Exploring genetic associations and drug targets for mitochondrial proteins and schizophrenia risk

Introduction
Schizophrenia (SCZ) is a highly heritable psychiatric disorder characterized by profound disturbances in thought, emotion, and behavior, along with significant cognitive impairments1. It is also a high-homogeneity, polygenic mental disorder that affects an estimated 1% of the world’s population2. According to the Global Burden of Disease (GBD) 2019 estimates, the number of schizophrenics has increased from 14.2 million to 23.6 million between 1990 and 20193. It poses a heavy burden and threat to human life and health. However, we remain unclear about the pathomechanisms of schizophrenia, which undoubtedly pose a challenge for the treatment and rehabilitation of the disease.
Mitochondria are key intracellular organelles with functions such as adenosine triphosphate (ATP) production, participation in metabolism, regulation of cell signaling, and apoptosis, which play a crucial role in maintaining cell survival and normal function4. Once mitochondria are dysregulated in the presence of multiple stressors, a variety of biological effects and diseases are triggered, including mitochondrial dysfunction, oxidative stress, inflammation, cell death, metabolic diseases, and psychosomatic disorders5,6,7,8. Accumulating evidence points to dysregulated mitochondrial quality control such as aberrant energy production, abnormal mitochondrial morphology, disturbed mitochondria-associated gene expression, and aberrant cell death, all of which are intimately related to the pathogenic mechanisms of SCZ9,10,11. Nevertheless, the observational studies mentioned above were subject to confounding factors and reverse causality, limiting their ability to provide causal evidence. More convincing evidence still needs to be complemented for the pathogenic mechanism of mitochondrial dysregulation in SCZ.
Cross-sectional studies are known to have a low level of evidence due to their tendency to be susceptible to confounders, reverse causation, and multiple biases. Although cohort studies enable causal inferences to be drawn, they are labor-intensive and resource-intensive. Advancements in genomics have led to the widespread adoption and application of Genome-Wide Association Studies (GWAS) in scientific research. A technique for genetic analysis that was applied on the basis of the results of GWAS analyses was launched namely Mendelian Randomization (MR)12. The MR approach uses genetic variation as instrumental variables (IVs) to determine whether risk factors have a causal effect on outcomes. Since individual genotypes are established at conception, this method effectively prevents reverse causality between genotype and outcome. This ushers in new opportunities to delve into the mitochondrial pathogenesis of SCZ.
Subsequently, to further validate the pathogenic mechanism of mitochondria in SCZ, the present study utilized the GWAS dataset of multi-ethnic SCZ from the Psychiatric Genomics Consortium (PGC) database13 and the GWAS dataset of 66 mitochondrial proteins from the European ancestry cohort14 to comprehensively and objectively assess the causal relationship between mitochondria-related proteins and SCZ.
Methods
Study Design
The study design is illustrated in Fig. 1. We performed a bidirectional Mendelian randomization (MR) study, utilizing single nucleotide polymorphisms (SNPs) as instrumental variables (IVs), to evaluate the causal relationship between mitochondria-associated proteins and SCZ. These SNPs were required to meet three key assumptions: (1) strongly associated with the exposure; (2) influence the outcome solely through their effect on the exposure; and (3) not be correlated with any confounding factors15.
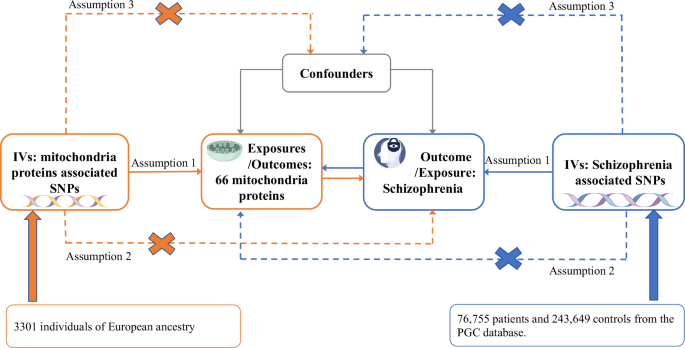
Framework and Hypotheses for a Bidirectional Mendelian Randomization (MR) Study on the Association Between 66 Mitochondrial Proteins and Schizophrenia. IVs instrumental variables, PGC the Psychiatric Genomics Consortium.
