Electrocatalytic mechanism for overall water splitting to produce sustainable hydrogen by 2D Janus MoSH monolayer
Introduction
Two dimensional (2D) monolayered materials have gained significant attention in recent years due to their unique properties, such as high surface area, tunable electronic properties, and excellent mechanical strength1,2. These materials have shown great potential in various applications, including energy storage, catalysis, and electronics. One of the most promising applications of 2D monolayered materials is in electrocatalysis for renewable energy production, such as water splitting for hydrogen production. The use of 2D monolayered materials as electrocatalysts offers several advantages over traditional catalysts, including high catalytic activity, low cost, and environmental sustainability1,3. One of the most commonly used 2D layered materials for electrocatalysis is Pt. However, Pt is a rare and expensive metal, which limits its widespread use in practical applications4. Other 2D layered materials that have been previously reported for electrocatalysis include transition metal dichalcogenides (TMDs) such as MoS2 and WS2. However, TMDs suffer from poor stability and low catalytic activity, which limits their practical applications5. Another 2D layered material that has been previously reported for electrocatalysis is graphene. However, graphene has poor electrocatalytic activity for HER/OER, which limits its practical applications6.
Encouragingly, the recently developed 2D ML-MoSSe material, where Se atoms entirely replace the S atoms on one side of MoS2 (as depicted in Fig. 1), possesses an inherent polarized electric field, characterized by an average electrostatic potential difference of 0.75 eV between the S and Se atomic layers7,8. This unique feature renders it superior in promoting the separation of photogenerated charges compared to MoS2. Furthermore, the internal electric field within MoSSe induces band bending, surpassing the conventional 1.23 eV band gap limit observed in photocatalytic water-splitting reactions3. However, it’s worth noting that both MoSSe and MoS2 exhibit limited catalytic activities in their basal planes for water-splitting reactions9,10,11, which poses challenges for their practical utilization. Therefore, enhancing the catalytic activity at the surface we have replace H atoms in place of Se atoms which transform into Janus MoSH from Janus MoSSe stands as a viable strategy for enhancing the catalytic efficiency of Janus MoSH monolayer.

a Fully optimized structure with top and side view, (b) phonon dispersion spectra, (c) fully optimized structure with top and side view before and after AIMD simulations, and (d) the change of total energy at room temperature (300 K) as a function of time with a (5 × 5 × 1) supercell of the Janus 2D MoSH monolayer.
Taking inspiration from the successful experimental synthesis of a Janus MoSH monolayer12, there has been a growing focus on Janus 2D materials due to their potential for remarkable photocatalytic capabilities13,14. However, the electrocatalytic mechanism of 2D Janus MoSH monolayer for water splitting is not well understood, and further investigation is needed to optimize its performance. In this work, we present a detailed analysis of the structural stability, electronic properties, charge transfer mechanism, and electrocatalytic properties of 2D Janus MoSH monolayer for overall water splitting. Due to the presence of asymmetric structure display intrinsic electric field, therefore finite dipole moment is found in 2D Janus MoSH monolayer. Our results provide insights into the electrocatalytic mechanism of 2D Janus MoSH monolayer and pave the way for the development of efficient and sustainable electrocatalysts for renewable hydrogen production.
Results
Structural stability of Janus MoSH monolayer
Initially, we have optimized the Janus 2D MoSH monolayer as shown in Fig. 1a. The dotted line represents the unit cell of the Janus 2D MoSH monolayer, which has three atomic thick layers. The optimized lattice constant of Janus MoSH monolayer is found to be 3.01 Å which is slightly smaller than the 2D MoS2 (3.17 Å)15 and Janus MoSSe (3.25 Å)16. The bond lengths between Mo-H and Mo-S are 2.02 Å and 2.36 Å, respectively. Also, the thickness of the Janus MoSH monolayer is found to be 2.64 Å. The calculated lattice constant is good consistent with previous literature12. To check the structural stability, we have calculated phonon dispersion spectra, Ab-initio molecular dynamics calculations, and mechanical properties of 2D MoSH monolayer. Figure 1b displayed the phonon description spectra of the MoSH monolayer. All the acoustical as well as optical modes are positive frequencies at the high symmetry K-points. Therefore, the MoSH monolayer shows dynamical stability. Furthermore, Fig. 1c, d shows the ab-initio molecular dynamics simulations for the MoSH monolayer at room temperature for 5 ps. It was seen that there is no breaking of atomic bonds between Mo-S and Mo-H. Also, the fluctuation of the total energy of the MoSH monolayer system displayed a very small. It means that the MoSH monolayer shows thermal stability. We have further investigated AIMD simulations at room temperature (300 K) of 2D MoSH monolayer in the presence of both O2 and H2O atmospheres which are presented in Supplementary Fig. 1 and Supplementary Fig. 2 in the Supporting Information (SI) file. Investigated results display structural stability of 2D MoSH monolayer as well as maintain crystalline material in both water (H2O) environment and oxygen (O2) environment.
