Determinants of patient satisfaction in clozapine users: results from the Clozapine International Consortium (CLOZIN)

Introduction
Traditionally, treatment success in schizophrenia research has relied mostly on symptom alleviation ascertained by raters other than the patient. Over recent years, however, patient perspectives are increasingly recognized as critical in the evaluation of treatment outcomes for individuals diagnosed with schizophrenia spectrum disorder (SSD)1. In this context, the recognition of patient satisfaction as a valuable tool for evaluating treatment outcomes and effectiveness has gained momentum2. Patients’ viewpoints and judgments may differ from that of healthcare professionals or family members, particularly when patients have limited insight into their illness, as is often the case in individuals diagnosed with treatment-resistant schizophrenia (TRS)3,4. For instance, individuals with TRS often rate the severity of their symptoms much lower than their treating physicians do4. This discrepancy also extends to concerns about adverse drug reactions (ADRs): healthcare professionals are often most concerned about rare, potentially life-threatening ADRs (such as agranulocytosis) and about the need for regular blood monitoring, while patients are primarily worried about common ADRs (such as weight gain, sleepiness, and hypersalivation) and less so about undergoing regular blood tests5,6,7,8. These differences between users of clozapine and prescribers underscore the importance of considering patient satisfaction in evaluating treatment success.
Several studies have explored the subjective experiences of clozapine treatment from the perspective of clozapine users. When comparing patient satisfaction between clozapine users and users of other antipsychotic medications, clozapine consistently receives higher ratings9,10. Clozapine is also deemed superior when users compared it to their previous antipsychotic treatments11,12. In all studies, the majority of participants seem generally satisfied with clozapine treatment11,12,13,14,15,16. Participants on average report a high level of satisfaction with the positive effects of clozapine, such as the reduction of positive symptoms and improved social functioning and quality of life, outweighing the side effects they experience11,15. However, to the best of our knowledge, the largest study on patient satisfaction among clozapine users published to date was based on 130 participants. Moreover, little research has been conducted on the specific factors contributing to patient satisfaction among clozapine users, and previous studies have primarily linked satisfaction to patients’ subjective assessments of clozapine treatment outcomes, such as response, while lacking analyses linking the clinician’s perspective to patient satisfaction10,11,13,15,16. Furthermore, previous studies have not examined the explained variance of the contributing factors and have investigated relatively few ADRs. Thus, it is unclear which variables contribute most to patient satisfaction in people using clozapine. This issue is of particular importance because of the continued high rates of underutilization of clozapine across industrialized countries, even though it remains the most effective antipsychotic available17,18,19.
Here, we therefore evaluated patient satisfaction in a comprehensively ascertained international dataset of 480 patients diagnosed with SSD using clozapine. We explored the satisfaction levels of patients undergoing clozapine treatment and examined which demographic variables (such as sex, age, education level, and marital status) and clinical factors (including illness duration, insight, ADRs, symptom severity, and treatment response) are associated with patient satisfaction about clozapine. We thus aimed to deepen the understanding of variables contributing to patient satisfaction in clozapine users for clinicians as well as patients, so that these variables can be measured and if needed addressed prior to and during treatment with clozapine, thus potentially increasing chances of successful treatment.
Methods
Study design and patient population
We used data from the ongoing CLOZapine INternational (CLOZIN) study20,21,22, a large cross-sectional international study. For this study, participants were recruited from inpatient and outpatient mental health care settings in 7 different countries between 2017 and 2024: 166 participants were recruited in the Netherlands; 76 in Germany; 110 in Spain; 9 in Finland; 50 in Serbia; 27 in Austria, and 42 in Italy. We complied with the Declaration of Helsinki (2013). Written informed consent was obtained from all participants and the study received approval from the respective local Institutional Review Boards in all participating countries.
