to SARS-CoV-2 [22]. However, clinical data for BCMA-targeted CAR-T cell recipients who became infected with SARS-CoV-2 during the Omicron wave is scarce. In this study, we described the clinical characteristics, outcomes, and potential risk factors associated with COVID-19 in patients with R/R-MM who have received prior BCMA-targeted CAR-T cell therapy during the Omicron wave of the COVID-19 pandemic.
Methods
Patients
We retrospectively reviewed data from patients with R/R-MM who had received BCMA-targeted CAR-T cell therapy at the First Affiliated Hospital of Zhejiang University School of Medicine (ChiCTR1800017404, NCT05430945). Our inclusion criteria were: (1) ≤75 years of age; (2) diagnosed with R/R-MM; (3) received BCMA-targeted CAR-T cell therapy for R/R-MM from July 2018 to December 2022 and (4) had undergone antigen or nucleic acid testing for COVID-19 between November 2022 and February 2023. Any patients who had been diagnosed with COVID-19 before November 2022 were excluded. The final follow-up time was on April 30, 2023. Patients were categorized into infected and non-infected groups based on the results of antigen or nucleic acid tests.
Patient consent statement
We got written informed consent forms from all the patients for data collection. This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Review Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (Approval No. IIT20240976A).
Data collection
Age, sex, MM disease stage, and the efficacy and complications associated with BCMA-targeted CAR-T cell therapy for R/R-MM were recorded. Vaccination, diagnosis, clinical symptoms, treatments, and outcomes were collected from the patients’ charts, electronic medical records, and follow-up telephone interviews.
CAR-T cell therapy procedures
The manufacture and infusion of BCMA-targeted CAR-T cells have been described previously [23]. Normal T-cells were collected from each patient and transduced with BCMA CAR. All patients received fludarabine (30 mg/m2/d for 3 days) and cyclophosphamide (500 mg/m2/d for 2 days) before BCMA-targeted CAR-T cell infusion. Cytokine release syndrome (CRS) was graded by the Lee criteria [24].
Definitions
The primary outcome was COVID-19 incidence at the end of the follow-up period. The secondary outcome was diagnosis of severe COVID-19. COVID-19 severity was classified as mild (no abnormal chest imaging), moderate (abnormal chest imaging but oxygen saturation measured by pulse oximetry [SpO2] ≥94% on room air), and severe (SpO2 < 94% on room air or lung infiltrates >50%) [25]. COVID-19 resolution was defined as being alive with all clinical symptoms resolved or negative antigen or nucleic acid test [26]. Duration of COVID-19 was defined as the interval between COVID-19 diagnosis and resolution.
Statistical analyses
The demographic, clinical characteristics and outcomes of the patients were analyzed. Continuous variables were expressed as medians and ranges and were compared using the Mann–Whitney U-test. Categorical variables were presented as frequencies and percentages and were compared using χ2 and Fisher’s exact tests. COVID-19 resolution and overall survival (OS) probabilities were assessed using the Kaplan–Meier estimator. The odds ratio (OR) for COVID-19 and severe COVID-19 were estimated using logistic regression model. The hazard ratio (HR) of COVID-19 duration and OS were calculated using Cox regression model. Variables with P-values of ≤0.1at univariate analysis were considered for multivariate analysis. All analyses were performed using SPSS version 26.0 (IBM, Chicago, IL, USA) and GraphPad Prism 9 version 9.0.0 (GraphPad Software, San Diego, CA, USA). A two-tailed significance P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 49 patients (30 male, 19 female) with R/R-MM who had received BCMA-targeted CAR-T cell therapy at the First Affiliated Hospital of Zhejiang University School of Medicine were enrolled. The median age was 60 (42–75) years. The most common heavy chain types were IgG (n = 19, 38.8%) and IgA (n = 18, 36.7%). Only one patient was light chain type, and one was non-secretory. Most of the patients were classified as Durie-Salmon (DS) stage III (n = 39, 79.5%), DS sub-A (n = 38, 77.6%), and R-ISS stage II (n = 26, 53.1%). All were diagnosed with R/R-MM, and about one-quarter of the patients (n = 13, 26.5%) had received auto-hematopoietic stem cell transplantation before CAR-T cell therapy. During the CAR-T cell treatment, CRS was observed in 93.9% of the patients (n = 46), and 30.6% (n = 15) were classified as having severe CRS (≥grade 3). The overall response rate reached 98.0% (n = 48), including 67.3% of the patients achieved complete remission (CR) (Fig. 1a). The baseline clinical characteristics of the patients before COVID-19 infection are summarized in Supplementary Table 1.