Data sources
In this study, we extracted detailed data on 66 mitochondria-associated proteins for 3301 individuals of European ancestry from the INTERVAL cochort14. The aptamer-based multiplex protein assay (SOMAscan) technique was employed to precisely quantify 3622 plasma proteins in these healthy participants16. This analytical method enabled an in-depth examination of the causal relationship between these proteins and schizophrenia. We used GWAS data on SCZ from the PGC database involving 76,755 patients and 243,649 healthy controls of multiethnic ancestry (Europe, East Asian, African American, and Latino). The study adhered to rigorous technical quality control standards across the 90 cohorts included in the PGC primary sample and was analyzed using a two-stage GWAS methodology13. Technical quality control applied to the PGC core cohort involved several measures, including SNP deletion rates < 0.05 (before sample removal), subject deletion rates < 0.02, autosomal heterozygosity bias ( | Fhet | < 0.2), and SNP deletion rates < 0.02 (after sample removal).
Gene-drug interaction
Candidate drug interaction targets were identified by MR analysis of genes related to mitochondrial proteins that are causally associated with schizophrenia. We explored and identified genes related to mitochondria-associated proteins using the GWAS Catalog (https://www.ebi.ac.uk/gwas/). We then investigated the DGIdb to identify and assess potential therapeutic targets and their associated pharmacological mechanisms17.
Statistical analysis
All SNPs used in our MR analyses were identified as independent predictors of exposure through GWAS, with those in linkage disequilibrium (R² < 0.001 within 10 Mb) excluded. The genome-wide significance threshold (P < 5 × 10−8) was met to fulfill the MR hypothesis criteria. When the count of IVs is limited to 10 or fewer, the selection threshold should be set at a p-value of 1 × 10−5. The F-statistic is employed to evaluate the strength of instrumental variables in predicting a particular exposure. It is calculated by averaging multiple F-statistics related to the exposure. Hence, the average F-statistic of SNPs reflects their importance in MR analysis18. A mean F-statistic exceeding 10 indicates the robustness of the SNPs used in the MR analysis.
We assessed individual genetic variants using Wald ratio estimates, which were summarized as the main analysis results using the IVW method. To validate our results, we performed additional analyses using the weighted median (WM), MR-Egger, Simple mode, and Weighted mode methods. The MR-Egger intercept was specifically employed to assess the impact of horizontal pleiotropy19. The Cochran’s Q test was employed to assess the heterogeneity of causal effect estimates for each mitochondria-associated protein across the variants. In our study, we utilized Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis to detect outliers and perform subsequent corrections.
Associations with significance levels below 0.05 were considered strong evidence of causality. We corrected false discovery rates (FDR) for results with significant associations. Statistical analyses were performed using the TwoSampleMR (version 0.5.9) and MRPRESSO (version 1.0) packages within the R software environment (version 4.2.3). Adherence to the STROBE-MR guidelines ensures comprehensive reporting of the study.
Results
Impact of 66 mitochondria-associated proteins on the pathogenicity of SCZ
After excluding SNPs that exhibited linkage disequilibrium and pleiotropic effects, we meticulously evaluated the genetic instruments associated with each of the 66 mitochondria-associated proteins (P < 1*10−5) (Additional Table S1). Using the IVW method, we determined a significant negative causal relationship between the pathogenicity of SCZ and a mitochondria-associated protein, superoxide dismutase (Mn) [SOD] (P = 0.024; FDR = 0.453) (Figs. 2a and 3a; Additional Table S2). Positively causally associated with genetic susceptibility to SCZ were the per-sulfide dioxygenase ETHE1 (ETHE1) (P = 1.26*10−5; FDR = 8.32*10−4), Calcium uptake protein 3 (CALU3) (P = 0.027; FDR = 0.453), and Complement Element 1 Q Subcomponent Binding Protein (C1QBP) (P = 0.008; FDR = 0.274) (Figs. 2a and 3a; Additional Table S3-5). The mean F-statistics of the four mitochondria-associated proteins ranged between 21.69 and 39.81, indicating significant pathogenicity of the SCZ (Additional Table S7). The analyses conducted using IVW, WM, weighted mode, simple mode, and MR-Egger methods strongly indicate that directional pleiotropy is minimal. Moreover, the MR-Egger intercept test indicated minimal horizontal pleiotropy (Additional Table S8).
