Exploring ionic liquids and deep eutectic solvents for emerging contaminant removal from water and wastewater

Introduction
In recent years, due to the growing concerns over water pollution and the challenge of emerging contaminants, there has been a marked increase in research aimed at identifying efficient and environmentally friendly materials for water and wastewater treatment1. Ionic liquids (ILs) and deep eutectic solvents (DESs) have emerged as forefront solutions in this area, attributed to their unique properties and adaptability for contaminant removal2. ILs consist of organic heterocyclic cations paired with either organic or inorganic anions. In contrast, DESs are mixtures of different hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD). The adsorption mechanisms for ILs are mainly driven by ionic interactions, mainly Coulomb forces, while for DESs, which are eutectic blends of distinct components, hydrogen bonding predominates3.
ILs are recognized for their remarkable thermal stability, negligible vapor pressure, and excellent solvating capabilities, while DESs offer a cost-effective and simpler alternative due to their formation from HBD and HBA4. Their ability to be precisely tailored to target specific contaminants, is a crucial attribute for the effective removal of a broad range of emerging pollutants, including pharmaceuticals, agrochemicals, endocrine disrupting chemicals, and industrial chemicals from water and wastewater that pose risks to both aquatic ecosystems and human health5,6. The tunability of ILs and DESs allows for maximization of pollutant removal from water and wastewater by optimizing key physicochemical properties to enhance selectivity towards specific contaminants1,7,8. Moreover, their environmentally benign nature aligns with the shift towards sustainable technologies, offering compatibility with existing separation techniques and promising avenues for advancing water and wastewater treatment capabilities9.
This review article is dedicated to examining the cutting-edge applications of ILs and DESs in treating water and wastewater, spotlighting their distinct tunability and efficiency, whether applied independently or as enhancements to existing treatment techniques. The objective here is to shed light on how ILs and DESs improve the effectiveness of adsorption and membrane-based technologies. The article also touches on sustainable attributes and the environmental considerations related to the deployments of ILs and DESs for water and wastewater treatment. The review details a visionary roadmap for the future applications of ILs and DESs in water and wastewater treatment, emphasizing their role in addressing water contamination challenges and shifting treatment paradigms towards sustainability. It highlights the importance of collaborative efforts to explore novel applications, optimize processes, and ensure environmental soundness and safety. By integrating ILs and DESs into existing treatment frameworks and exploring their potential in biological treatment and advanced oxidation processes (AOPs), the review envisions transformative solutions for sustainable water and wastewater management practices.
Tunability of ILs and DESs
The tunability of ILs and DESs, evident in their diverse physicochemical properties, features the potential of tailored design for water and wastewater treatment. This tunability enables precise adjustments in viscosity, polarity, and hydrophobicity, offering a versatile and task-specific resource for contaminant capture in water and wastewater particularly favoring hydrophobic DESs (HDES) for enhanced separation efficiency after treatment10. The two most commonly reported types of HDES are ionic-HDES and non-ionic HDES11. These HDES are composed of long-alkyl-chain quaternary salts or unsaturated alcohols as HBA, combined with fatty acids or carboxylic acids as HBD (Fig. 1a). The design basis of the aforementioned HDES lies in the Kamlet-Taft solvatochromic parameters in which π indicates dipolarity/polarizability, α indicates hydrogen bond donating ability/acidity, and β indicates hydrogen bond accepting ability/basicity of the solvents12. As illustrated in Fig. 1b, the alkyl chain length of DESs can influence the solvatochromic parameters of the HDES, in addition to the structure and mole fraction of the HBA. For instance, DL-menthol exhibits higher hydrogen bond acidity (1.56–1.79) compared to thymol (1.05–1.13)13.
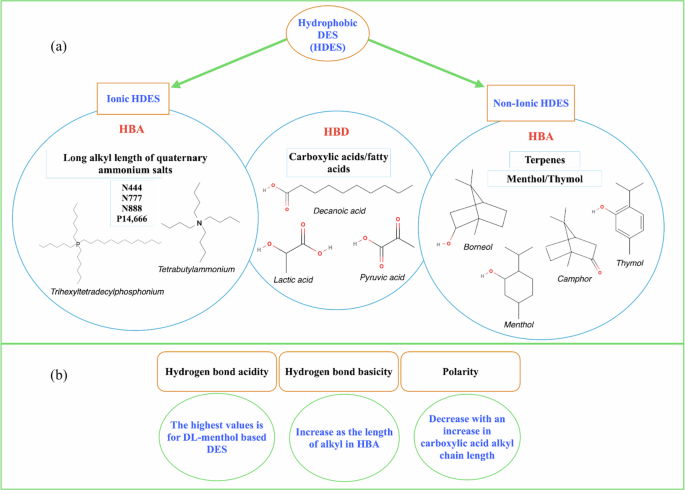
a Tunability of the ionic and non-ionic HDES based on HBD and HBAs, tetrabutylammonium (N444), trioctylmethylammonium (N777), trihexyltetradecylammonium (N888), and trihexyltetradecylphosphonium (P14,666), b Tunability of HDES based on Kamlet-Taft solvatochromic parameter. Synthesized from13.
