Evaluate the stability of synthesized allicin and its reactivity with endogenous compounds in garlic

Introduction
Garlic (Allium sativum L.), with typical pungent smell and taste, has been widely used in the food industry from ancient times to the present. In addition, it also has some pharmacological effects such as anti-atherosclerotic, antimicrobial, and anticancer activities, receiving wide attention from scholars all over the world1,2. Researchers find that allicin and its degradation products are closely associated with its special flavor and biological properties3.
As a traditional way to prepare allicin, it was usually obtained from garlic using organic reagent extraction and semi preparative liquid chromatography (HPLC). However, high amounts of solvents were used, and the extraction efficiency was poor due to the instability of allicin and time-consuming operation4,5. Under the trend of green environmental protection and increasing of efficiency, allicin could be obtained in quantities using chemical synthesis6,7.
High-speed counter current chromatography (HSCCC) is an advanced liquid-liquid chromatography technique that employs both a liquid stationary phase and a liquid mobile phase, effectively eliminating irreversible adsorption. This method offers several advantages, including minimal sample loss, reduced contamination, and high separation efficiency. Additionally, HSCCC allows for rapid and large-scale preparative separations, making it a valuable tool for isolating and purifying compounds in various applications, such as traditional Chinese medicine ingredient separation8, biochemistry natural product chemistry9, bioengineering10 and environmental analysis11. However, to our best knowledge, there is no literature report on the HSCCC is applied to isolate organosulfur compounds of garlic.
Due to the presence of thiosulfinic acid and allyl groups in the allicin structure, allicin is easily converted into other compounds even at mild conditions12,13. Previous studies have showed that the degradation products of allicin mainly include diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulphide (DATS), vinyldithiins (VDTs), (E/Z)-ajoene14,15. The instability of allicin have reduced its positively biological properties, and restricted its application16. Therefore, it is of great significance to study the factors that affect the stability of allicin. Although it has been reported that allicin degrades quickly at high temperature or different pH solutions17,18. However, the degradation kinetics of allicin in different environmental conditions were not systematically studied. Reports have suggested that the protein in milk19 and vegetables with high phenol compounds20 decreased the garlic odor. However, there is little literature investigated the endogenous substances effect on allicin and its degradation.
In this study, we optimized the synthesis conditions of allicin, and isolated of which with HSCCC applied. Moreover, the effects of temperature, concentration, pH, amino acids, and polyphenolic compounds in garlic on the stability of allicin were investigated. These findings play a crucial role in optimizing the conditions for allicin storage and application, improving its stability, and extending its practical utility in both pharmaceutical and food industries.
Results and discussions
The synthesis and isolation of allicin
At present, the most common method to prepare allicin is oxidizing DADS with H2O2, using acetic acid as a catalyst21. Due to the low yield of allicin and the large number of by-products after reaction, the synthesis method of allicin was optimized in this study. The reaction time, temperature, the addition amount of H2O2, and the addition amount of HAc significantly affected the generation of allicin (Fig. 1A–C). The results showed that the amount of allicin initially increased and then decreased as the reaction time increased at each temperature (20, 30, or 40 °C, Fig. 1A). The yield of allicin was highest (18.07 ± 0.77 mg) at 20 °C after reacting for 10 h, which was significantly higher than the yield at higher temperatures (30 and 40 °C). Moreover, the generation of allicin was higher (P < 0.05) when the addition of oxidant (H2O2) was equal amount to DADS (2:2, Fig. 1B). As the addition of HAc increased, the concentration of DADS was decreased, and the yield of allicin didn’t increase when the ratio of HAc:DADS reached 2:10 (Fig. 1C). According to the above results, three factors were invested for optimizing synthesis allicin using response surface design.