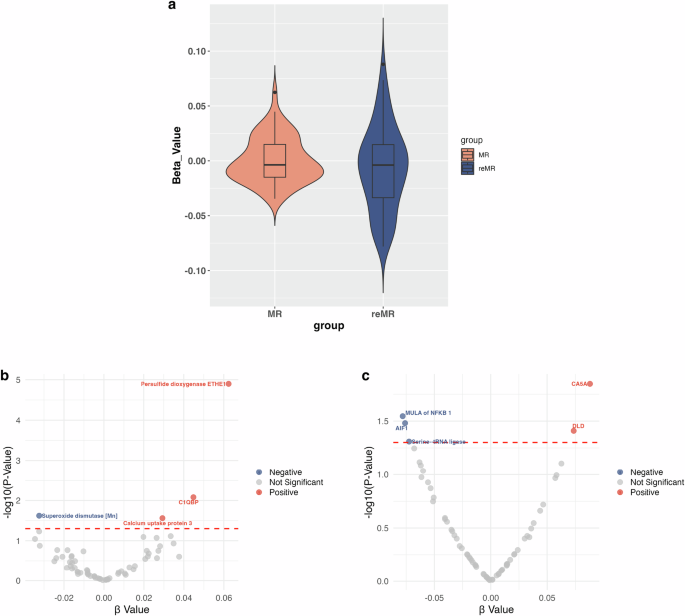
a Violin plot of the MR analysis. The Beta value is used as the Y-axis and groups are used as the X-axis. The distribution of Beta values in the MR group is more concentrated, with values fluctuating between 0.09 and −0.06. Conversely, the reMR group exhibits a broader distribution of Beta values, ranging from 0.13 to −0.12. MR Mendelian randomization, reMR, Reverse Mendelian randomization. b Volcano plot of 66 mitochondria-associated proteins on schizophrenia. c Volcano plot of schizophrenia on 66 mitochondria-associated proteins. AIF1 Apoptosis-inducing factor 1, CA5A Carbonic anhydrase 5A, C1QBP Complement component 1 Q subcomponent-binding protein, DLD Dihydrolipoyl dehydrogenase, MULA Mitochondrial ubiquitin ligase activator, RNMT rRNA methyltransferase 3.
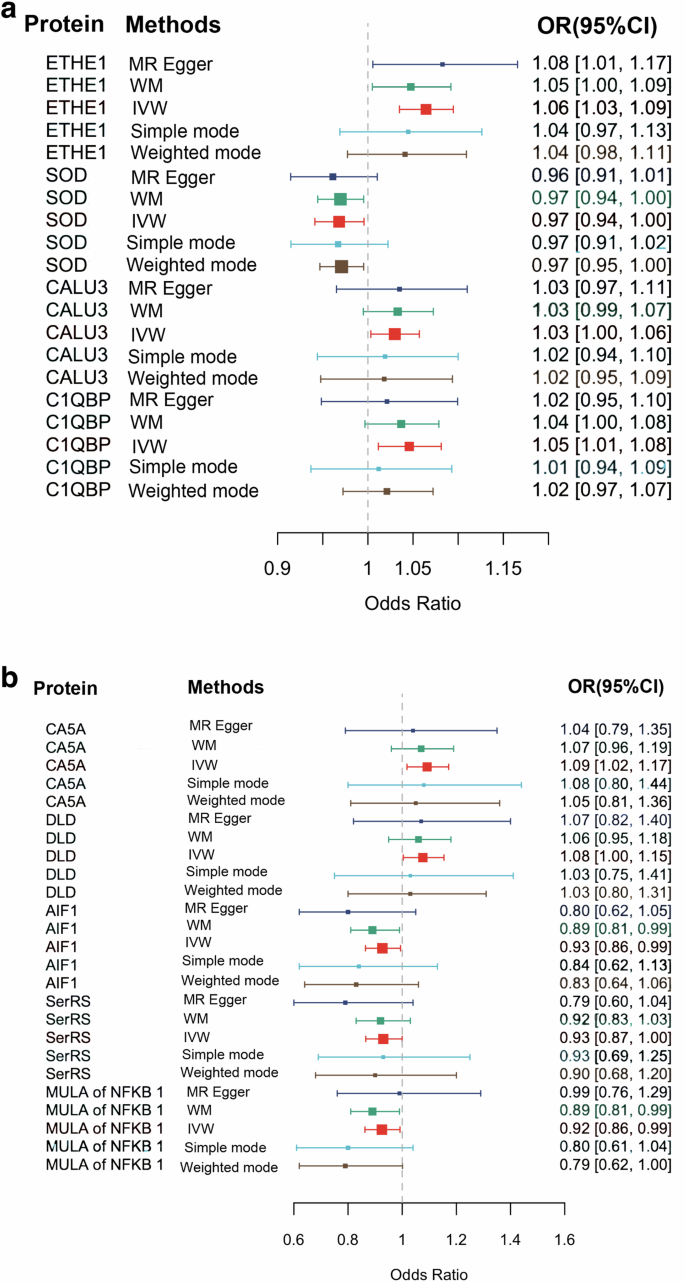
a The effects of four mitochondria-related proteins with significant genetic risk for schizophrenia. b The effects of schizophrenia with significant genetic risk for five mitochondria-related proteins. AIF1 Apoptosis-inducing factor 1, CALU3 Calcium uptake protein 3, CA5A Carbonic anhydrase 5A, C1QBP Complement component 1 Q subcomponent-binding protein, DLD Dihydrolipoyl dehydrogenase, ETHE1 Persulfide dioxygenase ETHE1, MULA Mitochondrial ubiquitin ligase activator, RNMT rRNA methyltransferase 3, SerRS Serine–tRNA ligase, SOD Superoxide dismutase [Mn], IVW inverse variance weighted, WM weighed median, MR Mendelian randomization.
Impact of the pathogenicity of SCZ on 66 mitochondria-associated proteins
A total of 178 SNPs met the criteria for instrumental variables, showing significant associations with SCZ (P < 5 × 10⁻⁸) (Additional Table S6). The IVW method revealed a remarkable negative causal relationship between the pathogenicity of SCZ and three mitochondria-associated proteins: Mitochondrial ubiquitin ligase activator of NFKB1 (MULA of NFKB1) (P = 0.012; FDR = 0.185), Apoptosis-inducing factor 1 (AIF1) (P = 0.006; FDR = 0.185), and Serine—tRNA ligase (SerRS) (P = 0.014; FDR = 0.185) (Figs. 2b and 3b). In addition, the pathogenicity of SCZ had a notable causal effect on two mitochondria-associated proteins including Dihydrolipoyl dehydrogenase (DLD) (P = 0.017; FDR = 0.185) and Carbonic anhydrase 5 A (CA5A) (P = 0.012; FDR = 0.185) (Figs. 2b and 3b). The mean F-statistic for SCZ was recorded as 46.03 (Additional Table S7). The findings from the IVW, WM, Weighted mode, Simple mode, and MR-Egger methods collectively suggest minimal directional pleiotropy. Furthermore, the MR-Egger intercept test indicates that horizontal pleiotropy is negligible (Additional Table S9).
Sensitivity analysis
Sensitivity analyses were crucial, and we reinforced the IVW findings by incorporating results from the WM, Weighted mode, Simple mode, and MR-Egger methods. Cochran’s Q test was utilized to assess heterogeneity, while Egger’s intercept test was employed to determine directional pleiotropy (Additional Table S8 and S9). Forest plots were utilized to depict the causal impact of each SNP related to the four mitochondria-associated proteins on the pathogenicity of SCZ (ETHE1, SOD, CALU3, & C1QBP) (Additional Figs. S1–4). Moreover, the forest plot illustrates the causal effect of each SNP associated with SCZ on five mitochondria-associated proteins (CA5A, DLD, AIF1, SerRS, and MULA of NFKB1) (Additional Fig. S5-9). The leave-one-out analyses revealed that no single genetic variant was responsible for any causal relationships (Additional Fig. S10-18). Scatterplots of SNPs depict the magnitude of the MR effect estimated by each method, highlighting the consistency of direction across the five different approaches (Additional Fig. S19-27). The funnel plot reveals no asymmetry around the blue line (Additional Fig. S28-36). Finally, the MR-PRESSO test did not identify any outliers.