The customizability of ILs and DESs is showcased in two key areas. Firstly, their physicochemical properties can be tuned to make them hydrophobic, thereby making them tailored adsorbents for targeted emerging contaminants such as pharmaceuticals, agrochemicals, endocrine disrupting chemicals, and industrial chemicals (Fig. 2)11,14,15,16,17,18,19. Secondly, they serve as versatile agents for enhancing other treatment methods, including adsorption and membrane technologies, by enhancing the system’s overall efficiency and specificity. ILs and DESs optimize adsorption processes through tailored molecular interactions, enhancing selectivity and efficiency in contaminant removal20,21,22. For example, in membrane technology ILs and DESs can be utilized as components for manufacturing membranes to enhance their stability and their antimicrobial properties or they can be utilized as surface modifiers to improve the selectivity23,24. This dual capability establishes ILs and DESs as promising components for developing advanced treatment strategies. The capability also positions ILs and DESs as standalone solutions and components enhancing the efficacy of water and wastewater treatment processes.
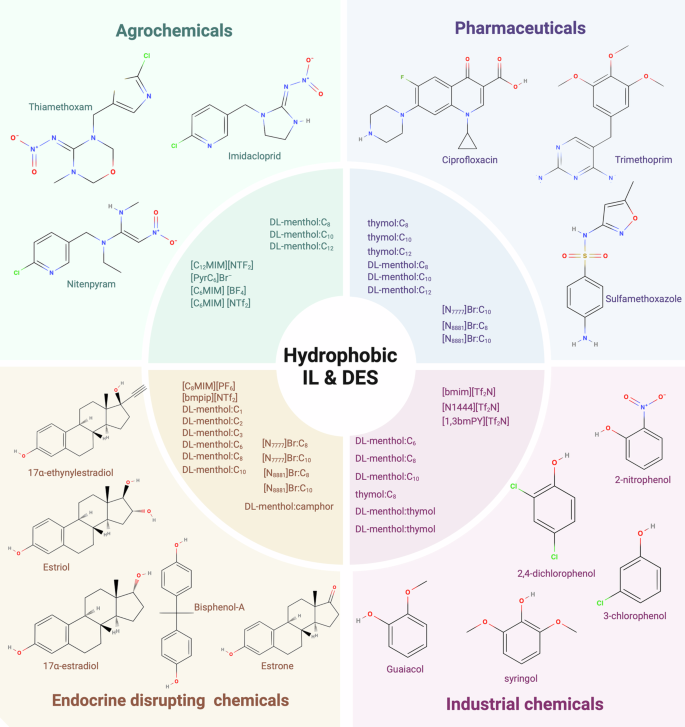
DL-menthol and hexanoic acid (DL-menthol:C6), DL-menthol and octanoic acid (DL-menthol:C8), DL-menthol and decanoic acid (DL-menthol:C10), DL-menthol and dodecanoic acid (DL-menthol:C12), DL-menthol and camphor (DL-menthol:camphor), DL-menthol and thymol (DL-menthol:thymol), thymol and hexanoic acid (thymol:C6), thymol and octanoic acid (thymol:C8), thymol and decanoic acid (thymol:C10), thymol and dodecanoic acid (thymol:C12), tetrabutylammonium bromide and octanoic acid ([N7777]Br:C8), tetraoctylammonium bromide and octanoic acid ([N8881]Br:C8), tetraoctylammonium bromide and decanoic acid ([N8881]Br:C10), 1-octyl-3-methylimidazoliumhexafluorophosphate([C8MIM][PF6]), 1-butyl-1-methylpiperidiniumbis(trifluoromethylsulfonyl)imide([bmpip][NTf2]), 1-butyl-3-methylimidazoliumbis(trifluoromethylsulfonyl)imide([bmim][Tf2N]), tetrabutylammoniumbis(trifluoromethylsulfonyl)imide([N1444][Tf2N]), 1,3-bis(methylpyridinium)bis(trifluoromethylsulfonyl)imide([1,3-bmPY][Tf2N]), 1-heptyl-3-methylimidazoliumbis(trifluoromethylsulfonyl)imide([C7MIM][NTf2]), N-butylpyridinium bromide([PyrC4]Br),1-hexyl-3-methylimidazoliumtetrafluoroborate([C6MIM][BF4]), 1-octyl-3-methylimidazoliumbis(trifluoromethylsulfonyl)imide ([C8MIM][NTf2]). Synthesized from refs. 11,14,15,16,17,18,19.
The vast array of ILs formulations, encompassing various cation and anion pairings, extends an arsenal for tackling environmental challenges. These ILs are engineered to engage specific interactions, such as electrostatic attractions, hydrogen bonding, and van der Waals forces, to isolate and remove a wide array of water contaminants, particularly challenging substances such as heavy metals and persistent organic pollutants25,26. The diversity of ILs and DES formulations, including amino acid, imidazolium, carboxylic acid, sugar and sugar alcohol, and pyridine based ILs and DESs, provides a broad toolkit for contaminant removal27. ILs and DESs harness distinct mechanisms for contaminant capture ranging from hydrogen bonding and dipole-dipole interactions to π-π interactions facilitating the removal of both hydrophilic and hydrophobic contaminants, including highly soluble, short-chained substances such as perfluoroalkyl and polyfluoroalkyl substances (PFAS)28.