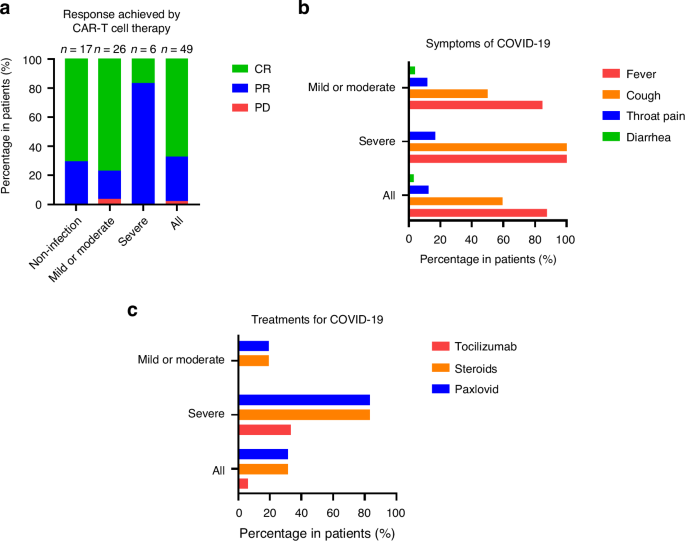
a The percentage of responses achieved by BCMA-targeted CAR-T cell therapy in patients with or without COVID-19. b Symptoms of COVID-19 in patients with COVID-19. c Treatments for COVID-19 in patients with COVID-19. BCMA B-cell maturation antigen, CAR chimeric antigen receptor, COVID-19 coronavirus disease 2019.
Characteristics of COVID-19-infected and non-infected patients
Among the total 49 enrolled patients, most (n = 43, 87.8%) had not received SARS-CoV-2 vaccination. For those who had received vaccination before November 2022, 4.1% (n = 2), 6.1% (n = 3), and 2.0% (n = 1) patients received one, two, and four doses, respectively. During the Omicron wave of COVID-19 (November 2022 to February 2023), 69.4% (n = 34) patients remained in the CR stage, while 6.1% (n = 3) and 24.5% (n = 12) patients were classified as being in partial remission and progression disease (PD), respectively. The median IgG level during COVID-19 infection period was 691.5 (158.0–1473.0) mg/dL. Thirty-two (65.3%) patients were diagnosed with COVID-19, and 17 without, at a median follow-up time of 24 (2–56) months post-BCMA-targeted CAR-T cell therapy. The baseline characteristics of the patients with or without COVID-19 are shown in Table 1. In univariate logistic regression analysis (Supplementary Table 2), no significant factors were observed indicating the risk of COVID-19 incidence in recipients of BCMA-targeted CAR-T cells.
Of the 32 patients with COVID-19, mild, moderate, and severe COVID-19 occurred in 15 (46.9%), 11 (34.3%), and 6 (18.8%), respectively. The clinical characteristics between mild or moderate and severe COVID-19 are summarized in Table 2. The patients diagnosed with severe COVID-19 were older than those with mild or moderate COVID-19 (68 vs. 56 years, P = 0.006). None of the patients <60 years of age experienced severe COVID-19. The rate of CR achieved by CAR-T cell therapy was higher (76.9 vs. 16.7%, P = 0.011) in the mild or moderate COVID-19 group, and the duration of COVID-19 infection was shorter (9.5 vs. 28 days, P = 0.025) in this group. Univariate and multivariate analyses revealed the advanced age (OR = 1.367, 95% confidence interval [CI] = 1.017–1.838, P = 0.038) and CR achieved by CAR-T cell therapy (OR = 0.012, 95% CI = 0.000–0.674, P = 0.032) were independent risk and independent protective factors, respectively, for severe COVID-19 (Table 3, Supplementary Table 3).