Gene-drug interaction analysis
Through an exploration of the GWAS catalog, we identified nine genes associated with mitochondria-associated proteins including SOD, ETHE1, C1BQP, AIF1, CAC5, CALU3, DLD, SerRS, and MULA of NFKB1. Specifically, nine mapped genes for ETHE1 were identified, including HRG and F12 (Additional Table S10). SOD has two mapped genes including F12 (Additional Table S11). Six mapped genes were found for C1QBP, including C1R, GPLD1, and BCHE (Additional Table S12). CA5A mutates to three genes, including CA5A, CFH, and PON1 (Additional Table S13). MULA of NFKB1 matched six mapped genes, including HRG (Additional Table S14). Only one mapped gene, CFH, was found for CALU3(Additional Table S15). We discovered two AIF1-mapped genes (Additional Table S16), a SerRS-mapped gene (Additional Table S17), and a DLD-mapped gene (Additional Table S18).
A thorough search of the DGIdb database identified HRG, F12, GPLD1, C1R, BCHE, CFH, PON1, and CA5A as promising therapeutic targets. Notably, F12 is targeted by Bortezomib and Antithrombin alfa (Additional Table S20). GPLD1 is targeted by Lonafrnib (Additional Table S21). BCHE is targeted by 24 drugs including Memantine Hydrochloride, Irinotecan Hydrochloride, Immunotoxin, Phenothiazine, and Eptastigmine (Additional Table S23). CFH is targeted by Eculizumab and Meningococcal group B vaccine (Additional Table S24). PON1 is targeted by Simvastatin and Quercetin (Additional Table S25). CA5A is targeted by Sulthiame and Ethoxzolamide (Additional Table S26).
Discussion
For the first time, this bidirectional MR study revealed a potential causal relationship between mitochondria-associated proteins and SCZ pathogenicity from a genetic perspective. The results showed that the levels of ETHE1, CALU3, and C1QBP were positively correlated with SCZ risk, whereas the levels of SOD were inversely correlated with SCZ risk. In addition, SCZ genetic susceptibility showed suggestive evidence of elevated levels of DLD and CA5A and reduced levels of MULA of NFKB1, AIF1, and SerRS. Of importance, the DGIdb data enabled us to identify eight potential targets for pharmacological intervention involving HRG, F12, GPLD1, C1R, BCHE, CFH, PON1, and CA5A.
Superoxide dismutase (SOD) encompasses a group of critical antioxidant enzymes that play a pivotal role in protecting cells from oxidative damage induced by free radicals20. Among the three SOD isoenzymes, manganese superoxide dismutase (MnSOD), or intra-mitochondrial SOD, stands out as the primary antioxidant enzyme crucial for the detoxification of superoxide radicals21. The significant connection between oxidative stress, particularly mediated by SOD, and the pathogenesis of SCZ has been widely explored22,23. There is growing evidence of abnormal SOD activity in the peripheral blood and brain of patients with SCZ24 as well as in the cerebrospinal fluid25. On the other hand, the MnSOD gene is considered a potential linkage region for SCZ26. Notably, the Ala9Val polymorphism in exon 2 indicates that an alanine-to-valine substitution could modify MnSOD activity in the human brain27. Furthermore, the results of the Meta-analysis revealed that reduced MnSOD activity may be associated with the risk of developing chronic SCZ28. Our results provide robust support for these conclusions. Based on gene-drug interaction analysis, we found that the mapped gene F12 of SOD and six drugs. Among the six drugs identified, the clinical use of human C1-esterase inhibitors (C1INH) appears to hold the most promise. C1-esterase inhibitor (C1INH) is a key multifunctional plasma glycoprotein and a major anti-inflammatory protein in circulation, playing a unique role in the regulatory networks of the complement, contact, coagulation, and fibrinolytic systems29. The clinical application of C1INH has proven to be an effective treatment for hereditary angioedema30. Due to its anti-inflammatory properties, the clinical therapeutic potential of C1INH is also being explored for other diseases, including COVID-1931. Targeting the inflammatory mechanisms, C1INH may represent a promising therapeutic approach for SCZ.