Participants were included when they were aged 18 years or older, had been diagnosed with an SSD according to the fourth or fifth edition of the Diagnostic and Statistical Manual of Mental Disorders, and were using clozapine. To accurately represent the real-world clozapine user population, we did not set a minimum duration for clozapine treatment. Furthermore, we explained to potential participants that gaining a comprehensive understanding of the factors associated with patient satisfaction and dissatisfaction was crucial, encouraging participation from both individuals who were satisfied with the treatment and those who were not. Participants were excluded if data regarding their satisfaction with the treatment were unavailable for analysis.
Exposures and outcomes
Dependent variables
In the current study, the primary outcome measure was patient satisfaction with clozapine treatment. Participants were asked to rate their satisfaction with clozapine treatment during the single study visit using a scale ranging from 1 to 10. A rating of 1 indicated ‘very unsatisfied with clozapine,’ while a rating of 10 denoted ‘very satisfied with clozapine’, with a score of 5 representing ‘neutral satisfaction.’ Participants were explained the meanings of these scores before they rated their satisfaction. They were instructed to choose one of these scores or a score in between these ratings that best matched their satisfaction with clozapine during the entire use period.
Independent variables
Demographic and clinical characteristics were gathered through participant interviews, with supplementary information obtained from their treating physicians in cases of uncertainty. The following variables were included as independent variables in the analysis based on prior literature or their potential association with patient satisfaction: age, sex, diagnosis, relationship status, highest educational attainment, smoking status, body mass index (BMI), use of recreational drugs, illness duration, clozapine dose, clozapine dose frequency, and the use of antipsychotic polytherapy compared to clozapine monotherapy9,10,11,12,13,15,16. Furthermore, participants were asked if they experienced common ADRs associated with clozapine; and if so, which ADRs they experienced (no restrictions to the number of ADRs they could list). This was done using a standardized questionnaire (Supplementary Fig. 1, further explained in ref. 22). The initial responses to this questionnaire about the occurrence or experience of ADRs were provided by the participants themselves. If a participant was unsure about their answer or if the researcher had doubts about the reliability of the response, the treating physician was consulted for verification. As explained previously22, the total number of clozapine-associated ADRs experienced by each participant was then summed to create a new variable: ‘total number of ADRs’.
Severity of schizophrenia symptoms was assessed by the treating physician or trained researcher using the Clinical Global Impression-Severity (CGI-S) scale, ranging from 1 (indicating normal, not ill) to 7 (indicating the most severe level of illness; Supplementary Fig. 2), as also explained before23. Additionally, the treating physician evaluated the treatment response level using a 10-point scale, as per criterion A of the Alda scale24,25. This criterion entails assessing the extent of response (activity of the illness under adequate clozapine treatment) on a scale ranging from 0 (no change or worsening of symptoms) to 10 (complete response; Supplementary Fig. 3)24,25.
Moreover, treatment adherence was assessed during stable periods based on criterion B4 of the Alda scale24,25. A score of 0 indicates excellent compliance, with all documented clozapine blood levels within the therapeutic range, while a score of 1 indicates good compliance, with 80–99% of blood levels within the therapeutic range. A score of 2 indicates poor compliance, with <80% of blood levels within the therapeutic range (Supplementary Fig. 3). The term ‘therapeutic range’ refers to the target clozapine serum level considered appropriate for the patient, as determined by the treating physician.
Finally, the treating physician recorded the use of any additional medication during stable periods by rating criterion B5 of the Alda scale24,25. Here, a score of 0 indicates no additional medication use except for occasional sleep medication (1 per week or less), a score of 1 indicates the use of low-dose or occasional antidepressants or antipsychotics, or prolonged use of sleep medication, and a score of 2 indicates systematic use of antidepressants or antipsychotics (Supplementary Fig. 3).
Statistical analysis
Baseline demographics were summarized using descriptive statistics. Continuous variables are presented as mean ± standard deviation (SD), median, minimum, and maximum. Categorical variables are reported as frequencies and percentages.
