Evaluation of electrical impedance spectroscopy of bovine eyes for early detection of uveal melanoma

Introduction
Uveal melanoma (UM) is an aggressive intraocular cancer associated with a high mortality rate due to distant metastases as well as significant risk for permanent vision loss1,2. UM is the most common primary intraocular malignancy in adults, accounting for 85–95% of all ocular melanoma cases3,4. Primary treatment with radiation is an option for the majority of UM patients, with the exception of patients with very large tumors which require surgical removal of the eye with enucleation1,5. In addition to increased risk for severe vision loss due to radiation or loss of the eye because of increased tumor size, larger UMs are associated with higher risk for metastatic spread6,7. There is currently no curative therapy for metastatic UM8,9. Therefore, it is beneficial to detect and treat UM tumors early to preserve the globe, maximize vision outcomes, and improve survival.
UM tumors may develop in the iris, ciliary body, and choroid of the uveal tract10,11. Tumors in the iris can be directly visualized by the patient or family members as well as by general health care providers and are lower risk for metastasis than posterior tumors arising in the ciliary body or choroid. In contrast, ciliary body and choroidal tumors are more challenging to detect, requiring specialized examination or imaging techniques, and are more aggressive malignancies with higher risk for metastasis. Due to their anatomical location behind the iris, small ciliary body tumors in particular, often require specialized ophthalmic ultrasound for detection11,12. Routine dilated fundus examinations can detect choroidal tumors and large ciliary body tumors, but few individuals undergo routine examinations of this kind in the absence of symptoms, which are a late sign in most UM patients. Therefore, a need exists for an accessible method that can be used by patients, primary care doctors and general eye care providers to enable early detection of ciliary body and choroidal UM tumors.
Clinical studies and experiments have shown that impedance of cancerous tumors is different than healthy tissues in the absence of the tumors13,14,15,16,17. Cancer affects the cell surface charge, transmembrane potential, ionic concentrations, and cell membrane permeability14,16,18. Cancerous tissues have different morphology, cell density, cell shapes and blood flow than normal tissues. This changes the capacitance of the cell membrane and the conductivity of intracellular fluid (ICF) and extracellular fluid (ECF), and thus, modifies the electrical impedance of the tissues. For instance, electrical impedance spectroscopy (EIS) was used successfully to distinguish healthy tissues on skin from cutaneous melanoma13,15.
Here, we evaluated the capabilities of EIS for detection of abnormalities and simulated intraocular masses in the uveal tract. We investigated if the presence of inserted tissue induces a change in the electrical properties of the bovine eye ex vivo. Our finite element analysis (FEA) showed that by placing probes on the cornea, the electrical current flows to the lens and ciliary body as well as the retina and choroid. In addition, FEA showed that the presence of a tumor in uveal tract significantly changed the current density in the eye models. We also used an electrical impedance analyzer to measure the impedance of bovine eyes ex vivo and determine if the presence of an additional tissue mass (simulating presence of intraocular tumor) in the globe was associated with any abnormalities in the electrical impedance. This work is a preliminary study in investigating the utility of measuring the electrical properties of eyes to identify abnormalities connected to additional tissues with tumor-like features in bovine eyes.
Results
In the following subsections, we described the mechanism of UM detection with EIS, investigated electrode placement on cornea and change of impedance due to presence of inserted tissues in eye. We analyzed the presence of UM in the eye models through FEA and measured impedance of bovine eyes with inserted tissues.
Detection of uveal melanoma with EIS
To identify abnormalities associated with UM, we measure the impedance of both eyes where the fellow eye is used as reference. The results from the eye suspicious to UM is compared with the impedance from reference eye, knowing that bilateral UM is exceedingly rare (1 in 50 million)19; therefore, the abnormal differences in the impedance of eyes may relate to the risk of UM. The presence of tumor in one eye induces changes in the impedance of the organ and affects the impedance difference between two eyes.
We created a computer model of eye with two electrodes on the cornea to analyze the electrical current density and impedance of the eyes. The current flowed from one electrode to the cornea and from cornea to lens, vitreous humor (VH), and uveal tract to measure the impedance of the organ as illustrated in Fig. 1a. The presence of a tumor changes the current density and affects the overall impedance of the eye. To study the electrical properties of eyes, we used the equivalent circuit of tissues, composed of cells and ECF with complex electrical impedance (ZL) as shown in Fig. 1b. The resistance of ICF (RIC) and ECF (REC) contribute to the resistance of the circuit, and the cell membranes form a bioelectric capacitance responsible for electrical reactance of the tissue20,21. The capacitive reactance XCM is inversely proportional to the frequency XCM = 1/ (2π f CCM); therefore, high frequency currents pass through the ECF, cell membranes, and ICF22. The capacitors around the cells exhibit large reactance at low frequencies, and as a result, low-frequency currents mostly do not enter cells and pass through ECF with resistance REC. Figure S1 shows the FEA of current density at very low frequencies in a healthy eye, in which the current mostly flowed through the cornea and a small portion of current (<7%) reached the choroid and retina in the back of the eye.