An enzyme that acts as an anti-oxidative stress agent is the holosulfide dioxygenase ETHE1, which is a human genetically encoded peroxisulfide dioxygenase32. This enzyme plays a key role in the metabolism of sulfur-containing compounds and contributes to the protection of cells from oxidative damage33. Previous studies have demonstrated that mutations in ETHE1 cause an inborn metabolic defective disorder known as ethylmalonic acid encephalopathy34. Current research in psychiatric disorders has increasingly focused on indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO), which are pivotal enzymes in the catabolism of tryptophan to kynurenine35,36. Kynurenine catabolic metabolites contribute to the pathology of neuropsychiatric disorders, including Parkinson’s disease37, bipolar mania38, and SCZ39, through both neurotoxic and neuroprotective mechanisms. The study confirmed TDO positivity in frontal cortex samples from both schizophrenic patients and controls using RT-PCR quantification and immunohistochemistry40. Recently RCTs have shown that aberrant expression of IDO levels correlates with negative symptoms and pro-inflammatory cytokine levels in first-episode drug-naïve SCZ patients41. This study offers further compelling evidence for the combined pathogenic role of kynurenine metabolism and chronic inflammatory processes in SCZ (FDR < 0.05). However, the relationship between these enzymes and the pathogenic mechanisms of SCZ remains under investigation, and our study points to a new direction for cracking the SCZ treatment challenge.
Ca2+ is known to be one of the most pleiotropic second messengers. Specifically, Ca2+ ions are responsible for decoding a variety of extracellular and intracellular stimuli ranging from endocrine to gene expression, muscle contraction, synaptic transmission, and are also involved in oxidative stress (OS)42. In pathological conditions such as ischemia-reperfusion injury, heart failure, and neurodegenerative diseases, mitochondrial Ca2+ overload occurs and leads to metabolic disturbances, with activation of Ca2+-dependent proteases and excess reactive oxygen species (ROS) production contributing to cell death43. Recent research on first-episode psychiatric disorders has pointed to structural alterations or abnormalities in the number of some mitochondrial ion channels, such as Ca2+, as being associated with increased susceptibility to SCZ44. In animal models of psychosis, mitochondrial dysfunction in Ca²⁺ handling has been observed, potentially resulting in reduced phosphorylation capacity and increased production of hydrogen peroxide (H₂O₂). This impairment of a fundamental mitochondrial function may play a causal role in acute psychotic episodes, including SCZ45. The theoretical hypothesis refers to the fact that in response to environmental changes, Ca²⁺ overload induces intense OS at the cellular level, which is disseminated throughout the body via the mitochondrial electron transport chain, potentially contributing to the onset of an acute episode of SCZ (Fig. 4). Calcium uptake protein 3 (CALU3) is a protein involved in the transmembrane transport of calcium ions in the cell membrane46. The results of the current study are the first to demonstrate that CALU3 is firmly associated with OS in SCZ and to identify HRG and F12 as drug targets. This provides new insights for the forthcoming animal models and clinical studies.