First, for our primary analyses, to assess which variables were associated with satisfaction with clozapine, we examined the associations between patient satisfaction and the following independent variables: age, sex, diagnosis, relationship status, highest educational attainment, smoking status, BMI, use of recreational drugs, illness duration, clozapine dose, clozapine dose frequency, the use of antipsychotic polytherapy compared to clozapine monotherapy, the total number of ADRs, the specific ADRs, symptom severity, and response. To that end, a linear regression model was employed, with patient satisfaction designated as the dependent variable, and age and sex included as covariates. In the model with ‘age’ as the independent variable, only ‘sex’ was added as a covariate, and vice versa.
For our secondary analyses, to assess the variance in patient satisfaction explained by all significantly associated independent variables (in the primary analyses), we employed multiple linear regression with patient satisfaction as the dependent variable. Thus, all variables significantly (after Bonferroni correction) associated with patient satisfaction in our primary analysis (p < 1.9 × 10-3) were included as independent variables in the model, along with age and sex. As described above, the ‘total number of ADRs’ was calculated by summing the occurrences of all specific ADRs. Therefore, ‘total number of ADRs’ and the occurrences of specific ADRs (such as hypersalivation) were assessed in separate models. Variables demonstrating collinearity, identified by a variance inflation factor (VIF) exceeding 10, were also analyzed independently to mitigate the risk of multicollinearity.
To mitigate the risk of type 1 errors, we applied Bonferroni correction for multiple testing. Specifically, we adjusted the significance level (alpha) to account for the number of independent tests conducted, which totaled 26. This resulted in a corrected significance threshold of p < 0.05/26 = 1.92 × 10-3.
The outcomes are presented using the explained variance (R2) of the model, with the unstandardized regression coefficients (B) for each variable, their respective 95% confidence intervals, and p values.
All statistical analyses were conducted using IBM SPSS Statistics Version 29.0.1.0.
Results
Demographic and clinical characteristics
Baseline characteristics are shown in Table 1 and Supplementary Table 1. The study included 480 participants, of whom 149 (31.1%) were female. The mean age of the participants was 43.9 years (SD 11.9). The majority of participants (41.2%) were rated as demonstrating a moderate response to clozapine treatment by their treating physicians, and 33.2% were classified as ‘moderately ill’ on the CGI-S scale during clozapine treatment. The mean duration of clozapine treatment was 7.6 years (SD 7.3). On average, participants experienced 3.6 clozapine-associated ADRs (SD 2.2). The top 3 most prevalent clozapine-associated ADRs in our study population were weight gain (68.3%), hypersalivation (55.2%), and prolonged sleep duration (50.2%; Supplementary Table 2). Patient satisfaction, assessed on a scale of 1 to 10, showed a mean score of 7.4 (SD 1.9; median 8; range 1 to 10; Supplementary Fig. 4).
Primary analyses
We tested independent variables together with age and sex as covariates in a linear regression model for their associations with patient satisfaction. We found that better response (B = 0.42, 95% CI 0.33 to 0.51, R² = 0.19, p = 3.9 × 10⁻¹⁸; Fig. 1A) was associated most significantly with higher patient satisfaction. In addition and in descending order of significance, greater symptom severity (B = −0.10, 95% CI −0.57 to −0.29, R² = 0.050, p = 2.1 × 10⁻⁹; Fig. 1B), a higher total number of clozapine-associated ADRs (B = −0.16, 95% CI −0.24 to −0.09, R² = 0.057, p = 3.2 × 10⁻⁵), and recreational drug use (B = −1.32, 95% CI −2.01 to −0.62, R² = 0.050, p = 2.1 × 10⁻⁴) were associated with lower patient satisfaction.