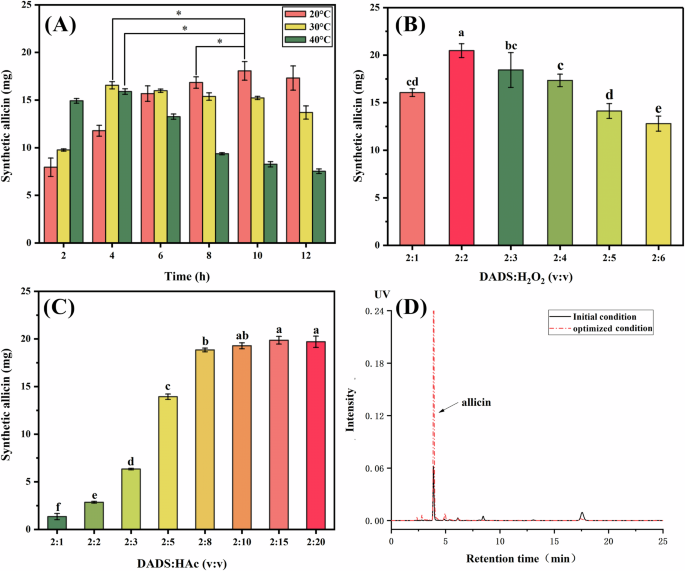
A Temperature and reaction time, B H2O2, C HAc, D HPLC chromatograms of allicin before and after optimization of synthetic method.
The results of ANOVA were shown in Table 1 for regression analysis by Design Expert data analysis software. The ratio of H2O2, DADS, and HAc was significantly (P < 0.05) affects the production amount of allicin, the P < 0.01 of the overall model, the quadratic equation model reaches the extremely significant level, and the lack-of-fit index is not significant (P > 0.05), indicating that the regression Eq. (1) fits the data well, and the quadratic regression equation is as following:
According to the results, the optimal synthesis conditions were 6.18 h, DADS:H2O2 = 2:1.67, DADS:HAc = 2:9.0, and the predicted value of theoretical allicin synthesis under these conditions was 23.15 mg. Considering the actual operation, the optimal synthesis process obtained by the regression equation was modified to 6.5 h, DADS:H2O2 = 2:1.6, DADS:HAc = 2:9.0, and the obtained allicin amount of 22.45 ± 0.23 mg (the yield is 80.95%), which is basically consistent with the software optimization synthesis amount, indicating that the results validated the accuracy of the model. The yield of allicin synthesis was only 65% before optimization, and the optimization scheme significantly improved the yield of allicin using area normalization method (Fig. 1D). At the beginning, Stoll and Seebeck22 first reported this method to synthesis of allicin, to our best knowledge22, Iberl et al.23 reported the yield of synthesis allicin was only 25%23, using HAc oxidizing DADS. Lawson and Wang24 reported a more detail protocol based on this method24, with DADS: H2O2 = 2:3, and DADS: HAc = 2:10, however, the yield of allicin was not mentioned.
Isolate allicin using HSCCC system
In previous reports, high purity of allicin was usually obtained by semi-preparative liquid chromatography7,25. Albrecht et al.21 obtained high purity allicin with silica gel chromatography using n-hexane and ethyl acetate (2:1) as eluent21. In this study, high-speed counter current chromatography (HSCCC) was used to isolate crude synthetic allicin, with four-phase solvent system (Hexane:ethyl acetate:methanol:H2O = 2:0.5:2:0.5). As shown in Fig. 2, it could be obtained a total of 6 main fractions. Compared to the standard reference of our previous report (Zhang, et al.2), it was found that fraction A was (E/Z)-ajoene (85% purity), fraction B was allicin (92.37%), fraction E was 2-VDT (96.8%). The mixure fraction of C, D, and F were discarded, as they were of low content and composed of various components. Therefore, allicin can be prepared and separated in large quantities by this system. In this separation system, 500 mg crude allicin was injected at a time, and 100 mg allicin (92.37% purity) can be separated through the system. Therefore, HSCCC could be a potential effective way for preparing high purity of allicin and other organosulfur compounds in large quantities in one injection.