Leveraging the intrinsic tunability of ILs, one can introduce functional groups such as −NH2 for enhanced water solubility or −CF3 for targeting non-polar contaminants, thereby optimizing the materials for specific treatment tasks. Amino-based ILs (includes −NH2) exhibit strong hydrogen bond basicity (β between 0.88 and 1.38), which enhances their ability to dissolve polar compounds, including biopolymers, due to their high polarity and hydrogen bond accepting capabilities. In contrast, trifluoromethanesulfonyl (Tf2N) groups (includes −CF3) increase hydrophobicity, making ILs effective for the removal of non-polar contaminants through their low hydrogen bond acidity and high dipolarity/polarizability (π), which facilitates interactions with non-polar substances29,30. Specific contaminants removed include phenolic compounds and dyes using amino-functionalized ILs, and heavy metals and organic pollutants using Tf2N-functionalized ILs. For instance, the composite Fe3O4-NIL-DAS@lac, which includes amino groups, has demonstrated effective removal of phenol, 4-chlorophenol, and 2,4-dichlorophenol from water through electrostatic interactions and hydrogen bonding. Similarly, the ionic liquid [HMIM][Tf2N], functionalized with trifluoromethanesulfonyl groups, has been shown to effectively remove chromium(VI) due to its hydrophobic nature and low hydrogen bond acidity, which facilitates the adsorption of heavy metal ions from aqueous solutions4.
Building on the inherent tunability of DESs for tailored environmental applications, functional enhancements are further realized through the strategic incorporation of specific functional groups. These groups, such as -COOH and -OH for hydrophilic modifications, significantly improve the water compatibility and dispersion of materials such as multi-walled carbon nanotubes (CNTs) and phenolic resins, which are essential for applications in contaminant removal from water and wastewater. Adding -Si-O-Si- group further amplifies materials’ affinity for water, utilizing hydrogen bonding and intermolecular interactions to ensure optimal dispersion31. Conversely, introducing hydrophobic functional groups, including -C=O and -COOC-, enhances the affinity of materials such as nanofibers and metal-organic frameworks towards non-polar contaminants, thereby improving their efficiency in removing such contaminants32,33. The modulation of DES viscosity and basicity achieved through adjustments in water content and temperature or by introducing basicity-enhancing agents such as guanidine (Gu), expands the utility of DESs, particularly in water and wastewater treatment. Gu, due to the presence of an imino group (=NH), and higher basicity relative to urea, exemplifies the chemical versatility of DESs, enhancing solubility in CO2 and offering precise control over chemical properties for specialized uses34.
When integrated into the pyrolysis for the production of adsorbents, ILs or DESs introduce varied functional groups. This modification shifts the materials’ affinity towards water or oil, enabling the targeted removal of particular pollutants. IL-enhanced CNTs incorporate oxygen-containing functional groups like -OH, -COO, and -CONH2, which boost the adsorbents’ hydrophilicity and affinity for water, thereby significantly enhancing their ability to remove heavy metals35. This enhancement in hydrophilicity complements the modifications where ILs impart positive charges to the surfaces of CNTs, optimizing their effectiveness in targeting specific contaminants. The positively charged surfaces of IL-modified CNTs have been shown to interact favorably with the anionic form of sulfamethoxazole, leading to improved removal efficiencies through electrostatic interactions36.
Building on the advancements achieved through IL-modified adsorbents, recent explorations into DES-modified adsorbents have further expanded the potential applications and efficiencies of adsorbent materials. Recent studies have demonstrated the enhanced adsorption capabilities of activated carbon when modified with DES, noting a significant increase in adsorption capacity due to the DES-induced expansion in surface area and pore volume37. Expanding the scope of DES applications, formulations with menthol, thymol, and various fatty acids have shown substantial potential in extracting phenolic compounds from water, with efficiencies exceeding 80%38. A group of researchers has effectively used hydrophobic DESs to extract pesticides from aqueous solutions, achieving high extraction efficiencies39. However, a critical aspect lies in understanding the long-term implications of these modifications, considering factors such as environmental persistence and potential shifts in toxicity profiles. Introducing functional groups within ILs that specifically target emerging contaminants or incorporating renewable resources in DES formulations such as natural DESs (NADES) could pave the way for a more sustainable paradigm.
Eco-friendliness
ILs and DESs stand out for their eco-friendliness, encompassing inherent green properties, the potential for biobased synthesis, and efficient recoverability after use. The inherent green properties of ILs and DESs are reflected by their low vapor pressure, which enhances their safety in handling and minimizes the potential for air pollution. Their exceptional fire safety characteristics contribute to reduced risks of degradation and flammation, reinforcing their environmental safety40,41. Based on life cycle assessment studies, ILs and DES are considered greener alternatives due to their lower environmental impacts, high efficiency in specific applications, and reduced global warming potential compared to many conventional solvents42,43,44. Furthermore, their synthesis from biobased/renewable feedstocks aligns with sustainable practices, making them valuable in environmental applications. However, deviating from these practices and choosing the wrong materials for IL and DES tuning and synthesis can also make them unsustainable and toxic.
There is a stark contrast between sustainable and unsustainable practices in the production and tuning of ILs and DESs. On the eco-friendly side, there are components like short-chain molecules that decompose easily, dual positively charged di-cationic species that could reduce toxicity, and naturally occurring carboxylate anions that are environmentally benign45. The selection of these eco-friendly cations and anions for the production and tunability practices can make ILs less toxic and increase their biodegradability. These practices were demonstrated in a study by Montalbán et al., which showed that shortening alkyl side chains and avoiding fluorinated anions lessen aquatic toxicity, making ILs safer for aquatic environments45. Making solvents more eco-friendly through careful tuning and material selection has also proven to be true for DESs. A study showed that through altering the choice of HBD and HBA, adjusting the mole ratio and concentration of components, and introducing specific organic solvents, the toxicity of DESs was reduced46.