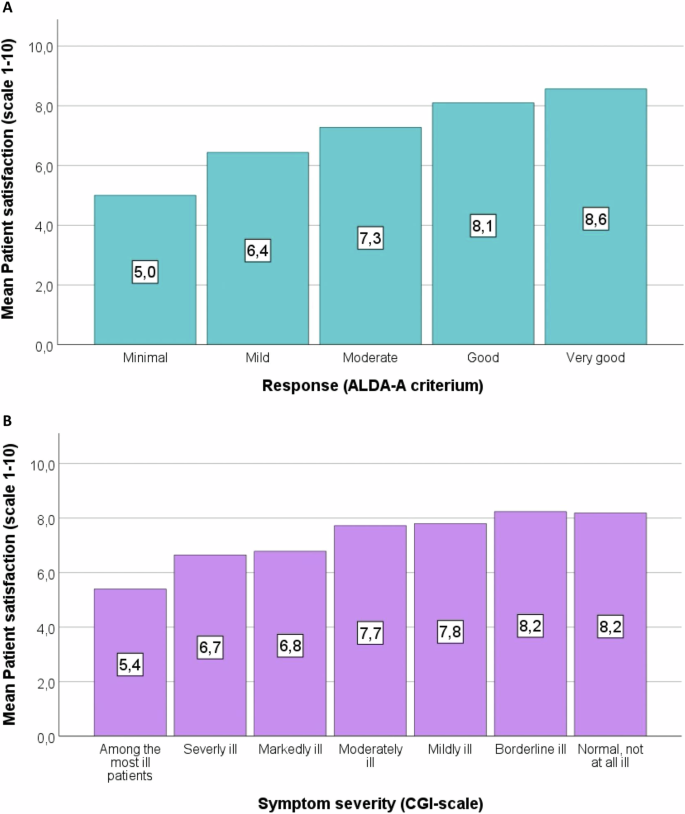
Bar charts illustrating the association of mean patient satisfaction rating with response as rated using the Alda-A criterium (A) and with symptom severity rated using the CGI-scale (B). The numbers denoted in each bar refer to the mean patient satisfaction rating of that group. Criterium A of the Alda scale was used to determine an association between clinical improvement and the treatment on a scale of 0 (no change or worsening) to 10 (complete response)24,25. For illustrative purposes, a score of 1–2 is labeled as ‘minimal’ response, 3–4 as ‘mild’ response, 5–6 as ‘moderate’ response, 7–8 as ‘good’ response, and 9–10 as ‘very good’ response. Abbreviations: CGI Clinical Global Impression.
Regarding clozapine-associated ADRs, hypersalivation (B = −0.72, 95% CI −1.07 to −0.39, R² = 0.056, p = 3.5 × 10⁻⁵) and prolonged sleep duration (B = −0.57, 95% CI −0.92 to −0.22, R² = 0.042, p = 1.4 × 10⁻³) were significantly associated with lower patient satisfaction (Supplementary Table 3).
Secondary analyses
As secondary analyses, we ran two multiple linear regression models, with patient satisfaction as the dependent variable: one model included ‘total number of ADRs’ as an independent variable, along with sex, age, and all variables with statistically significant associations with patient satisfaction identified in the primary analysis; the other model included ‘hypersalivation’ and ‘prolonged sleep duration’ as independent variables, along with sex, age, and all variables with statistically significant associations with patient satisfaction identified in the primary analysis. Multicollinearity was not detected among any of the independent variables (all had a VIF < 5). The model including the total number of ADRs as an independent variable demonstrated an R² of 0.24 (24%), with response and the total number of ADRs showing significant contributions to the model (B = 0.37, 95% CI 0.25 to 0.48, p = 1.5 × 10⁻⁹ and B = −0.14, 95% CI −0.21 to −0.06, p = 6.1 × 10⁻4; Table 2). Similarly, the model including hypersalivation and prolonged sleep duration as independent variables yielded an R² of 0.24, with response and hypersalivation showing significant contributions (B = 0.66, 95% CI 0.45 to 0.88, R² = 0.088, p = 5.9 × 10⁻⁹ and B = −0.59, 95% CI −0.95 to −0.22, R² = 0.033, p = 1.8 × 10⁻3, respectively; Table 3).
