Functional assessment of IDUA variants of uncertain significance identified by newborn screening

Introduction
As newborn screening (NBS) for Mucopolysaccharidosis Type I (MPS I) and other metabolic disorders continues to expand across the United States, the number of variants of uncertain significance (VUS) identified increases in parallel, creating unique challenges in diagnosis and management of these disorders1,2,3,4,5,6. Second-tier glycosaminoglycan (GAG) analysis can be useful in confirming a diagnosis but often cases with VUS and pseudodeficiency variants create uncertainty and stress among patients, families, and providers. The lack of clear diagnoses can also delay medical management and treatment decisions. One of the primary reasons for the high prevalence of VUS identified in IDUA during NBS for MPS I is the lack of published functional studies for previously identified IDUA variants. To address this need, we developed a biochemical platform capable of determining the relative specific activity (RSA) of a variant α-iduronidase7. RSA value represents the relative activity divided by the relative amount, with activity and abundance of a variant enzyme determined relative to the WT enzyme. In addition to providing a measure of enzyme amount, the western blot analysis also provides mechanistic insight into enzyme trafficking and lysosomal processing. The first version of this platform relied on viral-mediated delivery of variant-bearing IDUA into an IDUA-knockout Hap1 cell line. While effective in stratifying different IDUA variants, the platform was time-consuming and costly, hindering its broader utility to rapidly characterize newly identified IDUA variants within a clinically useful timeframe.
Here we present a revised platform for IDUA variant resolution using transfection in IDUA-KO HEK293 cells that increased the efficiency by which different variants can be studied. In order to calibrate the platform and make it applicable for use in variant classification (PS3 ACMG variant classification criteria8), we characterized thirty-five IDUA variants, including several established benign and pathogenic variants. These analyses revealed a remarkable range of effects on enzyme activity and processing, and uncovered that certain variant enzymes are prone to aggregation. This aggregation may cause impaired trafficking to the lysosome of these variant enzymes, and create a damaging effect of the variant that is distinct from effects on its inherent enzymatic activity. These findings suggest additional functional analyses – beyond the single measure of relative specific activity—may be important to accurately resolve the pathogenic or benign status of newly identified VUS. The utility of this platform for IDUA variant interpretation is discussed along with the challenge associated with resolving variants whose activity fall in between known pathogenic and benign variants.
Methods
Generation of IDUA-expressing HEK293 IDUA-null cells
An IDUA-null HEK293 cell line was commercially generated using CRISPR-Cas9. The following guide RNAs were used:
gRNA-A1(matching forward strand): TGGCCCCTGCTGCTCGCGACTGG
GRNA-A2 (matching forward strand): ACCACGGATTGTCTTGGCTCCGG
The CRISPR editing created a homozygous one base-pair deletion at 4691 resulting in a frameshift and premature stop codon. We confirmed the functional null consequence of this change by activity and western blot. The HEK293 IDUA-KO cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Benchmark, Gemini Bio, West Sacramento, CA), Penicillin/Streptomycin (Mediatech, Manassas, VA) and sodium pyruvate. pcDNA3.1 expression clones containing either WT and variant-bearing IDUA DNA were commercially generated (VectorBuilder, Chicago, IL). The full-length coding region of human IDUA was synthesized using the reference NM_000203.5. DNA constructs were transfected into HEK293 IDUA-KO cells using Lipofectamine2000 (Thermo-Fisher, Waltham, MA). Starting 2 days post-transfection, transfected cell populations were enriched by G418 (800 µg/mL) selection. In case of variant sequences with very low expression or activity after selection, single-cell clones were isolated.