We have calculated the cohesive energy of the MoSH monolayer to check the energetically stable using the following relation,
where EMo, ES, EH and EMoSH are the total energy of a single Mo atom, single S atom, single H atom, and MoSH compound, respectively. n1, n2, and n3 represent the number of Mo, S, and H atoms present in the unit cell. The calculated MoSH monolayer is presented in Table 1. The range of cohesive energy of the considered monolayer is found to be −3.99 eV/atom which is very near to the previously reported 2D monolayer materials PtS2 (−5.01 eV/atom)17, Janus NbSH (−6.72 eV/atom)18, RuSSe (−5.97 eV/atom), RuSTe (−5.74 eV/atom), and RuSeTe (−5.54 eV/atom)19 and other materials20,21. Such types of larger cohesive energy materials suggest that monolayers have high energetic stability and mechanical strength22.
Apart from this, we have calculated the mechanical properties for the mechanical stability of the MoSH monolayer. Also, we have calculated the in-plane Young’s modulus and Poisson ratio along different θ directions relative to the positive x-direction are as follows23,
The calculated elastic stiffness constants values of C11, C12, C22, C66 are calculated and it satisfy the necessary mechanical equilibrium conditions24 for mechanical stability: ({{rm{C}}}_{11},{{rm{C}}}_{22}-{{rm{C}}}_{12}^{2} >)0 and C11, C22, C66 > 0 (her are the values of each elastic constant C11=122.54, C12=33.41, C22=122.54, C66=44.57 N/m). From the calculated elastic constants, these considered systems satisfied the mechanical stability conditions. It means that the MoSH monolayer system is mechanically stable. Further to understanding mechanical properties, we have calculated Young’s modulus E(θ) and Poisson’s ratio ν(θ) as presented in Table 2. The values of Young’s modulus and Poisson’s ratio are 113.43 N/m and 0.27 which are near to 2D MoS2 and Janus MoSSe monolayer.
Electronic properties of Janus MoSH monolayer
To understand the electronic properties of 2D Janus monolayer, we have calculated the electronic band structure without spin-orbit coupling (SOC) and with SOC, projected density of states, electron localization function, linear charge profile, and electrostatic potential as depicted in Fig. 2. The electronic band structure without SOC effect is shown in Fig. 2a. It is clearly seen that the electronic band line crosses the Fermi level which means that it behaves like a metallic nature and good consistent with previously reported work12. The Mo d-orbital is mainly responsible for crossing the Fermi level as shown in the orbital contributed band structure Fig. 2a. The contribution of Mo d-orbitals at the Fermi level is confirmed by the projected density of states (see Fig. 2a). The S p-orbital has a small contribution near the Fermi level as well as other energy levels in the valence and conduction band. Also, it was seen that the H s-orbital has a contribution at a very deep energy level of around −4 eV in the valence band. Also, we have calculated the electron localization function to see the nature of bonding such as physical and chemical bonding (see Fig. 2b). In Fig. 2b, the color map red and red refer to the presence and absence of localized electrons. It was seen that the bond between Mo and S shows the green color lies between the color map, which means that electrons are localized between the Mo-S bond. Apart from that, electrons are less localized between the Mo-H bond as compared to the Mo-S bond because the color map (light blue color) shifted towards the absence of localized electrons. It was also confirmed by Bader charge analysis calculations. Due to the lack of vertical mirror symmetry, Janus MoSH monolayer inherently form electric dipole caused by charge migration between their upper and lower surfaces. Consequently, MoSH structure exhibit a noticeable electrostatic potential difference on their surfaces, which influences the reduction and oxidation potentials. Therefore, incorporating a dipole correction is essential for system with asymmetry. This study subsequent discussions are based on simulation results that account for this dipole correction. Due to the presence of an asymmetric structure of Janus MoSH monolayer, Mo atoms transfer different amounts of electrons to the neighboring H and S atoms. From Bader charge analysis, transition metal Mo atoms transfer 0.43 e− and 0.51 e− to H and S atoms, respectively, which means it shows strong out-of-plane polarization.