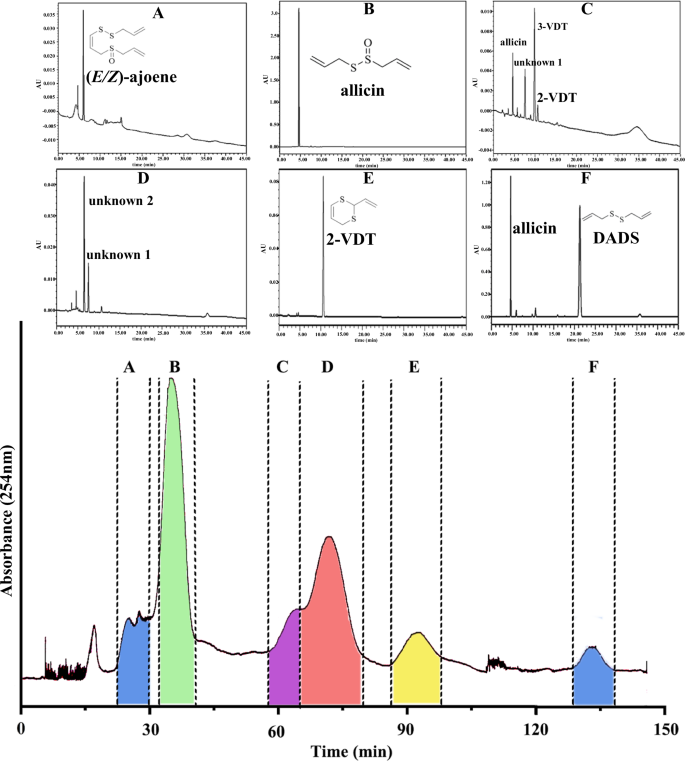
A 85% purity of (E/Z)-ajone, B 92.37% purity of allicin, C, D, F mixtures obtained from HSCCC, and E 96.8% purity of 2-VDT.
Effect of concentration and temperature on the stability of allicin
The concentration and temperature are important environmental factors to affect the stability of allicin. As shown in Fig. 3A, B, higher retention of allicin was observed at lower concentration, which indicated that higher concentration resulted in faster degradation of allicin. The allicin content decreased by 44.64% within 1 day at the initial concentration of allicin (60 mmol L−1), and the allicin was completely degraded until 12 days. At a low initial concentration of allicin (3.25 mmol L−1), the content of allicin remained 53.93% on the 8th day and 32.36% until 30 days. Li, et al.26 reported that the higher concentration of synthesized allicin solutions lead to increased degradation rates of allicin26. In contrast, Wang, et al.13 reported that the higher concentrations of allicin in garlic extract were associated with greater stability13. The discrepancy between these findings may be attributed to the various components in garlic extract, which could protect it from degradation.
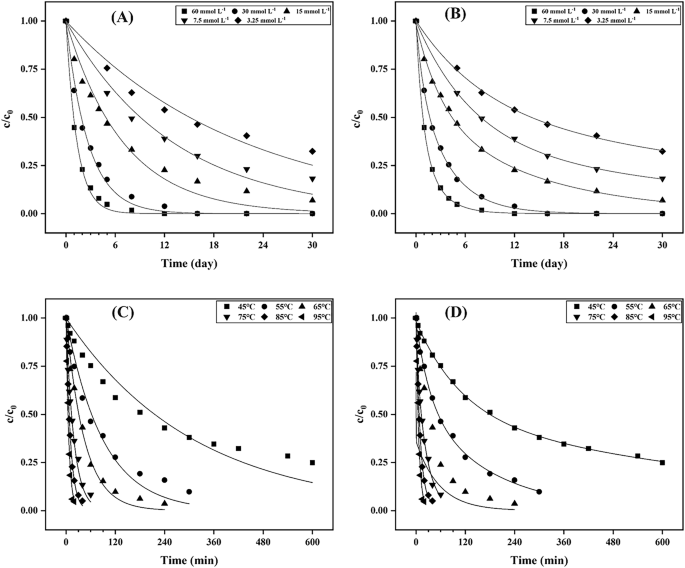
Experimental data were fitted with first-order reaction model (A, C), experimental data were fitted with two first-order reactions model (B, D).
Allicin is thermally unstable, its degradation rate increasing as temperature rise (Fig. 3C, D). Allicin rapidly degrades at a temperature ≥75 °C, with complete degradation occurring within 60 min. Allicin placed at 45 °C for 420 min and its content decreased to 32.22%, which was 100 times faster than which at room temperature. When the temperature raised from 80 °C to 85 °C, polysulfides were formed quickly through the condensation reaction according to the results of previous research27. Wongsa et al.28 suggested that allicin was decreased by 71%, 80% and 85% after blanching for 5 min at 70, 80 and 90 °C, respectively28. Therefore, our results indicated that maintaining allicin at low concentrations and applying mild temperatures effectively slow down its degradation.