α-Iduronidase activity assays
α-iduronidase activity was measured in cell lysates and culture media using established methods7. Cultured cells were harvested by trypsinization and cell pellets were lysed by sonication in Dulbecco’s phosphate-buffered saline containing Ca2+ and Mg2+, 0.1% Triton X-100, and protease inhibitor cocktail (Thermo Scientific, Waltham, MA) on ice. Protein concentrations were determined using the BCA assay kit (Thermo Scientific, Waltham, MA). For α-iduronidase activity assays total protein was adjusted to 0.1 or 0.01 mg/mL. Lysates from clonal cells expressing WT-IDUA were adjusted to 0.01 mg/mL and serial dilutions (1/3, 1/9, 1/27, 1/81, 1/243) used to create a standard curve. Enzyme reactions were initiated by mixing 25 µL of cell lysate with 25 µL of 4-methylumbelliferyl iduronide (4-MUI; 100 µM) in 0.4 M sodium acetate buffer (pH 3.5) in 96 well black plate at 37 °C for 1 h. Reactions were stopped by adding 200 µL of 0.1 M glycine buffer (pH 10.4) and then fluorescence (excitation/emission = 355 nm/460 nm) was measured using a Cytation 5™ Cell Imaging Multimode Reader (BioTek, Winooski, VT). The relative activity of each variant α-iduronidase was calculated using the standard curve along with the ratio of WT/variant concentration.
Western blot analyses
The relative abundance of α-iduronidase in lysates used for activity assays was calculated by Western blot7. A concentration gradient of WT-IDUA cell lysates was included on each gel. Immunoblots were performed using α-iduronidase antibody (Invitrogen, 1:1000, overnight) and images captured using the ChemiDoc™ imaging system (BioRad, Hercules, CA). Pixel densitometry was determined Image Lab software version 5.2.1 (BioRad).
Determining the relative specific activity (RSA) value
RSA value represents the relative activity divided by the relative amount, with activity and abundance of a variant enzyme determined relative to the WT enzyme7. Relative activity represents the activity of variant enzyme relative to WT enzyme in lysates normalized for the same total protein concentration. The relative amount of variant enzyme is determined using semi-quantitative western blotting (e.g., densitometric analysis of western blots that include equivalent WT and variant lysates taken at equal exposure). We resolve a range of WT lysate concentrations and compare the amount of variant enzyme to estimate the amount of variant enzyme relative to the WT enzyme.
Aggregation analyses of variant α-iduronidase enzymes
Protein aggregation was examined using native gel electrophoresis9. PAGE was performed using cell lysates generated for analyses of α-iduronidase activity. The following modifications were made: 1) native sample loading buffer was used instead of 4x Laemmli sample buffer, 2) polyacrylamide gels were prepared without SDS and 3) elution buffer containing 0.0375% SDS was used instead of 0.1% SDS. For testing the effects of heat on enzyme aggregation, lysates were incubated in 40 °C water bath for 15 min (or on ice) before running native PAGE.
Measurements of dermatan and heparan sulfate glycosaminoglycans
Cell lysates were reconstituted in distilled water and sonicated. An aliquot of the lysate was digested overnight with Chondroitinase B, Heparinase I, II, and III (Galen Laboratory Supplies), and Keratinase II GlycoFineChem). The resulting disaccharides (D0a4, D0S0, D0A0, g0A6, g6A6) were quantified using LC-MSMS10.
Human research participants and informed consent
We confirm that we have complied with all relevant ethical regulations including the Declaration of Helsinki for the patient samples included in this part of the study. Informed consent was obtained for all patients, including consent from parents for patients under the age of 16. Consent forms were approved by the Self Regional Healthcare Institutional Review Board (IRB) under study #Pro00085001: A comprehensive research project to determine the causes of suspected genetic conditions using clinical, laboratory, and/or research testing.
Analysis of non-reducing end (NRE) glycosaminoglycans in urine
Semi-quantitative analysis of endogenous non-reducing end (NRE) GAGs was performed by UPLC-MS/MS according to the method described by Saville et al.11, and by Herbst et al.12. In summary, a volume of urine equivalent to 20 nmol of creatinine was derivatized with 0.25 M 1-phenyl-3-methyl-5-pyrazolone (PMP) containing 0.01 nmoles of internal standard (chondroitin disaccharide ∆di-4S sodium salt). Samples were then acidified with 5 M formic acid and excess PMP was removed by liquid-liquid extraction with chloroform (CHCl3). Semi-quantification of UA-HNAc(1S), the NRE that has been associated with MPS I11,12,13 was performed using a Xevo-TQS micro (Waters Technologies, Milford, USA). The concentration of UA-HNAc(1S) is expressed as apparent μmoles/mol creatinine.