a Projected electronic band structure and corresponding density of states without spin-orbit coupling and (b) electron localization function of the Janus 2D MoSH monolayer without spin-orbit coupling. c Projected electronic band structure within spin-orbit coupling, (d) average linear charge density, and (e) electrostatic potential of Janus 2D MoSH monolayer.
The effect of SOC on electronic band structure is shown in Fig. 2c. From Fig. 2c, it is clearly seen that the SOC has a strong effect on the electronic band structure. The degenerate bands are found in each high symmetry point. Moreover, both the valence and conduction band lines are significantly split with a large amount of energy difference by the SOC effect. Such type of spin-splitting is known as Zeeman-type spin-splitting25. Additionally, the linear charge profile and electrostatic potential of 2D MoSH monolayer are presented in Fig. 2d, e. The Mo atom has a larger linear charge and the magnitude of electrostatic potential also has a higher value. Similarly, the S and H atomic side for both linear charge and electrostatic potential values shows lower values as compared to the Mo atomic side due to the asymmetric structure of the 2D MoSH monolayer. Due to the intrinsic dipole of the Janus MoSH structure generating an intrinsic electric field, the vacuum energy levels on both sides of the surface differ, resulting in a vacuum energy level difference Δϕ. We have also calculated the work function of the 2D MoSH monolayer and it is found to be 4.88 eV. It is also seen that the a small electrostatic potential difference (0.42 eV) between the S and H sides on the y-axis generating dipole moment of 0.24 D in a 2D MoSH monolayer. Such types of asymmetric structures are very beneficial for the enhancing piezoelectric effect.
Figure 3 displayed the crystal orbital Hamilton population (COHP) calculation to analyze the chemical bonding between the atoms. From COHP analysis, we can see the contributions of bonding, antibonding, and nonbonding nature to the electronic band structure between the atomic pair interactions. The +y-axis and -y-axis show the bonding and antibonding characters in which red and blue colors displayed Mo-S and Mo-H atomic pairs in a 2D MoSH monolayer. It is observed that there is a significant contribution of antibonding for atomic pairs Mo-S at the Fermi level. Whereas atomic pair Mo-H shows no significant antibonding contribution below the Fermi level. Also, the negative values of integrated COHP show strong covalent interactions and vice-versa. We found that the negative values of ICOHP analysis indicate the covalent interactions for the Mo-H pair as compared to the Mo-S atomic pair weaken the covalent interaction (relatively higher value as compared to the Mo-H atomic pair) due to the presence of antibonding contribution at the Fermi level. Consequently, Mo-H side display high catalytic performance and compared to Mo-S side in 2D MoSH monolayer material.

The zero vertical line shows the Fermi energy. The -pCOHP gives information about the contribution of a particular bond (bonding or anti-bonding) to the band energy. All the plots show bonding contribution in the up side (+y-axis) and the anti-bonding contribution is down side (-y-axis).
Hydrogen evolution reaction mechanism
In this section, we have calculated the hydrogen evolution reaction (HER) to analyze the performance of catalytic activity of 2D MoSH monolayer. For the ideal catalyst, the value of Gibbs free energy should be zero in case of HER activity26. The intrinsic electric field facilitates the separation of electrons and holes in the electrocatalytic water splitting process, contributing positively to the work performed. This contribution must be included in the overall energy calculations. Therefore, the HER performance for electrocatalytic water splitting using a 2D MoSH material with a vertical intrinsic electric field is calculated. To calculate the Gibbs free energy, initially we have taken three different active adsorption sites such as top of Mo and S/H atoms, between the hexagonal arrangement of atoms. After that we have taken most favorable active sites (i.e., lowest energy configuration) from possible adsorption sites as shown in Fig. 4a, b. It was seen the hexagonal lattice arrangement of atoms shows lowest energy configuration for S-side whereas top of the Mo atom shows lowest energy configuration for H-side as shown in Fig. 4a, b.