Discussion
To the best of our knowledge, this is the largest study to date examining patient satisfaction among clozapine users. We quantified the mean patient satisfaction rating with clozapine at 7.4 (SD = 1.9) on a scale from 1 to 10 (10 denoting maximum satisfaction scores). 82% of participants stated to be satisfied with clozapine treatment (score > 6). In univariable analyses, we found several variables to be associated with the level of patient satisfaction, including treatment response, symptom severity, total number of ADRs, hypersalivation, prolonged sleep duration, and recreational drug use. In multivariable analyses we found that treatment response contributed most to patients’ satisfaction (explained variance R² = 0.079), with those experiencing minimal response averaging a satisfaction score of only 5.0, compared to a mean score of 8.6 among those showing very good response to clozapine treatment. Hypersalivation and prolonged sleep duration were the only ADRs linked to lower satisfaction.
In line with previous research, clozapine users were generally satisfied with their treatment, with symptom reduction being the key contributor. Despite clozapine’s association with numerous and bothersome ADRs, users reported high satisfaction. Earlier studies similarly found that clozapine yields higher satisfaction rates compared to other antipsychotics9,10. As clozapine is typically prescribed for treatment-resistant symptoms, many patients likely underwent multiple unsuccessful antipsychotic trials before starting clozapine. Experiencing a significant reduction in symptoms and thereby a reduction in suffering, sometimes after years of unsuccessful medication trials, could therefore result in relatively high patient satisfaction. Consistent with our findings, Li et al. observed that patients remain positive about clozapine treatment despite its ADR profile and consequently posited that symptom-level and quality of life improvements may result in acceptance of ADRs26. As many view clozapine as a ‘last-resort’ option that finally relieves the distress associated with treatment-resistant symptoms, this may boost their commitment to treatment. Grover et al. further noted that clozapine often improves depressive symptoms, psychosocial functioning, and quality of life within three months of treatment and suggested that this relatively swift amelioration of well-being plays a role in patient satisfaction, outweighing concerns about ADRs27,28. Regarding the association between patient satisfaction and specific clozapine-associated ADRs, we found that people experiencing hypersalivation and prolonged sleep duration were generally less satisfied with clozapine. In previous work by Maher et al. hypersalivation was found to be the most prevalent adverse effect negatively impacting the quality of life in people treated with clozapine29. This may be due to the fact that hypersalivation is a visible ADR, which can be stigmatizing and functionally disabling, often leading to feelings of embarrassment and social impairment. However, despite experiencing hypersalivation, participants in our study reported a relatively high level of satisfaction with clozapine, with an average patient satisfaction score of 7.0 (compared to 7.8 in those who did not experience this side effect). This finding aligns with earlier research, which also found that users reported benefits of treatment and intended to continue taking clozapine despite the presence of hypersalivation15,30. When comparing patient satisfaction between those with and without prolonged sleep duration in our study population, the group experiencing this ADR had an average satisfaction score of 7.1, while those without it scored an average of 7.7. It is well documented that clozapine is associated with improvements in insomnia and sleep quality11. At the same time, a significant proportion of patients experience an increased need for sleep, excessive sedation, and extended sleep duration31. Grover et al. found that excessive sedation was perceived as the most distressing side effect reported by patients and an important reason for discontinuing clozapine treatment28. Grover & Naskar additionally found that hypersalivation and excessive sedation emerged as the two important factors leading to premature discontinuation of clozapine therapy27. Overall, our and previous findings highlight the nuanced relationship between clozapine’s therapeutic benefits and its ADRs, suggesting that while ADRs like hypersalivation and prolonged sleep duration may impact patient satisfaction, they do not jeopardize the overall acceptance of clozapine among patients.
In earlier research by Nordon et al., an association was found between patient satisfaction and age; we found a similar direction of effect with higher age being associated with higher satisfaction levels (B = 0.019, 95% CI .004 to .033, R2 = 0.15, p = 0.011; Supplementary Table 3 and Supplementary Fig. 5), but this association did not meet the Bonferroni-corrected significance level9. Interestingly -and similarly to our study-, Nordon et al. found no clear association between patient satisfaction and BMI or weight gain9. While evidence on this topic is limited, one may hypothesize that weight gain has a lesser impact on satisfaction than previously assumed, as many patients have already experienced significant weight increases from prior antipsychotic treatments. Consequently, the additional weight gain due to clozapine may not be viewed by patients as negatively impacting their overall wellbeing.