The first-order and two first-order kinetic models were used for fitting allicin degradation. The fitting results of two first-order kinetic equation showed that the fitting coefficient R2 > 0.994 at 45–85 °C, and the R2 = 0.9727 at 95 °C, and the fitting coefficient R2 > 0.998 at different concentrations, which were much higher than those of first-order kinetic equation (Supplementary Tables S1 and S2). Therefore, two first-order kinetic model is more effective in predicting allicin degradation. These results indicated that more than one degradation processes of allicin29.
Effect of pH on allicin stability and its degradation products
As mercaptans (RSH) and sulfinic acid (RS(O)H) are substances of weak acidity, pH is usually a vital factor in changes in volatile organic sulfides in Allium plants25. While previous studies have explored the effects of pH on volatile organic sulfieds in Allium plants13,26, the impact of pH and temperature on allicin stability and formation of its degradation products remains inadequately investigated. Fig. 4A showed the relationship between the degradation of allicin and pH values. Allicin contents were higher acidic environment (pH 3.0–6.0) compared to alkaline environment (pH 8.0–9.0). The result indicated that allicin is more stable in acidic environment, which is consisted with previous reports13,26. This result is attributed to the protonation of allicin in acidic environments26. Additionally, under neutral to alkaline conditions (pH 7.0–9.0) the contents of each degradation products were significantly higher than under acidic conditions (pH 3.0–6.0) (P < 0.05). This suggests that, under alkaline conditions, the degradation of allicin leads to increase in its degradation products, which exhibit greater stability in such environment. Interestingly, these results contrast with the behavior of allicin in garlic paste stored at different pH values (Fig. 4B). In this case both allicin and its degradation products showed more significant decrease under alkaline conditions (pH 8.0–9.0) (P < 0.05). Notably, when the pH was raised to 9.0, VDTs and DATS could be nearly undetectable. The result indicated the rearrangement reaction pathway is influenced by endogenous substances in garlic, supporting the findings of Prati, et al.30. Furthermore, Torres-palazzolo, et al.31 highlighted that the garlic matrix components could protect organosulfur compounds from degradation in digestive fluids, further emphasizing the importance of the garlic matrix in stabilizing allicin and its degradation products under varying pH conditions31.
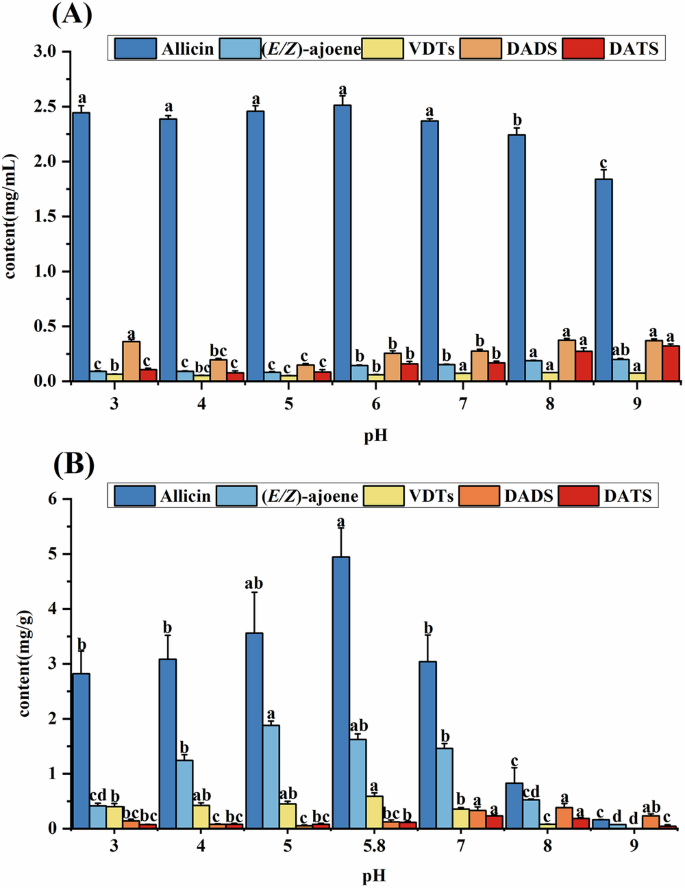
A allicin aqueous solution, B allicin in garlic paste. a–c The same compound with different letters differ (P < 0.05).