The tunability of ILs and DESs, along with their eco-friendliness, makes them more efficient in water and wastewater applications. For example, recent advancements have introduced room-temperature ILs synthesized with high-spin d5 iron (III), enabling their easy separation from mixtures through an external magnetic field for reuse, thereby enhancing their eco-friendliness by reducing waste47. On the other hand, the synthesis of HDES has made it possible to remove non-polar contaminants from water and wastewater. Moreover, their hydrophobic nature facilitates the separation of DESs from water, minimizing waste and enhancing environmental friendliness48.
Organic components and biomaterial feedstocks can be used for synthesizing ILs and DESs. These approaches use naturally occurring organic materials, which reduce the cost and environmental footprint of ILs and DESs. These material selection practices collectively support a sustainable approach in manufacturing ILs and DESs, aiming not only to reduce their toxicity but also to minimize their environmental impact. For example, using naturally occurring components such as choline and methoxy-choline for IL synthesis enables those ILs to biodegrade within 5 to 10 days49. Similarly, DESs synthesized from choline chloride and urea exhibited reduced environmental impacts50. The development of biobased ILs and DES from renewable resources like amino acids, lignin, and glucose highlights a shift towards more sustainable, non-petroleum-based chemical processes, offering an eco-friendly alternative for various applications, including water and wastewater treatment51,52. These advancements collectively support the significant eco-friendly potential of ILs and DESs, positioning them as a valuable asset in the water and wastewater treatment applications.
Deviating from sustainable practices for producing ILs and DESs can result in toxic and unsustainable conditions. The unsustainable side is characterized by long-chain molecules that are stubborn in the environment, single positively charged toxic mono-cationic species, and environmentally persistent fluorinated anions53. Using these unsustainable cations and anions to produce and tune ILs will make them toxic and persistent in the environment. Moreover, the unsustainable side also shows the use of hazardous substances and non-renewable petroleum-based feedstocks to synthesize ILs and DESs. The use of these hazardous and non-renewable materials for IL and DES synthesis signifies a detrimental cycle that potentially leads to increased environmental toxicity and pollution. Figure 3 shows 3 different pathways for synthesizing natural and eco-friendly DES. Figure 3a shows how acetic acid and menthol in 1:1 molar ratio for 10 min at 600 rpm can produce natural menthol-acetic acid DES54. Figure 3b illustrates that citric acid and xylitol can be homogenized through vortex, followed by treatment in an ultrasonic bath for 30 min and repeating the cycle 2 times can produce xylitol-citric acid NADES. Figure 3c presents how xylitol and malic acid can be vortexed and treated in a microwave reactor to produce xylitol-malic acid NADES55.
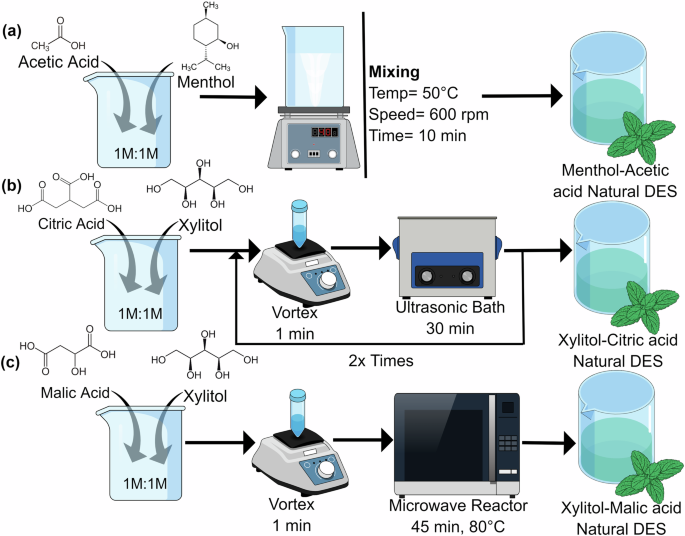
a Menthol-acetic acid, b Xylitol-citric acid, c Xylitol-malic acid. Synthesized from refs. 54,55.
Current applications of ILs and DESs
Membranes
Membrane-based technologies have emerged as a promising approach for pollutant removal in water and wastewater treatment and reuse, owing to their remarkable capabilities and straightforward operation56. However, it encounters challenges such as balancing flux and selectivity, reduced permeability, and susceptibility to organic fouling and biofouling57,58,59. To overcome these limitations, ongoing efforts focus on optimizing additives, improving mechanical properties, enhancing selectivity and permeability, and introducing antimicrobial and antibiofouling properties. In recent years, research on ILs and DESs has aimed to enhance membrane structures and mechanical properties and achieve specific selectivity and permeability for membrane applications. ILs play a versatile role in membrane fabrication, serving as components for various membrane types, including bulk ILs membranes (BILMs), emulsion IL membranes (EILMs), supported ILs membranes (SILMs), and poly ILs membranes (PILMs)60. In addition, both ILs and DESs can be utilized as physical additives or chemical modifiers to enhance membrane properties such as ion conductivity, minimal mass transport resistance, cost efficiency, effective separation, biodegradability, and chemical and thermal stability61.