Outcomes of patients with COVID-19
For 32 BCMA-targeted CAR-T cell recipients diagnosed with COVID-19, the main symptoms were fever (n = 28, 87.5%), cough (n = 19, 59.4%), throat pain (n = 4, 12.5%), and diarrhea (n = 1, 3.1%; Fig. 1b). Nearly half of the patients (n = 15, 46.9%) were hospitalized for COVID-19 treatment, including all of the severe cases. Twelve (37.5%) patients received oxygen inhalation, but none required mechanical ventilation. Steroids (n = 10, 31.3%) and Paxlovid (n = 10, 31.3%) were the most common drugs used to treat COVID-19, and all of the patients in the severe group had been administered at least one drug (tocilizumab, steroids, and Paxlovid; Fig. 1c). The median interval from CAR-T cell infusion to COVID-19 infection was 12 (1–52) months, and the median duration of COVID-19 infection was 10 (3–33) days (Supplementary Table 4).
As of the final follow-up on April 30, 2023, a total of 29 patients had achieved resolution from COVID-19. Among them, 20 of 25 patients in mild or moderate group and all four patients in severe group were tested negative for SARS-CoV-2 upon recovery. The 1-month resolution rates of COVID-19 in the mild or moderate and severe groups were 100% and 77.8%, respectively (P = 0.0003; Fig. 2a). Five of the patients died, at a median time of 25 (9–33) days following a COVID-19 diagnosis. Among the deceased, two had passed away due to the progression of MM following recovery from COVID-19—one from the mild or moderate group and the other from the severe group. Two others, both from the severe illness group, succumbed to ongoing complications from COVID-19. Additionally, one patient from the mild or moderate group died from progression of MM while still testing positive for SARS-CoV-2. The 3-month OS rates post-COVID-19 diagnosis in mild or moderate and severe groups were estimated to be 92.3% and 50%, respectively (P = 0.0092; Fig. 2c).
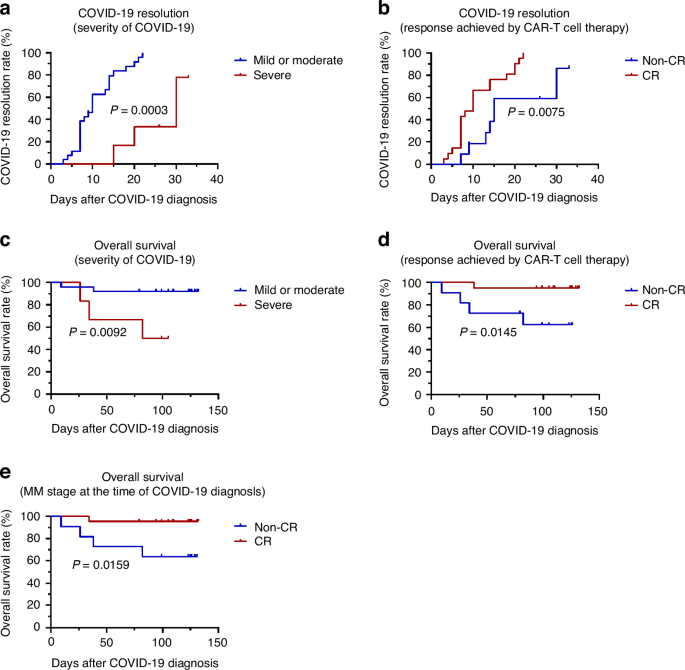
a COVID-19 resolution rates in the mild or moderate and severe subgroups. b COVID-19 resolution rates in the CR and non-CR subgroups (response achieved by CAR-T cell therapy). c OS rates following COVID-19 diagnosis in the mild or moderate and severe subgroups. d OS rates following COVID-19 diagnosis in the CR and non-CR subgroups (response achieved by CAR-T cell therapy). e OS rates following COVID-19 diagnosis in the CR and non-CR subgroup (MM stage at the time of COVID-19 diagnosis). BCMA B-cell maturation antigen, CAR chimeric antigen receptor, COVID-19 coronavirus disease 2019, CR complete remission.
