Clinically probable RBD is an early predictor of malignant non-motor Parkinson’s disease phenotypes
Introduction
Non-motor symptoms (NMS) in Parkinson’s disease (PD) are a significant determinant of quality of life and disability, especially in the later stages of the disease1. NMS are increasingly prevalent during PD progression, and they are critical determinants of quality of life and future risk for institutionalization2,3. NMS include mood disorders, cognitive impairment, hallucinations, autonomic dysfunction, apathy, and sleep disorders4.
NMS present treatment challenges3. Identifying those who will develop more severe NMS remains a significant hurdle. Currently, there is no reliable method to predict which patients are at a higher risk of a greater NMS burden. Additionally, we do not have a method to prioritize NMS screening and to tailor treatment based on possible risk for future disability5.
PD impacts various neural circuits, including those responsible for motor, cognitive, and affective functions. The diverse range of symptoms complicates the profiling of disease progression. The non-motor spectrum of PD remains insufficiently characterized without clear phenotypic profiles that are recognizable in the clinic setting. It remains unclear whether NMS in PD tend to co-occur or manifest as multiple distinct phenotypes. Identifying these clusters and profiles could significantly impact our understanding of disease trajectory and treatment selection. Additionally, it could aid in developing personalized screening tools and guide recruitment for clinical trials on disease-modifying treatments.
Rapid eye movement (REM) sleep behavior disorder (RBD) occurs in ~42% of patients with PD6, and it is often present several years before the onset of motor symptoms. A prospective study revealed that isolated RBD developed PD at a rate of ~6% per year7. Those with RBD had a higher rate of depression and cognitive impairment8. Potential differences in symptom profiles with and without RBD can possibly be used to define motor and non-motor phenotypes and potentially guide advanced treatment options for these patients9.
This study aimed to evaluate early probable RBD and its impact on the emergence of NMS. We then sought to characterize what impact early probable RBD has on the natural progression of PD compared to those who do not have early probable RBD.
Results
General characteristics
452 patients met the inclusion criteria, of which 180 (40%) had probable RBD. There was no significant difference in baseline age (t = −0.25, p = 0.4), time from PD diagnosis (t = 0.05, p = 0.5), United Parkinson’s Disease Rating Scale part III (t = 1.3, p = 0.19), or Hoehn-Yahr stage (t = 1.78, p = 0.07) between patients with early probable RBD and those without it.
Cognitive outcomes
The Montreal Cognitive assessment (MoCA) scores (Fig. 1a) in the first year after diagnosis were similar in both groups (t = 0.12, p = 0.54). However, at 5 years following diagnosis the group with early probable RBD had a lower mean score (t = 2.62, p = 0.035) that continued to decrease at a faster rate than those without early probable RBD (pcorr < 0.01 for each comparison).
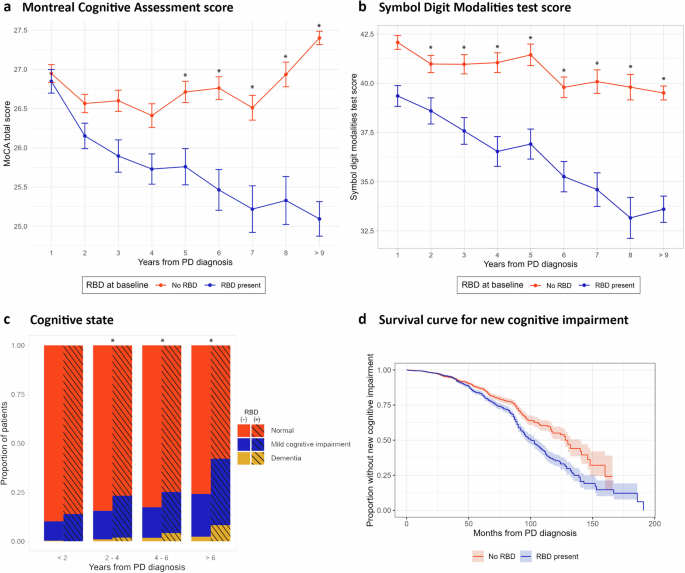
a Proportion of cognitive status through the study. b Montreal cognitive assessment (MoCA) score. c Symbol digit modality (SDM) test score. d Kaplan-Meier curve comparing patients with and without early probable RBD using “new onset of cognitive impairment” as the event. Error bars represent standard error of the mean.