ILs and DESs offer highly tunable properties, enabling the design of task-specific membranes. However, several factors influence their efficiency in membrane applications. For instance, while SILMs provide high selectivity and minimal solvent retention, ensuring membrane stability during prolonged and large-scale operations presents a significant challenge. The membrane performance and stability decline may be attributed to factors such as ILs’ viscosity or the immobilization process within the membrane’s pores62. ILs can be stabilized within a polymeric membrane using surface-grafting and filler-grafting techniques to mitigate such limitations. The electrostatic properties of ILs enable engagement with polymers via hydrogen bonding, van der Waals interactions, and coulombic forces, making them ideal additives for enhancing various membrane properties through surface or filler grafting63.
To address mechanical stability issues, membranes made from polyelectrolyte polymers, known as PILMs, have been developed. Poly ILs contain repeating units with either a cation or an anion, synthesized from ILs monomers and showing acceptable selectivity for metal ions. Incorporating multi-cation side chains on polyamide-based membranes (Fig. 4), is believed to enhance surface charge properties and strengthen stability62, addressing concerns such as the weakening or counterbalancing of membrane electro positivity by hydrolyzed acyl chloride groups from electrolyte monomers with low charge density, which may result in inefficient ion separation24.
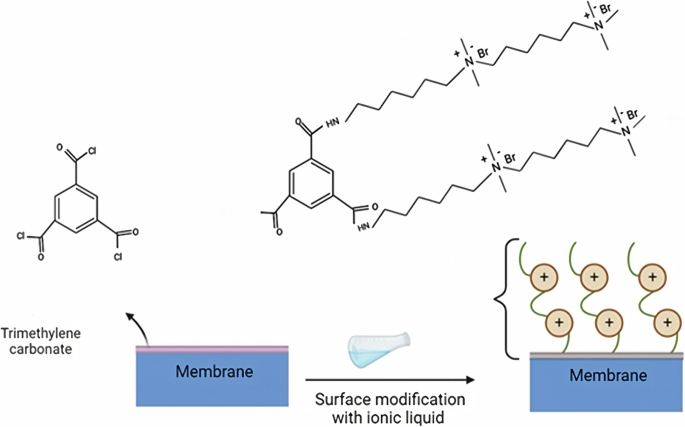
The polyamide-based membrane is modified with multi-cation side chains, represented by orange circles with positive charges and green curved lines. These side chains, synthesized from ILs monomers, enhance the membrane’s mechanical stability. Synthesized from67.
In some cases, increasing the number of cationic groups within the molecules of an ILs can lead to higher viscosity for tri-cationic variations compared to di-cationic ILs. The heightened viscosity observed in tri cationic ILs, particularly those with a short alkyl spacer, arises from increased inter-ionic coulombic interactions. As a result, it boosts van der Waals interactions while diminishing the rotational freedom of the cation, leading to larger and less flexible structures compared to di-cationic moieties64.
Modifying membranes with DESs has been shown to reduce thickness, thereby shortening transport distances and resistance for water molecules65. Moreover, enhancing the dispersion and density of hydrophilic components on the membrane’s effective surface creates a more tunable system. Also, it should be noted that by increasing the number of hydrophilic functional groups, the probability of generating hydrogen bonds with water molecules increases, leading to the formation of hydration layers on the surface of the membrane. Therefore, the hydration layer hinders the deposition of natural organic matter, including bovine serum albumin and humic acid, on the membrane surface and prevents organic fouling. The results of a study conducted by Zhang et al. showed that a polyimide ultrafilter embedded with DES-coated nanodiamond, in comparison with a pure polyimide ultrafilter, has a 30% higher flux recovery rate. Additionally, the irreversible fouling ratio in the modified membrane is about 35% lower than in the pure ultrafilter60.
Regarding the prevention of biofilm growth and antibiofouling capability, the strong antibacterial properties of quaternary ammonium-based ILs have attracted much attention. For example, the results of a study conducted by Soyekwo et al. on tri-quaternary ammonium-based ILs electrolyte monomer used for the surface modification of a polyamide nanofilter showed that the viability of Escherichia coli and Staphylococcus aureus cells on the modified surface was inhibited up to 2 log and 4 log, respectively, which can significantly decrease the probability of biofouling on the modified surface24. Achieving superior performance in structure optimization of DESs necessitates meticulous selections of HBD and HBA, significantly influencing absorption and selectivity by impacting hydrogen bond network organization and molar ratios. The composition and structure of DESs significantly impact membrane permeability, stability, and selectivity. For instance, DESs containing inherent water can impede mass transport, while those with hydroxyl groups may exhibit thermal fragility61. DESs containing amide groups tend to lack stability and durability in DES-based membranes66.
The pivotal role of membrane wettability, determined by the surface tension of imidazolium based ILs or DESs, lies between organic solvents and water. Measurement of the contact angle of ILs or DESs on the membrane support aids in determining wettability. Notably, the wettability diminishes with the elongation of the side chain in the moieties. This feature not only allows for the tailoring of properties towards substrates through the exchange of cations or counter anions but also serves as a mechanism for achieving reversibly switchable wettability (tunability of wettability), akin to on-off agents67. Furthermore, it significantly influences system stability by enhancing interaction with water molecules, effectively repelling foulants68.