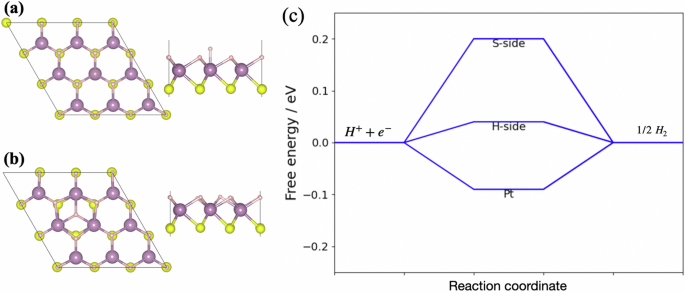
a, b Optimized structures in the presence of H species on both H-side and S-side of 2D MoSH monolayer, respectively and (c) free energy profile of HER on the 2D MoSH monolayer.
The Gibbs free energy are calculated to be 0.04 eV and 0.20 eV for H-side and S-side, respectively which is relatively lower than the Pt catalyst as shown in Fig. 4c. The value of Gibbs free energy 0.04 eV for H-side shows very near to the zero which means that it will be very excellent catalyst for hydrogen evolution reaction as compared to previously reported most of the 2D monolayer materials2. We found that the H-side has low value of Gibbs free energy therefore HER activity has been further calculate with different hydrogen coverages from 1/9 to 9/9. The Gibbs free energy for different hydrogen coverages 2/9, 3/9, 4/9, 5/9, 6/9, 7/9, 8/9 and 9/9 are found to be 0.063, 0.048, 0.061, 0.083, 0.064, 0.052, 0.048 and 0.057 eV, respectively. For the higher concentration of hydrogen coverages, the Gibbs free energy values are vergy near to the single H adsorption configuration. It means that the H-side of 2D MoSH monolayer display excellent performance of HER catalytic activity at higher hydrogen coverage.
Figure 5a, b displayed the projected density of states for H-adsorbed on the surface of 2D MoSH monolayer on both sides of H and S. The Mo d-orbitals has more contribution at the Fermi level whereas S p-orbitals have a very small contribution at the Fermi level for both cases i.e., H-side and S-side. It was also seen that the contribution of H s-orbitals has deep energy level between −2 and −6 eV. Furthermore, we have calculated the charge density difference plots of the most favorable configuration for HER mechanism which represents the charge redistribution with the adsorption of H-atom on both sides of 2D MoSH monolayer. The yellow and blue color shows the charge accumulation and depletion region, respectively as shown in Fig. 5c, d. It was clearly seen that the charge accumulation are present around the adsorbed H atom which is also confirmed by Bader charge analysis. To compare the catalytic performance of HER activity with previous reported data of 2D monnolayer material in the literature, we have calculated the volcanic profile in Fig. 6. The top of the volcanic curve shows excellent catalytic activity. From Fig. 6, we can see that the 2D MoSH monolayer displayed the higher catalytic performance as compared to previously reported 2D monolayer materials2. It means that, 2D MoSH monolayer is a promising candidates for HER catalytic activity.

Projected density of states of H species absorbed on the (a) H-side and (b) S-side of 2D MoSH monolayer. Charge density difference plot of H species absorbed on the (c) H-side and (d) S-side of 2D MoSH monolayer.

The values of overpotential in the present work are highlighted in red color.