Performance on the symbol-digit modality (SDM) test (Fig. 1b) was similar for both groups over the first two years. Following three years from PD diagnosis, the patients with early probable RBD had a lower score (F = 87.19, p < 0.001, individual comparisons pcorr < 0.01). The difference remained significant throughout the study, with patients who had early probable RBD experiencing a faster decline in their scores over the course of the study (mean decline: −9.07 vs. −3.32, p = 0.002). There were no differences in the Hopkins Verbal Learning Test (HVLT), the letter number sequencing (LNS) test, the Benton Judgment of Line Orientation (BJLO) test, or the semantic fluency test (SFT) at any time point during the study.
There was a higher prevalence of cognitive impairment in early probable RBD (M2 = 326.84, p < 0.001), with a higher proportion of mild cognitive impairment (MCI) and dementia. The difference is not significant in the first 2 years after diagnosis (p = 0.42), but it becomes significant in the 2–4-years (pcorr = 0.04), 4–6-years (pcorr = 0.01), 6–8-years (pcorr <0.002), and >8 years (pcorr < 0.04) intervals (see Fig. 1d).
In our survival analysis, we observed a higher incidence of cognitive impairment in patients with early probable RBD (log-rank test, p < 0.001) (Fig. 1d). Cox regression analysis identified the following hazard ratios (HR) for the presence of early probable RBD of 1.22 (1.01–1.48, p = 0.01), presence of depression 1.68 (1.38–2.06, p < 0.001), State-Trait Anxiety Inventory (STAI) score (0.98–1.02, p = 0.2), Questionnaire for Impulsive-Compulsive Disorders in Parkinson (QUIP) score 0.95 (0.85–1.07, p = 0.4), and Scales for Outcomes in Parkinson’s Disease – Autonomic Dysfunction (SCOPA) score 1.05 (0.92–1.06, p = 0.1).
A logistic regression model to predict the development of cognitive impairment revealed the following odds ratios (OR): time from diagnosis of PD 0.95 (0.89–1.02), presence of early probable RBD 0.95 (0.36–1.99), depression 2.15 (0.73–6.3), anxiety 1.01 (0.98–1.04), SCOPA score 1.05 (0.99–1.11), ICD 0.73 (0.26–2.01), apathy 2.6 (1.15–5.88), and hallucinations 1.68 (1.04–3.45). The accuracy of our model was 0.83, and the area under the receiver-operating curve (AUROC) was 0.86.
Apathy
Early probable RBD was associated with a higher prevalence of apathy across the study (M2 = 332.93, p < 0.001) (Fig. 2a). There was no difference during the first two years after PD diagnosis (pcorr = 0.54), however in the 2–4-year interval there was a significantly higher prevalence of more severe apathy degrees (pcorr = 0.02) that continued through the 4–6-years interval (pcorr = 0.003), 6–8 years interval (pcorr = 0.003), and more than 8 years after diagnosis (pcorr = 0.002).
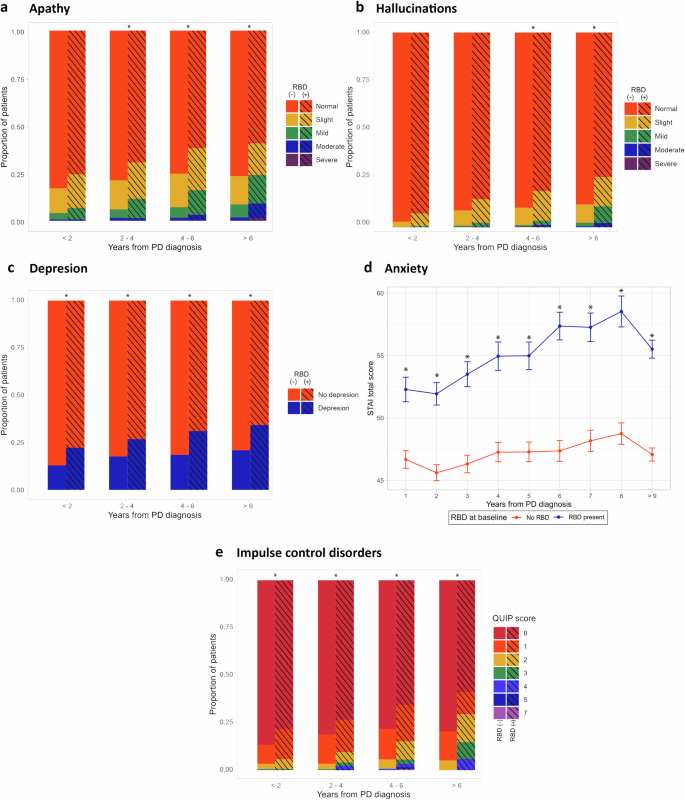
Asterisks represent a p-value under 0.05 after post hoc p value correction. a Comparison of apathy measured by the UPDRS part I. b Comparison of hallucinations as measured by the UPDRS part I. c Comparison of depression as measured by the GDS score ≥5. d Comparison of anxiety as measured by the STAI total score. e Comparison of ICD as measured by the QUIP scale.