Achieving a delicate balance is the key when using ILs and DESs in membrane technology. It involves optimizing flux and selectivity, balancing permeability with mechanical strength, and incorporating antimicrobial properties while maintaining membrane functionality. The introduction of ILs and DESs further contributes to this equilibrium by offering tailored solutions to enhance membrane performance. By carefully balancing those factors, researchers can design membranes that not only remove pollutants effectively but also exhibit long-term stability and efficiency in water and wastewater treatment and reuse processes.
Adsorption
ILs and DESs are promising adsorbents in water and wastewater treatment due to their tunable properties and ability to be incorporated into membrane technologies, effectively targeting a spectrum of emerging contaminants, pharmaceuticals, heavy metals, and PFAS5,27,69,70,71. The efficacy of ILs and DESs in adsorbing pollutants is rooted in diverse mechanisms, including ion exchange, hydrogen bonding, π-π interactions, and van der Waals forces72,73. Moreover, they enable the development of tailored adsorbents, such as functionalization of Zn-Al layered double hydroxides and CNTs, enhancing the selectivity and capacity for specific contaminants, thus offering significant potential for addressing water and wastewater treatment challenges37,38,74,75. Figure 5 presents an illustrative depiction of advanced adsorption processes utilizing ILs and DESs for the removal of various water contaminants. As highlighted in Fig. 5a, modification of adsorbents with ILs and DESs enhances their affinity for specific contaminants.
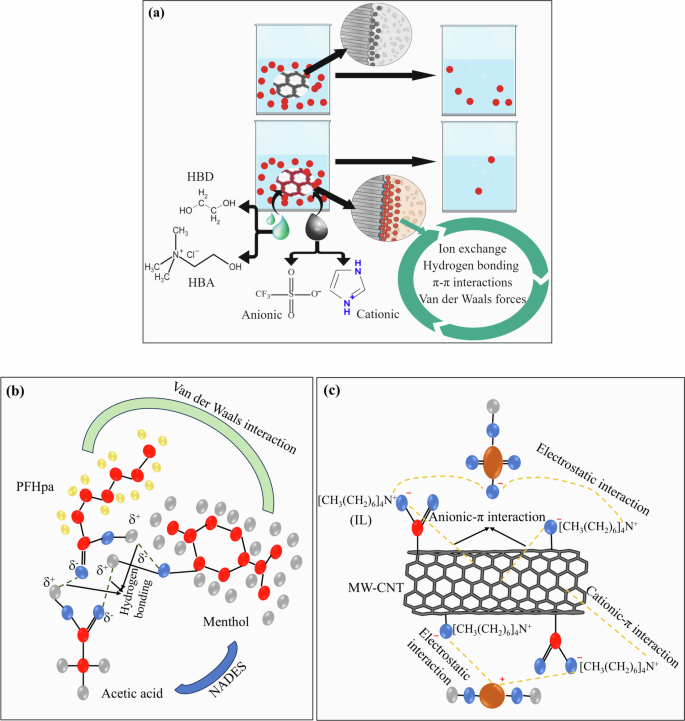
a General adsorption mechanisms of ILs and DESs that increases adsorbent performance (Red circle: contaminants, Gray droplet: ILs, Green droplets: DES, Circle with gray hexagons: typical adsorbent, Circle with red hexagons: modified adsorbent), b Hydrogen bonding and van der Waals interactions as the driving forces in the adsorption of PFHpa by a menthol and acetic acid-based NADES (Red circle: C atoms, Blue circle: O atoms, Gray circle: H atoms, Gold circle: F atoms)54, c Electrostatic and anionic and cationic-π interactions facilitate the removal of chromium species using ILs-functionalized oxidized MWCNT (Red circle: C atoms, Blue circle: O atoms, Gray circle: H atoms, Brown circle: Cr atoms). Adapted from80.
The types of ILs or DESs utilized, the functional groups present in the adsorbent, the species of pollutants in the solution, and the experimental conditions each have a distinct influence on the adsorption process. ILs can improve the adsorption affinity and capacity of sorbents for both organic and inorganic pollutants. Their molecular structure can be finely adjusted to target specific contaminants selectively. This customization enables the design of adsorbents with optimized interactions for various contaminant classes, such as π-π interactions for aromatic compounds, hydrogen bonding for polar substances, and electrostatic interactions for charged species36,73. ILs and DESs are emerging as promising materials for the efficient and selective extraction or separation of PFASs, including both short- and long-chain variants, from edible oils and water samples contaminated with these substances27. ILs and DESs can be specifically tailored to enhance their efficiency, selectivity, and stability. For example, an IL-modified natural clay enhanced the adsorption of perfluorooctanoic acid and perfluorooctanesulfonic acid, outperforming activated carbon. Long-chain ILs (C16) offered fast kinetics for sorption and high removal efficiencies due to the expansion of the clay’s interlayer, facilitating hydrophobic and electrostatic interactions with PFASs molecules21.