a Two electrodes are placed on cornea and the current flows from electrode to the eye and return to the second electrode. The presence of tumors in uveal tract changed the current density in eye, b The equivalent circuit of the biological tissues. Low frequency currents flow through intercellular fluid and high frequency currents flow through cells.
Analysis of electrodes and tumors on current density in eye models
The impedance of the eyes can be measured noninvasively through two corneal electrodes. The shape of electrodes and method of connection to the surface of cornea (needle inserted into cornea or a flat electrode placed on surface) affected the current density on the electrode and resultant impedance. In addition, the presence of tumors changes current density in the eye due to higher conductivity than healthy tissues15,20,23,24. We used FEA to investigate the electrical current density in the eye models and simulate the impact of probe shape and presence of tumors in the ciliary body and choroid as shown in Fig. 2. We analyzed two configurations of probes: flat probes with an annulus shape fabricated on a contact lens, and a needle probe with solid circular shape inserted into cornea of the eye models. The current densities in tumors in the ciliary body were lower for the contact lens than the needle probe inserted into cornea. Figure 2a shows that current in a 0.6-mm diameter tumor was approximately 65% lower for the contact lens. The current density in a 6-mm diameter tumor in the ciliary body was 55% lower for the flat probes on contact lens. Figure 2b shows the current distribution in the cornea near the location of the probe and contact lens. The current density is more localized for the probe while the maximum current in the cornea is 40% higher for the contact lens. The data show the shape and method of placement of electrodes on cornea affected the current distribution in the eye and in the tumor. Figure 2c shows that presence of a 0.6 mm diameter tumor in the ciliary body shifted the maximum current density from the center of the eye lens to tumor. Due to higher conductivity of the tumor, the maximum current density is 40% higher in the tumor compared to a healthy eye without tumor. Figure 2d shows that presence of a 4-mm diameter tumor in the anterior choroid near the ciliary body also changed the current distributions in the eye model. The maximum current is in the vitreous humor near the center of the lens in a healthy eye while the maximum current density shifted to the tumor in choroid and was roughly increased by 50% in the eye with melanoma in this model. The data from this modeling experiment demonstrates that the presence of either a ciliary body or choroidal location significantly changed the current distribution in the eye.
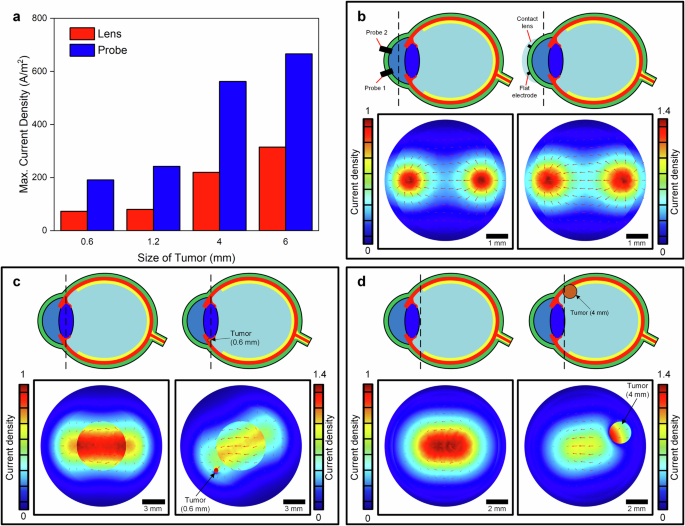
a Effect of probe on maximum current density in 0.6, 1.2, 4 and 6-mm diameter tumors in the ciliary body. A flat electrode on a contact lens injects lower current into the tumors than a solid circular probe inserted into cornea, (b) current density distribution in cornea for flat electrode on a contact lens and solid probe, (c) current density in a healthy eye and an eye with a 0.6-mm diameter in ciliary body, (d) current density in a health eye and an eye with 4-mm diameter tumor in choroid.