The factors associated with COVID-19 duration and OS after COVID-19 diagnosis are listed in Table 3, Supplementary Table 5, and Supplementary Table 6, respectively. Univariate and multivariate analyses revealed that male sex, compared to female (HR = 5.274, 95% CI = 1.584–17.562, P = 0.007); and CR achieved by CAR-T cell therapy, compared to non-CR (HR = 3.107, 95% CI = 1.025–9.418, P = 0.045) were significant independent protective factors for COVID-19 duration. CR achieved by CAR-T cell therapy, compared to non-CR response (HR = 0.064, 95% CI = 0.007–0.589, P = 0.015), was an independent protective factor for OS after COVID-19 diagnosis. While PD stage at the time of COVID-19 diagnosis, compared to CR stage (HR = 14.206, 95% CI = 1.555–129.819, P = 0.019), was an independent risk factor. Moreover, the 1-month resolution rates of COVID-19 in the CR and non-CR groups (achieved by CAR-T cell therapy) were 100% and 86.4%, respectively (P = 0.0075; Fig. 2b). The 3-month OS rates post-COVID-19 diagnosis in the CR and non-CR groups (achieved by CAR-T cell therapy) were 95.2% and 62.3%, respectively (P = 0.0145; Fig. 2d). And the 3-month OS rate was 95.5% in patients remained CR at the time of COVID-19 diagnosis, while the non-CR patients was 63.6% (P = 0.0159; Fig. 2e).
Discussion
This study revealed the characteristics, outcomes, and significant factors associated with COVID-19 in BCMA-targeted CAR-T cell recipients during the Omicron variant wave of the COVID-19 pandemic. Overall, among all 49 enrolled recipients of BCMA-targeted CAR-T cell recipients, 65.3% (32 of 49) patients were diagnosed with COVID-19 over the study period (November 2022 to February 2023), and 18.8% (6 of 32) of these were classified as severe cases. At the final follow-up (April 30, 2023), the resolution rate reached 95.6% in terms of the surviving patients with COVID-19. The 1-month resolution rate in the mild or moderate group was 100%, and that in the severe group was 77.8%. The 3-month OS rate post-COVID-19 diagnosis was 84.3% for all infected patients, and 92.3% and 50% for mild or moderate and severe groups, respectively. Advanced age was a risk factor for severe COVID-19, while CR achieved by CAR-T cell therapy was protective. Male sex and CR achieved by CAR-T cell therapy were protective factors in terms of COVID-19 duration. Finally, CR achieved by CAR-T cell therapy was also found to be a protective factor for OS after COVID-19 diagnosis, while PD stage at the time of COVID-19 diagnosis was a risk factor.