DESs tailored with specific hydrogen bond donors and acceptors have effectively extracted heavy metals by forming complexes. By virtue of their tunable molecular interactions, a choline chloride/1-(o-tolyl)biguanide DES-based superparamagnetic nanofluid achieved high recovery rates for PFAS in edible oils, demonstrating the potential of DESs for environmental cleanup74. However, advancing the development of ILs and DESs requires a focus on creating biocompatible formulations that exhibit low toxicity and have a minimal environmental impact. Natural hydrophobic DESs can present a wealth of untapped potential for sustainable applications. Hydrophobic natural DES (HNADES), a mixture of menthol and acetic acid, was used for the liquid-liquid extraction of perfluoroheptanoic acid (PFHpa) from water54. The interaction between the adsorbent and adsorbate was hydrogen bonding and van der Waals forces (Fig. 5b). The results showed high extraction efficiencies ranging from 80.2% to 90.1%. The study was carried out under various conditions, different initial concentrations of PFHpA and pH levels. The extraction efficiency was consistent across a concentration range of PFHpA and was not significantly affected by the initial pH of the aqueous solution54.
The adaptability of ILs and DESs to specific contaminant characteristics through molecular design enhances their efficacy as adsorbents. New generation techniques such as molecular imprinting, smart adsorbent, and electro-enhanced adsorption can add a new dimension to the applications of ILs and DESs38,54,75,76,77,78. He et al. found an IL, 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), as an effective additive for enhancing cellulose acetate imprinted membranes’ performance in treating salicylic acid-containing water38. This adaptability, combined with environmental sustainability considerations, suggests potential for further optimization and application expansion, particularly in water and wastewater treatment technologies.
Improvements in the coating of ILs and DESs on adsorbent surfaces can further enhance their performance. Strategies include the development of more robust and stable attachment methods to prevent leaching, increase the surface area for better contaminant capture, and incorporate functional groups or nanoparticles to improve selectivity and affinity. For example, functionalizing CNTs with DESs has been shown to enhance their specific surface area and chemical stability. CNTs functionalized with a ternary DES demonstrated a significantly increased adsorption capacity for mercury due to the introduction of -S-H functional groups, which improved chemisorption between mercury ions and the DES-functionalized magnetic graphene oxide79. In another study, functionalization of oxidized multiwalled CNTs with tetra-n-heptylammonium bromide IL enhanced the adsorption capacity for hexavalent chromium from 13.2 to 85.8 mg/g. As illustrated in Fig. 5c, the cation π interactions, electrostatic forces, and anion π interactions between the adsorbent and adsorbate were the driving force for this higher absorption efficiency80. Further expansion including development of ILs and DESs with enhanced thermal and chemical stability will enable their applications in extreme conditions, such as industrial wastewater treatment. Using renewable resources and minimum hazardous reagents in the development of ILs and DESs, will ensure a more sustainable approach. Data driven approaches and machine learning techniques can be used to determine the most suitable ILs or DESs for specific contaminant removal.
Key factors affecting removal efficiency include sorbent dosage, pH, contact time, concentration, and temperature. ILs customization enables selective contaminant capture, where pH significantly influences the interaction between the adsorbent and adsorbate by altering the surface charge density of the adsorbent and the ionizing capacity of the ionic liquid, showing different trends based on their nature75. Similarly, temperature is another significant factor affecting adsorption capacity, depending on whether the process is endothermic or exothermic81. Furthermore, the specificity of ILs and DESs towards target contaminants may vary depending on the composition of the water and wastewater matrix. Complex mixtures of contaminants can interfere with the adsorption process, leading to reduced efficiency and selectivity73. Therefore, a comprehensive understanding of the interactions between ILs and DESs and various contaminants is necessary to optimize their performance in water and wastewater treatment practices. One significant concern is the potential toxicity and environmental impact of ILs and DESs themselves. While they offer promising adsorption capabilities, their biocompatibility and eco-toxicity remain subjects of scrutiny. Therefore, further research into the environmental fate and toxicity of ILs and DESs is crucial to ensure their safe and sustainable use in water and wastewater treatment.
Future perspectives
A roadmap for future applications of ILs and DESs in water and wastewater treatment involves envisioning a paradigm shift toward sustainable and efficient solutions. Figure 6 outlines the fundamental pattern for moving forward with the applications of ILs and DESs in the water and wastewater treatment sector and provides a tentative timeline for advancing the applications of ILs and DESs, guiding necessary steps forward. In the next phase of development, research and innovation should focus on harnessing the unique properties of ILs and DESs to address pressing challenges in water and wastewater treatment. Collaborative efforts between academia, industry, and government will be crucial to explore novel applications, optimize treatment processes, and scale up technologies. Moreover, emphasis will be placed on ensuring the environmental sustainability and safety of ILs and DESs, while maximizing their effectiveness in removing pollutants, reclaiming resources, and enhancing water quality. Through strategic investment, interdisciplinary collaboration, and continuous improvement, the roadmap aims to pave the way for the widespread adoption of ILs and DESs as transformative technologies in the water and wastewater treatment sector, contributing to a more sustainable and resilient future.
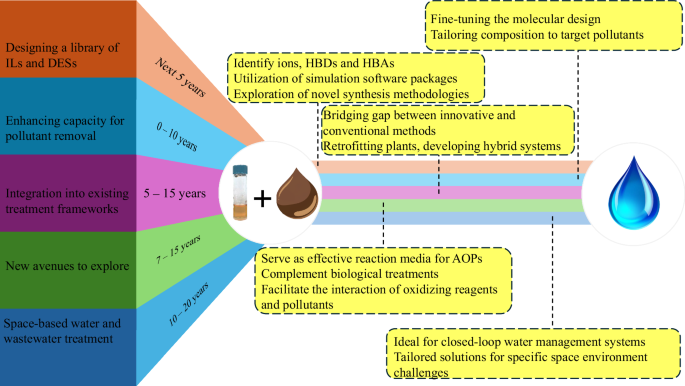
Key areas for research and development are highlighted to address emerging challenges and showcase the vast potential for sustainable and innovative solutions.