Hallucinations
The early probable RBD group had a higher rate of hallucinations when compared to those without early probable RBD (M2 = 480.05, p < 0.001). Additionally, the severity of hallucinations increased more rapidly in patients with early probable RBD starting at the 4–6-years interval (pcorr = 0.002) and remaining significantly higher in the 6–8-years (pcorr = 0.060) and after more than 8 years after PD diagnosis (pcorr = 0.04) (see Fig. 2b).
Mood disorders
The early probable RBD group consistently had a higher prevalence of depression (Fig. 2c) throughout the study (M2 = 480.05, p < 0.0001, pcorr < 0.01 for each comparison). Similarly, the mean total STAI anxiety score remained higher in the early probable RBD group across the study (for each time point pcorr < 0.01; see Fig. 2d). There was no significant differences in the rate of change in STAI scores (t = 1.15, p = 0.25).
Impulse control disorders (ICD)
The QUIP score (Fig. 2e) was significantly higher in patients with early probable RBD (M2 = 77.577, p < 0.001). This difference was statistically significant within the first two years of diagnosis (pcorr = 0.01), 2–4 years interval (pcorr = 0.001), 4–6 years interval (pcorr = 0.001), and beyond 6 years after motor onset (pcorr = 0.01).
Autonomic dysfunction
The early probable RBD group had a higher SCOPA total score at all follow-up intervals (F = 251.8, p < 0001). At a 5-year follow-up interval, the early probable RBD group had a higher prevalence of patients with autonomic dysfunction (t = 2.52, p = 0.013), defined as a SCOPA total score of more than 12.
Discussion
We identified a malignant non-motor phenotype in PD patients with early probable RBD. This aggressive disease profile is characterized by more severe forms of cognitive impairment, apathy, hallucinations, anxiety, depression, ICD, and autonomic dysfunction. The currently embraced natural progression model for PD places cognitive impairment, apathy, and hallucinations at around 10 years following motor symptom emergence10. We demonstrated the onset of these symptoms within the first 5 years after the onset of motor symptoms.
We observed that patients with early probable RBD experience the same NMS profile as those without early RBD, but they occur more frequently and with much greater severity. Interestingly, we also note a disproportionally greater incidence of autonomic dysfunction in the early RBD cohort. At a group level, we detected a strong co-occurrence of apathy, cognitive impairment and anxiety in PD patients with early probable RBD (Fig. 3). This raises the question as to whether these symptoms represent pathology along a common brain network. Previous studies have demonstrated a strong link between apathy and cognition11 and that the presence of apathy increases the risk of progression from PD mild cognitive impairment to PD dementia12. The interconnection between these two symptoms and anxiety remains incompletely characterized. However, dysfunction of the prefrontal networks and mesolimbic dopamine system may play a central role13. The relationship between the above symptoms and autonomic dysfunction remains largely unexplored in PD. Previous imaging studies have found a correlation between prefrontal cortex hypoperfusion and the severity of autonomic dysfunction in PD14. This may suggest that autonomic dysfunction may share a common pathway with these other symptoms, rather than existing as a separate issue. Further imaging studies are needed to clarify the underlying biological networks, which could potentially identify new targets for intervention.
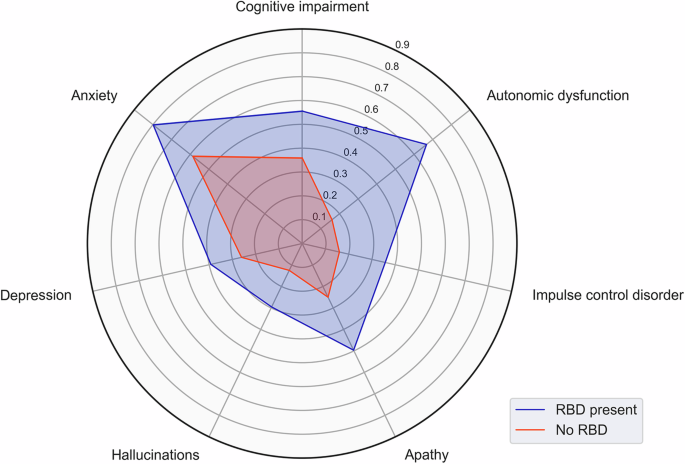
Proportions of patients affected with each NMS are displayed in the radial axis.