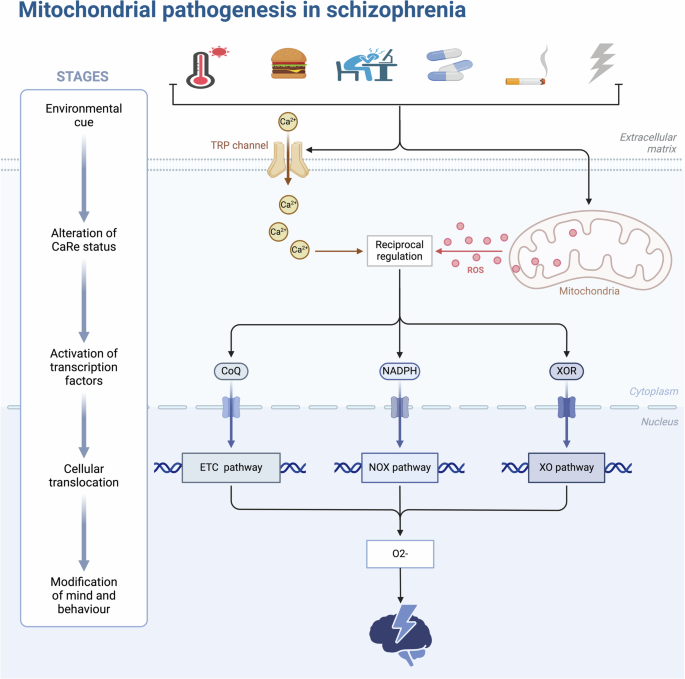
In response to alterations in the external environment (climate change, fatty diet, stress, drug side effects, heavy smoking and alcohol consumption, radiation, etc.), the human body undergoes oxidative stress at the cellular level and releases excessive reactive oxygen species through the mitochondrial electron transport chain, the NADPH magnesium oxide pathway, and the xanthine oxidase pathway, causing psychiatric and behavioral abnormalities. CoQ coenzyme Q, ETC Electron Transport Chain, NOX NADPH Oxidase, ROS Reactive Oxygen Species, XO Xanthine Oxidase. This figure was drawn by BioRender.
Complement C1q binding protein (C1QBP) is a multifunctional protein localized in the mitochondrial matrix that plays an important role in OS47. Mounting studies have revealed that C1QBP exerts pleiotropic effects on many cellular processes, ranging from mitochondrial homeostasis, mitochondrial oxidative phosphorylation, inflammation, and cancer48,49,50. Important pathologic mechanisms in SCZ include OS mechanisms. C1QBP is probably an important hidden fulcrum of the OS pathogenesis of SCZ. In addition, the results of gene–drug interaction analysis indicated that the approved drugs for BCHE, the drug target gene of this protein, include choline esterase inhibitor (ChEI), which undoubtedly also greatly enhanced our confidence to continue the exploration. Several of these ChEI, such as memantine, carboplatin, and tacrine hydrochloride, are commonly used in the clinical treatment of Alzheimer’s disease. Among the 40 drugs identified, Meptazinol, an analgesic, was also noted for its use in pain management51. Animal model research revealed that the novel dual-binding AChEI, Bis(9)-(-)-Meptazinol, significantly improved learning and memory abilities in APP/PS1 mice52. Recent clinical study has shown that multifunctional (-)-Meptazinol serotonin heterodimers alleviate OS-induced apoptotic neuronal death and memory deficits53. Based on its mechanism of action involving OS, we anticipate that Meptazinol may have potential clinical applications in the treatment of SCZ.
Mitochondrial ubiquitin ligase activator NFKB1 (MULA of NFKB1), which is located in the outer mitochondrial membrane, the main function of this protein is to activate ubiquitin ligases on mitochondria, which in turn affects the activity of NFKB1 (nuclear factor-κB1)54. MULA of NFKB1 is implicated in diverse cellular processes such as mitochondrial dynamics, apoptosis, mitochondrial autophagy, innate immune response, and mitochondrial and cellular metabolism55. However, MULA of NFKB1 dysregulation may affect a variety of pathological processes including inflammatory, cardiovascular, and neurological diseases56. Therefore, MUL 1 has been proposed as a potential target for the treatment of mitochondrial dysfunction-related diseases54. Currently, MULA of NFKB1 is recognized as a novel factor in the pathogenesis of inflammation associated with SCZ.
The mitochondria-associated protein AIF (apoptosis-inducing factor) serves as a critical effector of cell death. It is released from mitochondria in response to OS, translocating to the nucleus where it functions as a pro-apoptotic factor57. The majority of the discussion of AIF in previous studies has focused on pathomechanisms including colon, liver, and prostate cancers58,59,60 or among neurological disorders such as toilet sclerosis or Parkinson’s disease58,61. The current findings robustly associate AIF1 with SCZ, thereby enhancing our understanding of its role in the pathogenic mechanism involving OS.