Oxygen evolution reaction mechanism
Furthermore, we have calculated the oxygen evolution reaction (OER) mechanism to see the better catalytic performance of 2D MoSH monolayer. For that implicit water environment (solvation effect) as well as vertical dipole correction has been taken into account during the calculations. First, we have calculated the interaction of water molecule (H2O) with lowest energy configuration on the surface of 2D MoSH monolayer on both H-side and S-side. It was seen that the H-side of MoSH monolayer have lowest energy configuration which is presented in Fig. 7a. The adsorption energy are found to be −0.16 eV and −0.11 eV for H-side and S-side, respectively and which is relatively higher in magnitude as compared to 2D MoS2 monolayer and almost same as Janus 2D MoSSe monolayer2,27. Also, we have calculated the projected density of states after adsorption of H2O molecule on MoSH monolayer as shown in Fig. 7b. It was seen that Mo d-orbitals has more contribution whereas small contribution comes from S p-orbitals at the Fermi level same as pristine MoSH monolayer. While O p-orbitals and H s-orbitals have the significant contribution below the Fermi level which indicate the physical interaction between the H2O and MoSH monolayer surface. Apart from this, we have calculate the charge density difference profile for H2O adsorption on MoSH surface. The yellow and blue color represents the charge accumulation and charge depletion as shown on Fig. 7c. The accumulation region are presented between the MoSH surface and H2O molecules which means that significant charge transfer occurs between the interface. In addition, we have also calculated the adsorption energy values of −2.86 eV on H-side and −0.19 eV on H-side on the surface of MoSH monolayer for O2 molecules as shown in Supplementary Fig. 3 in SI. This implies that water and oxygen molecules, which have been adsorbed, can undergo significant activation when they interact with the activation site of 2D MoSH catalysts. It means that, higher interaction of H2O and O2 molecules with MoSH monolayer surface indicating the good catalytic activity for overall water splitting.

a Lowest energy configuration of H2O adsorption, (b) projected density of states and (c) the corresponding charge density profile for H2O adsorbed with top and side view on 2D MoSH monolayer. The charge accumulation and depletion regions are represented by yellow and blue color, respectively, and 2 × 10−3 e/Å−3 has been set as the isosurface value.
Figure 8 shows the OER mechanism on the surface of 2D MoSH monolayer and the reaction mechanism will be completed in four elementary reaction pathways2. (I) Initially H2O molecule splits into H+ and HO*, (II) after that HO* species split into H+ and O*, (III) then O* interact with new H2O molecule and it splitted into H+ and HOO* and (IV) finally HOO* dissociates into H+ and O2 molecule in which O2 molecule released from the surface of 2D MoSH monolayer catalyst. During each steps of four elementary reaction pathways, a cation H+ and an electron are released simultaneously. Figure 8a, b shows the all intermediates (i.e., O*, HO* and HOO* species) with lowest energy configurations for OER mechanism for H-side and S-side, respectively. The free energies profile for each elementary step indicates the uphill reactions as shown in Fig. 8c. The rate determining step for OER mechanisms calculated at electrode potential of U = 0. The calculated overpotential (ηOER) values are found to be 0.17 V and 0.45 V for S-side and H-side with the inclusion of dipole correction, respectively. Apart from this with the inclusion of implicit water environment (solvation effect) and vertical dipole correction, the values of ηOER are 0.11 V for S-side and 0.49 V for H-side as shown in Supplementary Fig. 4 in SI. The rate-determining step are found to be O* to HOO* and H2O to HO* for S-side and H-side as shown in Fig. 8c, respectively. The calculated overpotential ηOER value of H-side of 2D MoSH catalyst extremely lower than the previously studied 2D monolayer materials2 which means that 2D MoSH monolayer material displayed a better electrocatalyst candidate for OER mechanism.

a, b Optimized structures of OH, O and OOH species on the surface of 2D MoSH monolayer on both H-side and S-side, respectively. c The free energy profile of OER mechanisms on the 2D MoSH monolayer at the U = 0 V on both H-side and S-side represents with blue and red color, respectively.
Further, to check the charge transfer between the intermediates adsorbed species and MoSH monolayer on both surface as shown in Fig. 9. For that we have calculated the charge density difference profile for each species on both H-side and S-side in Fig. 9a–f, respectively. In the charge density difference plot, accumulation and depletion of charge are presented by yellow and blue color, respectively. It was seen that the charge accumulation are mostly located towards all intermediates adsorbed species where as depletion of charge appears towards the 2D MoSH monolayer surface side which are beneficial for OER catalytic mechanism. These investigated results suggest that the 2D MoSH monolayer facilitate HER and OER on separate surfaces, which can significantly suppresses the carrier recombination.

a OH, (b) O and (c) OOH species absorbed on the H-side of surface of MoSH monolayer. d OH, (e) O and (f) OOH species absorbed on the S-side of surface of MoSH monolayer.