Patients with MM are more susceptible to infection, including COVID-19. Studies have shown that the COVID-19 severity and mortality rates before the Omicron wave were higher in patients with MM vs. the cancer-free population [10,11,12]. Despite good responses to BCMA-targeted CAR-T cell therapy, the toxicity of this treatment still merits attention. For example, the deficiency of humoral immunity following BCMA-targeted CAR-T cell therapy for R/R-MM can increase the risk of pathogenic infection, particularly during the first 100 days after infusion [21, 27, 28]. One large systematic review and meta-analysis reported recently found that infection was the most common reason (in 50.9% of patients) for non-relapse-associated mortality following CAR-T cell therapy—with COVID-19 contributing to >50% of this mortality [29]. However, no statistical relationship was found between the interval of BCMA-targeted CAR-T cell infusion and COVID-19 diagnosis and COVID-19 severity or OS in this study. The infection rate in this cohort was 65.3%, which was higher than a previous large MM clinical cohort in USA (32.9%) [30]. Patients with MM treated with novel drugs (daratumumab, bortezomib, lenalidomide, carfilzomib and pomalidomide) also showed low (14.2%) frequency of COVID-19 infection before Omicron variant [13]. But the COVID-19 infection rate of MM patients who have been treated with daratumumab reached 80.7% after adjustment of “dynamic zero clearing” policy in China [31]. According to provincial-level administrative divisions’ online surveys, 82.4% of individuals became infected between December 2022 and February 2023 after lifting the strict COVID-19 control policy in China [32]. The high infection rate in our and other cohorts in China at the same time may be due to the susceptibility of the Chinese population. The same phenomenon was also observed in Hong Kong and South Korea in the equivalent time span [33]. The large-scale outbreak of COVID-19 infection after the opening of the COVID-19 policy provides a good model for us to study the clinical characteristics and outcomes of Omicron variant infection in BCMA-targeted CAR-T cell recipients. Regardless the high infection rate, almost all of the infected patients (95.6%) recovered from COVID-19 within one month, which was consistent with data from immunocompromised patients (75%) in a previous study on the Omicron variant [34]. This good result may be explained by the lower virulence of the Omicron variant compared to the other ones [35].
In this study, CR achieved by CAR-T cell therapy was a protective factor against severe COVID-19, COVID-19 duration, and OS. While PD stage at the time of COVID-19 diagnosis was regarded as a risk factor for OS. Previous studies also demonstrated that responsive disease was a significant protective factor against COVID-19 hospitalization in patients with MM [13, 36], and uncontrolled MM was identified as a risk factor for increased morality [11, 12, 37, 38]. In general, CR after anti-tumor treatment (including BCMA-targeted CAR-T cell therapy for R/R-MM) reflected a better immune condition in patients vs. non-CR. The possible reasons for the high risk of COVID-19 infection among patients who had not achieved CR after CAR-T cell treatment included MM progression, the use of fludarabine and cyclophosphamide, and the use of other anti-myeloma chemotherapy after CAR-T cell therapy failure. At the last follow-up time, we found two patients died of disease progression after COVID-19 resolution. Additionally, one patient who died with the positive SARS-CoV-2 was diagnosed with mild COVID-19 according to our criteria, but was in PD stage when infection. The clinically diagnosed cause of death was intracranial hemorrhage. Thus, equal attention should be paid to both disease control and infection prevention in these patients.
Our study showed that advanced age was an independent risk factor for developing severe COVID-19. According to a Chinese clinical study, advanced age was the only significant risk factor (OR = 1.14, 95% CI = 1.06–1.26, P = 0.002) that may contribute to severe COVID-19 in patients with MM during the Omicron wave [39]. Thus, focusing on monitoring older patients during COVID-19 outbreaks is essential.
When writing this report, the new Omicron variant of SARS-CoV-2 is circulating again, and the question of how to manage patients with long-term hematological diseases after CAR-T cell treatment remains a significant clinical challenge. The number of enrolled patients in this study was limited. However, we summarized the clinical data of BCMA-targeted CAR-T cell recipients with COVID-19 in a single center during an epidemic of the Omicron strain of SARS-CoV-2, thus providing evidence that may prove valuable for reducing the severity and mortality of COVID-19 infection after CAR-T cell treatment.
In conclusion, patients with R/R-MM who have received BCMA-targeted CAR-T cell therapy may be more susceptible to COVID-19. However, the severity of COVID-19 disease and the associated mortality rate in this patient population were significantly reduced during the Omicron wave of the pandemic. The main risk factor associated with the development of severe COVID-19 was advanced age, and the main protective factor against it was CR achieved by CAR-T cell therapy. Male sex and CR achieved by CAR-T cell therapy were significant protective factors for COVID-19 duration. CR achieved by CAR-T cell therapy was also considered a protective factor for the OS after COVID-19 diagnosis, while PD stage at the time of COVID-19 diagnosis was a risk factor. Thus, preventive strategies are vital for older patients with R/R-MM and those who do not respond to CAR-T cell therapy.
Responses