The initial research focus for the next few years, as depicted in Fig. 6, should be identifying pairs of ions, HBDs and HBAs, and facile synthesis procedures for ILs and DESs. Since there is a plethora of ions available to pair, designing a library for potential ILs and DESs, their properties, and targeted pollutants must be a priority. Several simulation software packages such as conductor-like screening models for real solvents and density functional theory can be utilized to study ILs and DESs, and their properties, followed by experimentation procedures to verify simulation results82,83,84,85. Software tools allow researchers to model and predict various characteristics of ILs and DESs, such as their structure, thermodynamic properties, and behavior in different environments. Furthermore, streamlining the synthesis of ILs and DESs and prioritizing the development of efficient, sustainable, and scalable synthetic routes to enhance accessibility and cost-effectiveness are essential for large-scale water and wastewater treatment applications. Moreover, there is a need to explore novel synthesis methodologies, including greener approaches such as microwave-assisted synthesis, enzymatic catalysis, and continuous flow reactors, which can enhance reaction efficiency and reduce environmental impact.
As shown in Fig. 6, the next crucial premise involves enhancing the capacity of ILs and DESs for pollutant removal through precise chemical structure modifications. By fine-tuning the molecular design of ILs and DESs, it is possible to optimize their affinity for specific contaminants, improve their selectivity, and enhance their overall performance in water and wastewater treatment processes. This may involve tailoring the composition of ILs and DESs to target particular pollutants, adjusting functional groups to increase binding affinity, or engineering novel solvent formulations with synergistic properties.
The logical progression in advancing the application of ILs and DESs in water and wastewater treatment is their seamless integration into existing treatment frameworks. This integration entails bridging the gap between innovative solvent technologies and conventional treatment processes, ensuring compatibility, efficiency, and practicality. Incorporating ILs and DESs into current treatment schemes allows leveraging their unique properties to enhance pollutant removal, resource recovery, and overall treatment performance. This may involve retrofitting existing treatment plants for ILs and DESs incorporations, developing hybrid treatment systems that combine conventional methods with solvent-based processes, or designing novel treatment configurations optimized for ILs and DESs applications. Collaborations among researchers, engineers, regulators, and industry stakeholders will be essential in navigating the technical, regulatory, and economic challenges of ILs and DESs as transformative tools in water and wastewater treatment.
The inherent properties of ILs and DESs open new avenues for exploration in biological treatment86 and advanced oxidation processes87. Microbial treatment often demands a conducive medium for electron transfer to biodegrade contaminants. ILs and DESs offer unique properties that can enhance the solubility and accessibility of contaminants, making them more readily available for microbial degradation. ILs and DESs can enhance the bioavailability of contaminants and in turn facilitate their biodegradation. Moving forward, it is imperative to holistically understand the collaborative mechanisms between biological treatment and ILs and DESs application in the biodegradation of recalcitrant pollutants and emerging contaminants of concern. Furthermore, the research focus must pivot towards developing ILs and DESs with heightened applicability and ease of disintegration to effectively tackle environmental concerns. Also, due to their inherent physicochemical properties, ILs and DESs are great candidates for microbial immobilization and biofilm formation.
ILs and DESs possess unique properties that can be harnessed to enhance the efficacy and efficiency of AOPs, such as photocatalysis, ozonation, and Fenton oxidation for degrading recalcitrant pollutants in water and wastewater. ILs and DESs can serve as effective reaction media, solubilizing hydrophobic contaminants and facilitating their interaction with oxidizing agents. Moreover, their tunable chemical structures enable the design of tailored solvents with enhanced selectivity and reactivity towards specific pollutants, thus improving treatment performance and minimizing undesirable byproducts. By integrating ILs and DESs into AOPs, it is possible to develop robust and sustainable treatment strategies for addressing emerging contaminants and other challenging pollutants in water and wastewater streams.
Another exciting future application of ILs and DESs is treating water and wastewater in outer space especially for human settlements on the moon and Mars. The unique properties of ILs and DESs make them well-suited for space-based applications, where resource recycling and efficiency are paramount. ILs and DESs can play a crucial role in water purification and recycling systems, enabling the efficient removal of contaminants and the recovery of valuable resources such as water and nutrients. Their low volatility, thermal stability, and versatility make them ideal candidates for closed-loop water management systems in space habitats, where water is a precious and limited resource. Furthermore, the tunability of ILs and DESs properties allows for tailored solutions to address specific challenges encountered in space environments, such as microbial control and corrosion prevention.
The multifaceted utilities of ILs and DESs in water and wastewater treatment underscore their transformative potential in addressing contemporary challenges and charting a course toward sustainable water management practices. However, surmounting existing hurdles, such as cost, scalability, and toxicity concerns, remains imperative. A critical aspect of ILs and DESs research endeavors must focus on developing cost-effective synthesis methodologies, exploring renewable feedstock alternatives, and conducting comprehensive environmental impact assessments. By perpetuating a culture of innovation and collaboration surrounding ILs and DESs, it is possible to propel the evolution of sustainable water and wastewater treatment solutions, safeguarding societal well-being and environmental integrity.
Responses