Additionally, we have investigated the projected density of states to better understand interaction of all possible intermediate species for OER mechanism. Figure 10a–f shows the PDOS of OH, O and OOH species absorb on both H-side and S-side of 2D MoSH monolayer, respectively. It was seen that O p-orbital has significant contribution at the Fermi level as well below the Fermi level it means that p-orbitals of O are hybridized with surface atoms of MoSH monolayer. From charge density difference and PDOS investigations which shows ionic character of bonding. These investigated results indicate the 2D MoSH monolayer displayed superior electrocatalyst for overall water splitting to produce hydrogen.

a OH, (b) O and (c) OOH species absorbed on the H-side of surface of MoSH monolayer. d OH, (e) O and (f) OOH species absorbed on the S-side of surface of MoSH monolayer.
Discussion
We have systematically investigated the structural stability, electronic properties, charge transfer mechanism, and electrocatalytic mechanism for hydrogen evolution reaction and OER for overall water splitting. The Janus MoSH monolayer exhibit asymmetric structure therefore it has dipole moment of 0.24 D with metallic behavior and it shows good conductivity for electrocatalytic performance. We have used implicit water environment (solvation effect) as well as dipole corrections to predict the electrocatalytic activity for overall water splitting. Our study demonstrates that 2D Janus MoSH monolayer exhibits excellent electrocatalytic activity for both HER (i.e., overpotential are 0.04 V and 0.20 V for H-side and S-side of catalyst, respectively) and OER (calculated overpotential values are found to be 0.11 V and 0.49 V for S-side and H-side of the catalyst, respectively) making it a promising candidate for overall water splitting. Our findings provide insights into the underlying mechanisms of electrocatalysis on 2D Janus MoSH monolayer and pave the way for the development of efficient and sustainable electrocatalysts for water splitting. Moreover, our study highlights the potential of 2D monolayer materials for various applications in renewable energy production. By understanding the unique electronic and structural properties of 2D Janus MoSH monolayer material, we can design and optimize them for specific applications, leading to the development of efficient and sustainable energy technologies.
Methods
All the calculations have been performed using first principles calculations as implemented in VASP software28. The generalized gradient approximation in the form of Perdew-Burke -Ernzerhof functional (GGA-PBE) has been used for an exchange-correlation interaction29. The van der Waals interactions with DFT-D3 were considered by Grimme et al.30. For the plane-wave basis set, we have used an energy cutoff of 500 eV and (28 × 28 × 1) k-meshes for (1 × 1 × 1) unit cell of 2D Janus MoSH monolayer for Brillouin zone integration within the Monkhorst–Pack scheme31. To describe the ion-electron interaction, we have used the projected augmented wave (PAW) potential32. Furthermore, we have used the (3 × 3 × 1) supercell with (9 × 9 × 1) k-meshes for the electrocatalytic mechanism of the 2D MoSH monolayer. In addition, a vacuum of 24 Å has been used in the transverse directions to prevent the physical interactions between the consecutive layers. The convergence criteria for Hellmann–Feynman force fell below 2 × 10−3 eV/Å during the structural optimizations. The energy convergence criterion has been set as 10−6 eV for the electronic wave function. The Janus MoSH monolayer shows asymmetric structure therefore vertical dipole correction is taken into account during all calculations33. We have also considered the implicit water environment (solvation effect) to investigate the electrocatalytic activity for HER and OER mechanism of 2D Janus MoSH monolayer.
It was seen that the standard DFT gives the underestimated band gaps therefore, we have used hybrid HSE06 functional for accurate band gap with a screening parameter of 0.2 Å−1 and mixing parameter (α) of 25%34. We have investigated the phonon dispersion spectra using PHONOPY code35 with (5 × 5 × 1) supercell sheet to check dynamical stability. Ab initio molecular dynamics (AIMD) calculations were used for thermal stability. Newton’s equation of motion is integrated using Verlet’s algorithm with time steps of 2 fs and a Nose-Hoover thermostat is used for AIMD simulations. The stability of 2D MoSH monolayer as well as 2D MoSH monolayer in the presence of water (H2O) and oxygen (O2) environment at room temperature (300 K) using canonical ensemble (NVT i.e., fixed particle number, volume, and temperature) from AIMD simulation for 5 ps. AIMD calculations help to determine whether the change in structure is reversible or not and it also provides information about the thermal stability of the used host material. Lobster software36 has been used to calculate the crystal orbital Hamiltonian population (COHP) for chemical bonding information.


Responses