Effect of polyphenols on the stability of allicin
As shown in Fig. 5A, the content of allicin in the reaction system with both polyphenols and peroxidase was significantly higher than it in the control group and the reaction system including allicin and polyphenols. The result suggested that the combination of polyphenols and peroxidase prevented the degradation of allicin. Further analysis in Fig. 5B demonstrated that the effect of when specific polyphenolic, such as apigenin, myricetin, and quercetin, were combined with peroxidase, the allicin content was maintained at 3.88–3.96 mg mL−1, significantly higher than that of control group. However, when only polyphenols were added without peroxidase, no significant effect on allicin stability was observed, indicating that polyphenols alone do not influence allicin degradation, but their effect is likely mediated through their interaction with peroxidase.
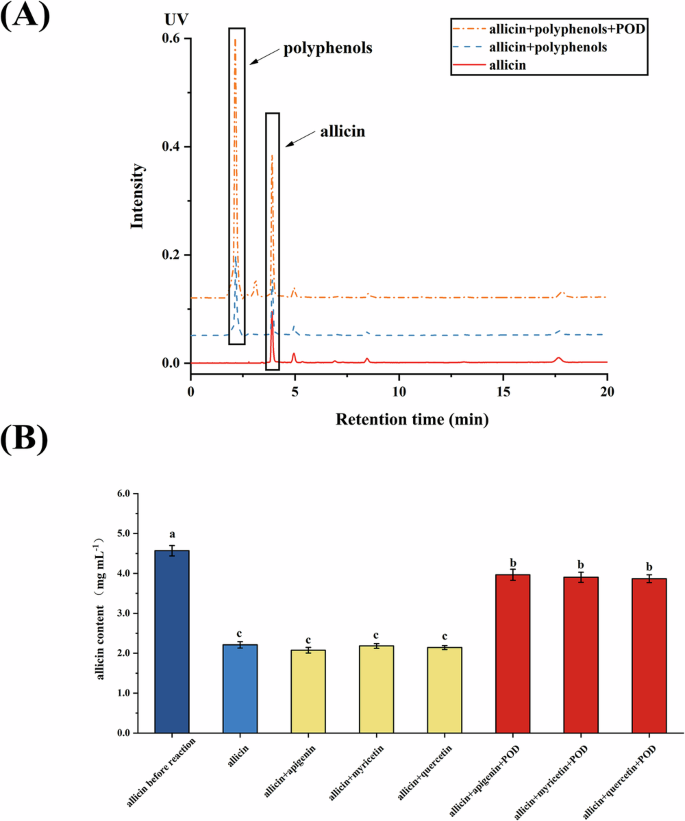
A HPLC chromatograms of polyphenolic extract and peroxidase (POD) on the stability of allicin, B effect of apigenin, myricetin and quercetin on the stability of allicin. a–c Allicin content with different letters differ (P < 0.05).
These findings align with previous studies by Castada et al.20 and Mirondo and Barringer32, who observed that exogenous phenolic compounds like rosmarinic acid reduced the levels of volatile polysulfides (such as DADS and DATS) when combined with peroxidase20,32. Their results indicated that rosmarinic acid and peroxidase enhanced the stability of allicin. These findings suggest that polyphenols, particularly when combined with peroxidase, can play a significant role in stabilizing allicin, with potential applications in food processing and storage to maintain the quality of garlic-derived products.
Effect of amino acids on allicin stability
Garlic undergoes greening discoloration during processing and storage, which is involved in amino acids and thiosulfinates. While previous reports have investigated the reaction model between free amino acids and 1-proplythiosulfonate, the reaction between amino acids and allicin (allyl thiosulfinate) has not been thoroughly explored. The effect of different amino acids on allicin stability was shown in Fig. 6A. Most amino acids had no significant effect on allicin stability (P > 0.05). However, cysteine and several basic amino acids (lysine, arginine, histidine), were found to reduce allicin content (P < 0.05). As cysteine contains sulfhydryl groups, it reacts with allicin to produce allyl cysteine disulfide (S-allyl mercapto cysteines (SAMC)33. The basic amino acids lysine, arginine, and histidine, which possess nitrogenous groups and exhibit alkaline properties after ionization, are likely to promote allicin degradation.