Results
Revised platform for functional characterization of IDUA variants
In an effort to streamline functional evaluation of IDUA variants identified through NBS, we generated an IDUA-null HEK293 cell line. IDUA-null cells were transfected with either wild-type or variant-expressing IDUA DNA vectors and IDUA-expressing cell populations enriched using G418 selection. In cases where enzyme activity or abundance was too low to reliably measure in the selected population, we isolated single cell clones with higher enzyme expression. Our prior study showed single clones yield more consistent protein abundance and activity measurements than enriched populations7. Despite this, many of the variant α-iduronidase enzymes studied here were reliably characterized from pools of G418-selected cell populations.
Enzyme activity was measured in the lysates of selected cell populations or single cell clones using the fluorescent simple sugar substrate, 4-methylumbelliferyl-α-iduronide (4MUI). The activity values were compared to those obtained from an equivalent amount of total protein present in control lysates generated from cells expressing WT-IDUA. The same lysates were analyzed by western blot with an anti-human α-iduronidase antibody. Relative amount of the WT or variant α-iduronidase protein was assigned using densitometry software. The relative specific activity (RSA) of each α-iduronidase variant was calculated by dividing the relative activity of lysate by the relative amount of enzyme in the same lysate. An overview of the workflow is shown in Fig. 1.

HEK293 IDUA KO cells are transfected with WT or variant-bearing IDUA expression vectors followed by antibiotic selection and, in some cases, single-cell cloning. Activity measurements using a fluorescent substrate for α-iduronidase are performed in the resulting cells along with quantitative western blot analysis to determine enzyme amount. These values are determined relative to the WT enzyme and combined to provide the relative specific activity (RSA) for each variant enzyme. This value represents the primary readout for each variant enzyme studied, but other secondary analyses can be performed to probe aggregation potential and ability to clear GAG accumulation.
Relative specific activity of variant α-iduronidases expressed in HEK293 cells can be used to estimate the pathogenicity of IDUA variants
A variety of IDUA variants were evaluated using this platform. For the purpose of validation, we included 8 variants associated with either severe or attenuated MPS I, and 4 known benign variants. We further analyzed variants classified as pseudodeficiency alleles (reduced enzyme activity but clinically benign), and multiple variants of uncertain clinical significance (VUS) identified in infants with an abnormal newborn screen for MPS I. The RSA value of each variant α-iduronidase plotted on the graph in Fig. 2 represents the average value from a minimum of three independent biological replicates. The amino acid position within the α-iduronidase protein structure is shown for each of the variants studied in Fig. 3, and the amino acids comprising the active site are shown in Supplementary Fig. 114. All four benign variants yielded RSA values that were indistinguishable from the wild-type enzyme. Nearly all of the variants associated with attenuated MPS I exhibited RSA values below 1%, while the variants associated with severe MPS I exhibited values up to 2 orders of magnitude lower than 1%. Of note, the RSA values of the three known pseudodeficiency variants tested (p.Gly409Arg, p.His82Gln, and p.Ala79Thr) were highly variable. The p.Ala79Thr variant exhibited an RSA of 5.4%, which is similar to the activity level detected for multiple variants present in patients with attenuated MPS I. The RSA values observed for p.Gly409Arg and p.His82Gln (70% and 24%, respectively) are in very close agreement with previously published results15,16. Although the values fall within an activity range not thought to cause MPS disease, these data demonstrate that the pseudodeficiency variants do alter enzyme function.

Graph showing the relative specific activity (% RSA) values (plotted on a log 10 scale) for every variant characterized in this study. Replicate analysis (n = 3–6) was performed on either cell populations after initial antibiotic selection, or 2-3 different single cell clones. Average RSA values are shown within the bars and standard error of the mean is depicted with the error bars. Bar colors denote the following classifications: dark gray – WT; light gray – VUS; blue – benign; green – known pseudodeficiency; yellow – associated with attenuated MPS I; orange – associated with attenuated or severe MPS I; red – associated with severe MPS I.