The incidence of depression, ICD, and hallucinations was relatively lower compared to other NMS. These symptoms may be linked by anatomical and functional commonalities. Cell loss and alpha-synuclein burden at the midbrain is a common substrate for depression15, hallucinations16,17, and ICD18,19. ICD commonly present in PD at later stages, at the time where hallucinations and depression are more prevalent20. In addition, depression and hallucinations present together earlier and more severely in patients with dementia with Lewy bodies (DLB)21. Therefore, these symptoms tend to co-occur across the PD-DLB spectrum. Previous studies have demonstrated that depression and impulsive behaviors are linked to dysfunction of a wide-spread network that includes the nucleus accumbens and its projections to the frontal and rostral cingulate cortices22,23. The circuitry underlying visual hallucinations in PD and DLB are less characterized, but some studies have linked them to the ventral attention network that also includes the cingulate cortex24,25. In combination with our observations on progression, these studies point at a common network dysfunction among depression, ICD, and hallucinations that occur in PD and DLB at different points of the natural history of the disease.
There are currently no biological models to explain this clinical phenotype adequately. Current theories on biological phenotypes of PD have proposed that it may start with pathological changes in the brain (“brain first”) or in the peripheral nervous system (“body first”). Some of the predictions of this model are that patients with “body first” phenotype will have earlier cognitive impairment, autonomic symptoms, depression, and RBD, which closely aligns with our results26. Our observations are consistent with the diffuse malignant phenotype, which has been observed in a data-driven clustering study by Fereshtehnejad et al. 27, where these patients had a higher rate of RBD, cognitive impairment, and autonomic symptoms. This classification has been correlated to post-mortem histopathological findings, including a higher burden of Lewy body pathology without an additional beta-amyloid burden28.
We hypothesize that this rapid progression of NMS may reflect an underlying anatomical connectivity abnormality that occurs in patients with early RBD. PD patients with RBD have decreased functional connectivity in the default mode network, the frontoparietal networks, and frontopolar-to-dorsolateral prefrontal cortices, which correlates with worse cognitive executive function29. These patients have brainstem noradrenergic dysfunction which is manifest by decreased functional connectivity between the locus coeruleus and global resting state networks30, which could correlate with the higher rates in apathy we observed in this cohort. Future imaging studies will need to elucidate if there are network level differences between these phenotypes. Identification of such phenotypes may have significant implications for advanced PD therapies.
A malignant non-motor phenotype is critically important when considering the possibility of surgical treatment for an individual patient. Pre-surgical cognitive impairment is an important predictor of worsening cognition after deep brain stimulation (DBS)31,32, and apathy has been described in patients after subthalamic nucleus DBS33. There are also several cases reported of new onset and worsening ICD after DBS in both the subthalamic nucleus and the globus pallidus pars interna34. Therefore, identifying patients who are at high risk for developing these NMS will be important to prevent further NMS deterioration following surgery. Additionally, patients who experience severe NMS may receive a smaller benefit in quality of life from surgical treatment. Horn et al.35 observed a depressed patient who showed less improvement in motor symptoms after DBS than their functional connectivity model had predicted. However, once the patient’s depression was treated, the motor symptom improvement matched their model’s predictions. This example reveals a potential quantifiable gap for improvement in motor symptoms after DBS that may be missed by failure to recognize NMS, or not having the proper treatment.
Our analysis was performed in one of the largest de-novo PD cohorts. The magnitude and broad scope of the PPMI have the advantage of a large sample size, systematization of standardized testing, and a long-term follow-up. On the other hand, our analysis was limited in some key respects. First, our definition of probable RBD was based on the RBD screening questionnaire which has demonstrated a sensitivity of 0.96 compared to the gold standard (video polysomnography). This tool has a specificity of 0.56 due to problems discriminating between sleepwalking and epilepsy36. We think that this is also a strong point in our study, as our results can be applied in patients who are screened with a simple clinical tool, rather than only to patients that are polysomnography-confirmed to have RBD. Second, our definition of apathy and hallucinations has been based on a single item from the Unified Parkinson’s Disease Rating Scale (UPDRS) part I. This single item partially captures the severity of the symptom, and thus, we focused our analysis on differences in the proportion of severe symptom categories. Third, we defined depression and anxiety based on standardized questionnaires rather than gold standard evaluation by a psychiatrist using accepted diagnostic criteria.
In conclusion, using RBD as an early clinical predictor of a malignant non-motor phenotype could impact the long-term management of these patients. Identification may benefit from early cognitive, mood, and apathy screening.