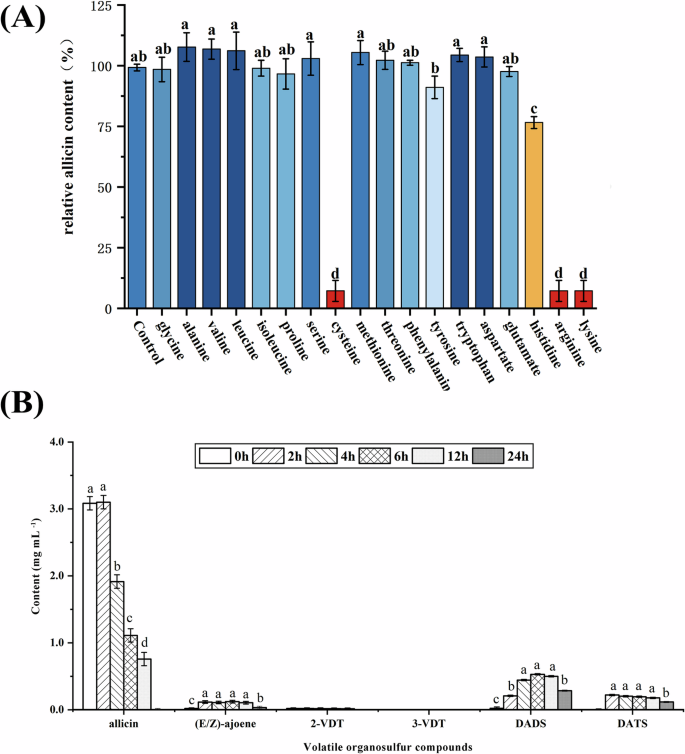
A Different amino acids on the stability of allicin, B effect of lysine on allicin and its degradations. a–d The same compound with different letters differ (P < 0.05).
Figure 6B illustrated the changes of each volatile organic sulfide during the reaction between allicin and lysine. The allicin content decreased by 37.90% and 63.96% after storing at 45 °C for 4 h and 6 h of reaction. Additionally, the contents of DADS and DATS increased significantly after 2 h (P < 0.05). Arginine and lysine are the most abundant free amino acids in garlic. Additionally, previous reports suggested that in garlic the degradation of allicin enriched DADS and DATS7,25. Therefore, it is likely that these endogenous amino acids contribute to the reduction of allicin stability, although further studies are needed to fully elucidate their role and reaction mechanisms.
Materials and methods
Materials
Analytical grade DADS (≥80% purity) was purchased from (Solarbio, China). Chromatographic grade DADS (98% purity) and DATS (98% purity) were purchased from Sima-Aldrich (China). Chromatography-grade acetonitrile and methanol were purchased from Oceanpak (Sweden). All of other analytical grade chemicals and solvents were purchased from Kaitong Chemical Technology Co., Ltd. (Tianjin, China). The experimental flowchart for optimizing allicin synthesis conditions, separating allicin using HSCCC, and evaluating its stability and reactivity is presented in Fig. 7.

The process involves optimization of allicin synthesis conditions, separation using HSCCC, and characterization of its stability and reactivity.
Synthesis of allicin procedure and optimization of synthesis conditions
Allicin was synthesized according to the method of Iberl et al.23. In brief, 200 μL DADS dissolved in 500 μL acetic acid (HAc), after that 100 μL 30% hydrogen peroxide was added at room temperature (25 °C) with stirring. Reaction lasts for 2 h and terminated with 5 mL distilled water.
To optimize the reaction conditions, the effect of reaction time, reaction temperature, the concentration of DADS (DADS: HAc), and the addition ratio of DADS:H2O2 were investigated. After that, DADS:HAc (2:1, 2:2, and 2:3), DADS:H2O2 (2:8, 2:10 or 2:15), and reaction time (6, 8, and 10 h) were used for optimization using response surface design. Allicin production is used as a response value to optimize independent variables (factors).
Isolate allicin using HSCCC system
HSCCC separation was performed using the TBE-300C instrument (Tauto Biotech, Shanghai, China) with a 300 mL multilayer helical tube (1.9 mm inner diameter) and a 20 mL sample quantification loop. The solvent was pumped using the TBP-5002 constant flow pump. Continuously monitor the effluent at 280 nm using the 8823B-UV detector (Beijing BINTA Instrument Technology Co., Ltd., Beijing, China). Record chromatograms using a portable recorder Model 3057-11 (Chongqing Instrument Automation Co., Ltd., Chongqing, China).