Implications of our work include the possibility of psychoeducation for patients using clozapine or considering its use. The findings are also clinically actionable in that clinicians should actively enquire about hypersalivation and prolonged sleep duration. Given the high prevalence of hypersalivation in clozapine users and the wide array of treatment options for this ADR, patient satisfaction may be boosted if hypersalivation and possibly other ADRs are adequately managed22. Furthermore, our findings may inform longitudinal and intervention studies aiming to optimize user satisfaction with this last resort treatment. Finally, our finding of overall high satisfaction ratings by patients on clozapine may help curtail ‘prescriber fear’, which in turn may help counter underutilization32. This discrepancy between patient satisfaction and prescriber concerns underscores the importance of involving patients in decisions regarding clozapine use through shared decision-making. Prior research has shown that shared decision-making, focused on the patient’s perspective, can increase the likelihood of prescribers recommending a clozapine trial33,34.
Notwithstanding the size and scope of this study, conducted across multiple centers and countries, we acknowledge several limitations. Firstly, we cannot fully rule out the possibility of selection bias as participants in the study had, on average, been using clozapine for an extended period and may have reported higher satisfaction levels and positive treatment responses relative to short-term users. Nonetheless, approximately 20% of participants had been on clozapine for less than 2 years and we found no significant association between patient satisfaction and the duration of clozapine treatment (Supplementary Table 3). Possibly, more satisfied clozapine users decided participating in this study. However, efforts were made to mitigate this by actively encouraging dissatisfied individuals to participate in the recruitment process, recognizing the value of their experiences. Secondly, the study’s focus was limited to a specific set of ADRs, not including certain possible ADRs, such as pneumonia and seizures (that may sometimes be challenging to link to clozapine use specifically). Additionally, the rare incidence of severe clozapine-associated ADRs curtailed statistical power to examine their possible associations with patient satisfaction. Thirdly, for the main outcome measure of patient satisfaction, we did not use a validated questionnaire but instead asked a single question that is also easy to implement in clinical practice. For the outcome measure of response, we used the Alda scale, which is validated for assessing response to long-term treatment with mood stabilizers in bipolar disorder24,25. We applied this scale to assess response to clozapine because schizophrenia, like bipolar disorder, requires a long-term perspective to accurately evaluate treatment efficacy. This is particularly true for clozapine, which is known for its effectiveness over extended treatment periods. Fourth and finally, the understanding of determinants of patient satisfaction with clozapine could be further enriched by future studies collecting additional data, such as qualitative data capturing the personal opinions and experiences of clozapine users. Future studies may also examine additional factors of possible relevance to patient satisfaction, such as the presence of comorbid depressive symptoms, the level of illness insight, and the impact of involuntary clozapine treatment. Future studies may also recruit people who are hesitant to start clozapine treatment, those who refused it, or those who discontinued the agent. This information may prove valuable as it could shed light on the discrepancy between the considerable hesitation among patients and clinicians to initiate clozapine treatment and the relatively high satisfaction with it. Furthermore, longitudinal designs additionally including patients on other antipsychotics may allow for assessments before and after clozapine initiation and before and after specific ADR occurrences, as well as comparative drug analyses. Together, these additions may foster a more nuanced perspective on the contributors to patient satisfaction and thus help lower barriers to clozapine prescribing and use.
In conclusion, patients diagnosed with schizophrenia are generally satisfied with clozapine treatment, with a reduction in symptoms showing the strongest association with patient satisfaction. Our findings may inform clinical decision-making and have the potential to reduce hesitancy among both patients and healthcare professionals to start clozapine. This could lead to more timely initiation of clozapine in the disease course, ultimately lowering underutilization and improving treatment outcomes for individuals with severe psychosis.



Responses