Methods
Study design and participants
We used data from the Parkinson’s Progression Markers Initiative (PPMI, www.ppmi-info.org) study. Briefly, the PPMI is a longitudinal multi-cohort observational clinical study. We specifically looked at the “de-novo Parkinson’s disease cohort” that followed patients with PD diagnosed within the last two years. Patients in this cohort were followed for five years and then had the option to continue the study with yearly visits. For a comprehensive description of the cohort and details on the assessments performed, see the study’s original description37.
Outcomes and variables
We analyzed the time from PD diagnosis, non-motor items of the Unified Parkinson’s Disease Rating Scale (UPDRS) part 1 (apathy and hallucinations), geriatric depression scale (GDS), state-trait anxiety inventory (STAI), Montreal cognitive assessment (MoCA), Hopkins Verbal Learning Test (HVLT), Benton Judgment of Line Orientation (BJLO), Letter Number Sequencing (LNS), Semantic Fluency Test (SFT), and Symbol Digit Modalities (SDM).
We defined cognitive impairment as performance under 1.5 standard deviations on two tests: HVLT (each modality counts as one test), BJLO, LNS, SFT, or SDM. Furthermore, patients with cognitive impairment were classified as mild cognitive impairment (MCI) if their cognition does not impair their ability to perform their activities of daily living or as dementia if it does38.
We use the term “probable RBD” in accordance with the International Classification of Sleep Disorders-3-TR criteria a recent trend in the field to define as “probable” when there is no formal polysomnography study to confirm the diagnosis39. We classified patients as “RBD present at baseline” if they had an RBD screening questionnaire score above five36.
For Fig. 3, we selected the latest timepoint tested for each symptom. This was 8 years for the case of most symptoms plotted, except for ICD, which was last robustly tested at 5 years. These symptoms were categorized based on previously published thresholds: depression was defined as a GDS of 5 or more40, anxiety was defined as a STAI total score of more than 5441, ICD was defined as a QUIP total score of 1 or more42, and autonomic dysfunction was defined as a SCOPA score of 12 or more43, or the presence of orthostatic hypotension during clinic visits, defined as a drop in >20 mm Hg in systolic pressure or >10 mm Hg in diastolic pressure. Apathy and hallucinations were defined as UPDRS part 1 item score of 1 or more.
Group-wise comparisons
Our analysis is based on comparisons between patients classified as RBD present at baseline and No RBD present at baseline. To compare continuous variables between groups, we used a paired t-test between each time point and a Bonferroni correction for multiple comparisons. We performed a Shapiro-Wilks test to ensure that each variable followed a normal distribution to ensure proper use of parametric statistical tests. To compare categorical data across different time points, we used a Cochran-Mantel-Haenszel test using the time interval bin as a control categorical variable. For this later test we report the M2 statistic for each test. We report p-values after the post-hoc corrected when noted as “pcorr”.
Survival analysis
A survival analysis was conducted using the new development of cognitive impairment as the event. This was defined as progression from normal cognition to MCI or from MCI to dementia. Patients with dementia at the baseline evaluation were excluded from this analysis. Our cohort was classified as probable RBD present and no RBD present as above and plotted over time using months from PD diagnosis. The resulting survival functions were compared using the log-rank test, and we performed a Cox regression to explore whether baseline probable RBD explains the event (new development of cognitive impairment) when including STAI score, QUIP score, SCOPA score, and presence of depression.
Logistic regression model
We fitted a logistic regression model using “new cognitive impairment at 5 years of PD diagnosis” as the dependent variable, and SCOPA score, QUIP score, STAI score, presence of depression as evaluated by the GDS, and time from PD diagnosis as independent variables. We performed a comprehensive grid search for hyperparameter optimization and then fitted the regression model with the parameters that had the highest accuracy performance.
Our model was trained with a random sample of 80% of our data and left out 20% for validation. We included in our model: time from PD diagnosis, STAI total score, SCOPA score, UPDRS part 1 item for apathy and hallucinations, and presence of depression based on the GDS at 5 years after PD diagnosis, and baseline probable RBD. We used hyperparameter optimization by cross validation, with the best performing model using L2 regularization.
We checked for multicollinearity using a variance inflation factor (VIF) threshold of five. We used the AUROC and accuracy as our primary performance metrics for our classification task.
Statistical methods
We used an alpha of 0.05 for all two-tailed hypothesis contrast tests. All analyses were performed using R Statistical Software (v4.3.1), save for the logistic regression model that was performed in Python (v3.11.9) using the statsmodels library and the scikit-learn library for hyperparameter optimization.
Responses