Serine-tRNA ligase (SerRS) is pivotal in protein biosynthesis, facilitating the attachment of serine amino acids to their corresponding tRNA molecules. This process enables tRNA to transport serine to the nascent protein chain during translation. Previous research has shown that SerRS actively competes with c-Myc for the promoter region of anti-vascular endothelial growth factor A in higher vertebrates62. Additionally, SerRS has been observed to directly bind to telomeres, leading to telomere shortening and subsequent induction of senescence in tumor cells63. Therefore, SerRS has a relatively clear future in cancer treatment. Research on SerRS in the context of SCZ remains unexplored, and our findings open new avenues for investigation in this area.
Our study identified another protein involved in OS, dihydrolipoyl dehydrogenase (DLD), which is an enzyme that plays an important role in cellular metabolism, particularly in the tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation in mitochondria. DLD, functioning as an oxidoreductase, is susceptible to oxidative modification by ROS originating from external sources64. Studies on ischemic stroke have confirmed that DLD serves as a source of ROS, and strategies aimed at reducing ROS production during reperfusion have been shown to mitigate tissue damage65,66. In studies of neurodegenerative diseases such as Alzheimer’s disease, evidence has been found for a neuroprotective effect of inhibiting DLD activity67,68. Moreover, it has been reported that down-regulation of DLD by UV light inhibits melanoma through mechanisms involving OS and dysregulated enzyme metabolism69. Our discovery may open a new arena for the application of DLD—the treatment of psychiatric disorders.
Carbonic anhydrases (CA), primarily expressed in the lungs, are enzymes featuring Zn2+ ions at their active sites. They catalyze the conversion of carbon dioxide (CO2) and water into protons (H+) and bicarbonate ions (HCO3–)70. The physiological functions of CA encompass CO2 and ion transport, maintenance of acid-base balance, regulation of respiration, and modulation of cellular metabolism71. The clinical application of the biological activity of human CA activators and/or inhibitors is particularly prominent in anticancer therapy72. Recent research has demonstrated that CA is resistant to ferrocytosis/apoptosis in the hypoxic state of malignant mesothelioma73. The efficacy and safety of novel diaminobenzenesulfonamide antitumor-selective CA IX inhibitors are being explored74. CA inhibitors have also been observed to exhibit neuroprotective effects75. We found quercetin, a flavonoid, as a potential drug targeting PON1, the gene mapped to CA5A. Quercetin is a compound with antioxidant, anti-inflammatory, antibacterial, antiviral, free radical scavenging, and immunomodulatory activities76,77,78. Given its properties, quercetin has shown considerable promise for clinical use in the treatment of neuropsychiatric disorders79,80. Animal model studies of depression have shown that quercetin alleviates depressive-like behavior by inhibiting corticosterone-induced neuroinflammation and oxidative damage in mice81,82. Quercetin nanogel further enriches the treatment of depression83. Similarly, emerging evidence suggests that quercetin has a beneficial effect on the clinical management of SCZ84. Recent findings demonstrated that quercetin attenuates OS and cytokine toxicity, modulates neurotransmission, and ameliorates SCZ psychosis-like symptoms85. CA5A drug target identification unlocks novel potential for its clinical application.
There are also limitations to our research. First, in order to have a sufficiently large number of SNPs for IVs, we relaxed the P threshold to 1 × 10−5 when screening for mitochondria-associated proteins. However, this could potentially increase the risk of weak IVs. Second, the GWAS data for mitochondria-associated proteins are derived from European ancestry, which may limit the generalizability of the study. Third, the number of mitochondria-associated proteins included in this study is relatively small. However, as GWAS techniques improve and more genetic data on mitochondrial proteins become available, this limitation is expected to be addressed. Fourth, the omission of confounder-associated SNPs during the screening process for IVs may have increased the risk of bias. Fifth, our study had limited causal association strength, with only one result passing the FDR correction. Finally, our analysis is constrained by the use of summary statistics from the GWAS, as we lack access to individual-level data. This limitation prevents us from exploring potential non-linear relationships between mitochondrial-related proteins and SCZ pathogenicity.
Conclusion
A comprehensive MR analysis identified four mitochondria-related proteins potentially contributing to SCZ risk, while reverse MR analysis indicated that SCZ may influence the levels of five mitochondria-related proteins. A thorough analysis of the DGIdb database identified eight potential therapeutic targets for SCZ. Further clinical investigations are essential to better elucidate the role of these mitochondria-associated proteins in the pathogenesis of SCZ.




Responses