Electrical impedance of bovine eyes and specificity of EIS
Bovine eyes have a similar anatomy to human eyes. We measured the impedance of preserved and fresh bovine eyes at a wide range of frequencies from 50 Hz to 500 kHz (Figs. S2–4). Figure 3a, b shows the absolute impedance of fresh eyes measured with needle probes inserted into the cornea and the contact lenses placed on the surface of cornea. The impedance was measured as 250 Ω at 100 kHz with the needle probe with diameter of 0.6 mm. We also measured the impedance of the same eye with two flat annulus probes on the contact lenses as 330 Ω at 100 kHz. At frequencies from 200 to 500 kHz, the difference for two probes was roughly 50 Ω that was 20% higher for the contact lens. The data show the impedance of the eye was measured lower with solid probes across the 500-kHz spectrum with higher differences at lower frequencies (roughly 50% at 10 kHz). The data implies that electrical conductance of needle probes inserted in the cornea is higher due to high-fidelity electrical contact with the eye. The contact lenses have a poor electrical connection with the cornea due to trapped air and fluid. The phase for the needle probes was measured to be −3° at 200 kHz while the contact lens showed the phase of −11°. The negative phase angle shows that the fluid of the eye trapped between contact lens and cornea, and thus increases the capacitance of the connection. We also measured the impedance of preserved and fresh bovine eyes as shown in Fig. 3c, d. The impedance of the fresh eyes was measured 5 h after the animals were slaughtered. The absolute impedance of the preserved eyes was significantly higher (75% increase at 100 kHz) than the impedance of the fresh eyes as measured from cornea with probes. The amount of fluid, blood and water in the fresh eyes contributed to the electrical conductance and reduced the impedance. The phase angle is much lower for the preserved eyes at frequencies below 35 kHz, showing that the equivalent circuit of the eye is more capacitive than fresh eyes. Figure 3e, f shows the impedance of an intact bovine eye changed from 700 Ω at 100 Hz to 300 Ω at 200 kHz. The impedance did not significantly change from 200 kHz to 500 kHz.

a, b Absolute impedance and phase of the eyes with contact lens and probes, (c, d) absolute impedance and phase of preserved and fresh bovine eyes, (e, f) absolute impedance and phase of intact bovine eyes, and eyes with cut and an additional muscle tissue inserted into eyes. g Current measured from different parts of bovine eyes at 10 kHz.
Next, we evaluated how the presence of a simulated intraocular tumor impacted the measured impedance. First, we made a 5-mm incision in the sclera, choroid and retina on the superior aspect of the eye to assess how this intervention changed the impedance. The impedance of the eye with an incision was increased compared to an intact eye (Figs. 3e, f and S5). The increase in impedance was 130 Ω from 50 to 200 kHz since the incision changed the current distribution. This shows an incision in the eye affects the impedance observed from cornea. We then inserted a piece of bovine muscle measuring 5 × 5 × 5 mm through the incision in the eye to simulate an intraocular tumor. Some tissues exhibited tumor-like features and mimic tumors and malignancies. We used muscle tissues that showed some similarities to tumors such as instability, hyperproliferation, resistance to cell death, and invasiveness25,26. The additional tissue mass in the eye reduced the impedance of the eye by 230 Ω across the spectrum (Fig. 3e). The impedance of the eye was 100 Ω lower than the intact eye without incision and additional tissue. In addition, Figs. S6–22 show the impact of incision into eyes and additional tissues on parallel resistance and capacitance of the eye as well as absolute impedance and phase angle. The data shows that the abnormalities including incision and extra tissue change the impedance and can be measured with electrodes on the cornea. Figure 3g shows the current measured at various parts of a fresh eye at 50 kHz when the electrical current is inserted into the eye through two electrodes in the cornea. The current in the middle of the eye was measured approximately 37.5% of the current in the cornea for the intact eye.
To analyze the effect of load from the brain, skull, and tissues on the impedance measurement of the eyes, we conducted an experiment on eyes that remained in place in a bovine head. Figure 4a shows the placement of two contact lenses with electrodes and insertion of two needle probes on the eyes. Absolute impedance and phase were measured for right and left eye to obtain the impedance difference between eyes (Δz) and investigate the effect of an incision and additional tissue in one eye as shown in Fig. 4b, c. The experiments were conducted 5 h after the animal was sacrificed. The incision was in the peripheral cornea. The impedance difference between two intact, natural eyes in the head was 590 kΩ at 1 kHz and phase difference (Δθ) was 0.67° at 20 kHz. When we cut the right eye and added a 60-mm3 piece of bovine muscle to the eye simulating an intraocular tumor, Δz was reduced to 125 kΩ and Δθ was increased to 1.13°. This shows the changes due to additional tissue and incision are detected by comparing the impedance of two eyes. The sensitivity was 7.75 kΩ per 1-mm3 of muscle tissue. Figure 4d, e shows the impedance and phase of eyes 5 and 20 h after slaughter time. The impedance was increased from 120 kΩ after 5 h to 600 kΩ after 20 h at 200 kHz. This is attributed to low level of conducting components such as water, ions, and blood in the aged tissues.

a Placement of contact lenses and probes to measure impedance from cornea, (b, c) absolute impedance and phase of right and left eye in a bovine head. The incision and additional muscle tissues into one eye changed the impedance and phase, (d, e) absolute impedance and phase of eyes 5 and 20 h after slaughter time.