The four-phase solvent system (Hexane:ethyl acetate:methanol:H2O = 2:0.5:2:0.5) was determined with suitable partition coefficient (KD) as described in our previous report34. For each separation, the upper phase (stationary phase) is first filled into the separation column at a flow rate of 20.0 mL/min. The device was then rotated forward at 800 rpm while the lower phase was pumped through the column as the mobile phase at a flow rate of 5.0 mL/min. After the hydrodynamic balance system is established, the sample solution was injected through the sample port (500 mg crude allicin dissolved in 4 mL lower phase and 4 mL upper phase). The separation process was maintained at 25 °C. Wastewater was continuously monitored at 254 nm with a UV detector and collected manually according to HSCCC chromatograms. Identification of compounds is referenced by commercial standards and previously prepared compounds in laboratories.
HPLC analysis of organosulfur compounds
The content of allicin and its degradations were determined according to our previous study2, with high performance liquid chromatography applied. A Shimadzu LC-20AT HPLC system with an InertSustain C18 column (4.6 mm × 250 mm, 5 μm i.d.) were applied. The HPLC conditions were as follows: mobile phase, ACN:water:MeOH (50:41:9, v/v/v); flow rate, 1.0 mL/min; column temperature, 25 °C; injection volume, 10 μL; and detector wavelength, 254 nm. Peak identification and quantification of individual compounds were conducted using the reference standards.
Effect of temperature on allicin stability
For stability analysis of allicin as a function of temperature, synthetic allicin in aqueous solution was diluted to concentration of 20.00 mg L−1 as mentioned above. Samples were stored at 45, 55, 65, 75, 85, 95 °C, respectively. The stability of allicin was evaluated for 30–1200 min.
Effect of concentration on allicin stability
For stability analysis of allicin as a function of concentration, the stability of allicin was evaluated in a series of concentration gradients allicin aqueous solutions including 3.25, 7.5, 15, 30, 60 mmol L−1 at 25 °C water bath for 40 d.
Allicin degradation kinetics
The effect of temperature and concentration on allicin retention (CA) was calculated by Eq. (2); Allicin degradation kinetics during storage was evaluated by using first-order reaction kinetic model (Eq. (3)) and two first-order reaction kinetic model (Eq. (4)).
where C0 represents allicin concentration (mg L−1) at the initial time and Ct represents the allicin concentration (mg L−1) at t moment. k, k1, k2 are rate constants (d−1 in concentration experiment; min−1 in temperature experiment); a1 and a2 represent the pre-exponential factors of different degradation reactions, and t is the storage time (d−1 and h−1).
Effect of pH on allicin stability
To investigate the effect of pH on allicin stability, allicin solution with pH ranging from 2.0 to 10.0 were prepared using phosphate buffers. The garlic paste was prepared by blending garlic cloves with deionized water (1:2, m:v), the pH of garlic paste was adjusted from 3.0 to 9.0, the original pH of garlic paste was 5.8. The contents of allicin, (E/Z)-ajoene, VDTs, DADS, and DATS were evaluated after stored at 25 °C for 48 h.
Effect of polyphenols and their analogues on allicin stability
The peroxidase and polyphenols were extracted following the method described in our previous report2. The apigenin, myricetin and quercetin were prepared at a concentration of 10 mmol L−1. 0.1 mL of peroxidase was added to 2.0 mL flavonoid solution to oxidized to quinoid type at 25 °C for 150 min. The polyphenols and their analogues was mixed with equal volumes of allicin and placed at 40 °C water bath for 4 h.
Effect of amino acids on allicin stability
Synthetic allicin solution was diluted to concentration of 20.00 mg L−1, and 18 kinds of amino acid solutions at a concentration of 10.00 mmol L−1 were prepared. Allicin mixed with different amino acids by equal volumes and placed at 40 °C water bath for 4 h.
Statistical analysis
All experiments were repeated at least three times, and all data was performed as the mean. Effect of environmental conditions and endogenous substance on the stability of allicin were analyzed by the one-way ANOVA test using data analysis software SPSS (PASW statistics 18). Data were analyzed using Duncan’s test and a statistically significant difference was considered as a P-value <0.05.
Responses