In addition, the FEA results (Fig. S23) show that EIS can distinguish UM from other eye diseases. For instance, UM changed the current density significantly more than glaucoma and retina infection. In addition, our experimental results showed a tear in the sclera and choroid changed the electrical impedance differently than existence of solid tissue in the bovine eye (Figs. 3e, f and 4b, c). This may be contributed to high electrical conductivity of malignant tumors.
Discussion
In this study, we conducted FEA to study the impact of tumors on the electrical properties of eye models and measured the impedance of bovine eyes ex vivo with inserted tissue masses simulating intraocular tumors. Our results showed that the presence of a simulated UM in the eye computer models significantly changed the current distribution and the maximum current density in the globe. In addition, we demonstrated that impedance of the eye was significantly reduced in bovine eyes after insertion of muscle tissue. The experiments showed that the electrical impedance of the globe was impacted by the presence of a scleral incision, as well as with the insertion of the tissue mass. A 5-mm incision increased absolute impedance by 100 Ω. However, after insertion of a 5 × 5 × 5 mm muscle tissue through the incision, the impedance decreased by 100 Ω as compared with an intact bovine eye. In addition, our results showed that the current density is higher in the cornea than VH, choroid and retina in normal eye models. The electrical current was reduced from the cornea to the retina with a factor of 25.6 at 1 kHz. Also, the existence of a tumor with high conductivity changes the current density inside the eye model with high current density occurring in the tumor (FEA).
Our ex vivo experimental data on bovine eyes demonstrate that EIS of eyes has enough sensitivity (7.75 kΩ per 1-mm3 of muscle tissue) to detect abnormalities in the uveal tract. The flat electrodes on the contact lens showed lower current density; however, this technique permits noninvasive measurement of the impedance of eyes. In future, contact lenses with electrodes could be designed to enable noninvasive measurement of eye impedances without causing discomfort. To reduce the effect of natural variability of eyes, the fellow eye will be used as reference (baseline). We will also conduct a clinical trial and measure impedance of both eyes in healthy participants (control) and patients with UM at various stages to find a pattern in impedances as our future work. We will use the clinical data to score eyes and identify abnormalities associated with intraocular tumors. In addition, our FEA showed that electrical signatures of UM can be distinguished from other eye diseases. As future work to improve the specificity, we will test EIS on patients with UM, glaucoma, retina infections, etc. to correlate the EIS of eyes with different eye conditions. We will create an EIS dataset from patients with different eye conditions to train an artificial intelligence (AI) model to identify the risk of UM.
Furthermore, to reduce the effect of eye movement on measurement, the users will consciously focus on a point preventing unnecessary eye movements. We will also use filtering and smart algorithms to separate unwanted signals due to natural motions from impedance data. In this study, the FEA and ex vivo results are preliminary proof of a concept that extra masses inside eyes could be detected by EIS. As our future research, we will conduct animal model and human subject study to evaluate the capability of EIS to identify abnormalities associated with UM in live species.
Methods
Finite element analysis of eye models
We analyzed the impedance, voltage distribution and current density of an eye model implemented in COMSOL Multiphysics® Software. The model consists of various parts of the globe such as sclera, choroid, retina, and vitreous humor. We used bovine eyes for analysis and experiments since their anatomy is similar to human eyes. The bovine eye diameter was 30 mm for simulations, and two probes with a diameter of 0.6 mm were placed on the cornea. Electric currents physics and stationery/dynamic study were used to find the current distribution in the eye. The injected current from the probe was 188 µA for the simulation.
Measurement of bioimpedance of bovine eyes
We used Multifunctional Impedance Analyzer (MFIA) from Zurich Instruments to measure the impedance, parallel capacitance, and resistance across various bovine eyes. To compare the results, the impedance of a bovine eye was measured with MFIA with accuracy of 0.1%. In addition, a voltage was applied to the cornea of the bovine eye. Two probes were inserted into the cornea as the current source and two in the backside near the retina to measure current flow in the back of the eye. The experiments were conducted at 700 mV input voltage. The maximum current density of 1.5 mA/mm2 occurred on the tips of the probes inserted into the cornea. The probes were cylindrical with a radius of 0.2 mm and height of 3 mm. The current density in the middle of the eye in VH with a distance of 15 mm from probes is reduced to 15 µA/mm2.
Measurement of current distribution in bovine eyes
An input voltage of 700 mV was applied to the eyes at frequency range 1 kHz to 100 kHz. Electrical current was measured through a digital multi-meter that measured the input current of 250 µA at 1 kHz and 132.8 µA at 100 kHz flowing through the probes toward cornea. While the current at cornea was 38.5 µA at 1 kHz and 29.8 µA at 100 kHz, the current at retina was as low as 1.5 µA at 1 kHz and 0.317 µA at 100 kHz. Table S1 lists the current measured through a digital multi-meter in different regions of the eye at different frequencies.
Responses