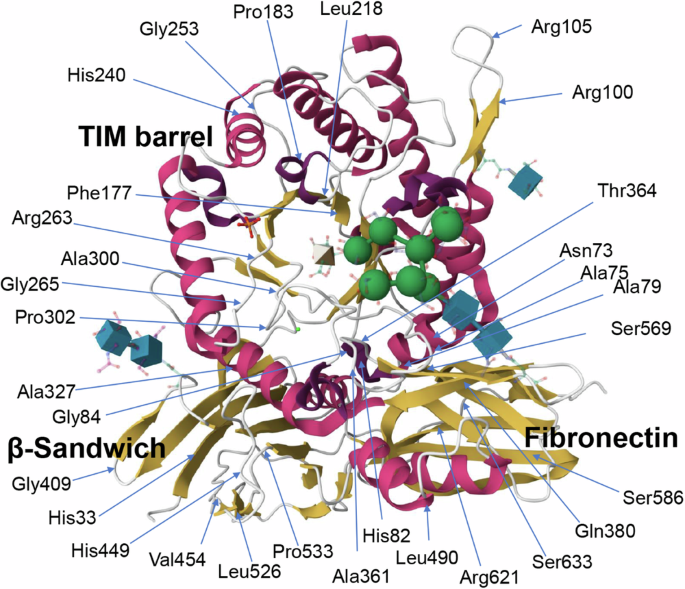
The location of specific amino acid residues corresponding to the variants we studied in this paper is shown. Domains within the crystal structure (PDB: 3W82; RSCB PDB)14,26 of the enzyme are also labeled.
Two other missense variants (p.Arg100Gly and p.Arg100Lys) exhibited RSA values indistinguishable from the WT enzyme, however, both of the corresponding nucleotide changes (c.298A>G, c.299G>A, occurring at the end of exon 2) have high SpliceAI scores, indicating that these variants may affect splicing rather than having a missense associated effect on enzyme activity. In light of the fact that the platform utilizes only the coding sequence, additional studies will be needed to fully resolve the pathogenicity of these variants. The SpliceAI scores for all variants investigated in this study are shown in Supplementary Table 1, along with predictions on the resulting impact of the splice effects. Only the nucleotide changes corresponding to the missense variants at amino acid position 100 (p.Arg100Gly and p.Arg100Lys) predicted a significant effect on splicing. We propose that all IDUA nucleotide variants selected for functional studies going forward be initially screened for possible effects on splicing as the current design of the platform can only gauge the effects of different amino acid substitutions on enzyme function.
Altered expression/stability and processing were observed for many variant α-iduronidases
In addition to their utility for gauging enzyme amount, western blot analysis also highlighted multiple differences in the proteolytic processing of the variant α-iduronidase enzymes. The wild-type enzyme undergoes a series of proteolytic processing steps within the secretory pathway that include removal of the signal peptide and digestion of peptide fragments from both the N- and C-terminus17. Much of this processing occurs within the lysosomal compartment. Overexpression of WT-IDUA enzyme primarily yielded three different protein species (~75 kDa, ~70 kDa, ~62 kDa). In cells treated with bafilomycin A, an inhibitor of lysosomal proton transport that neutralizes the pH in this organelle, the highest molecular weight species (75 kDa) accumulated, reducing the abundance of the two lower molecular weight species (Fig. 4A). These data support the likelihood that each of the protein species detected on western blot result from processing events in the lysosome. As expected, each of the benign α-iduronidase variants analyzed exhibited normal patterns of protein processing (Fig. 4B). This was more variable in the other forms of IDUA analyzed, with many variants showing limited enzymatic processing (Fig. 4C-F). The extent of processing, along with the RSA values and allele frequencies of each variant enzyme is summarized in Table 1. Our data suggest that increased levels of enzyme processing generally correlate with higher RSA values. One explanation for poor enzymatic processing may be inefficient lysosomal targeting. Alternatively, it is possible that lysosomal targeting is normal but certain variant forms of α-iduronidase are unstable within the low pH environment of the lysosome. This latter hypothesis is difficult to interrogate, as modulating lysosomal pH would itself prevent enzyme processing.

Representative western blots for variant α-iduronidases in this study. A WT IDUA-expressing cells were treated with bafilomycin A (BafA), or the protease inhibitors, E64d or pepstatin A (PepA) prior to analysis by western blot. Note that only bafilomycin A blocked proteolytic processing of the enzyme. B Comparison of WT to the four known benign variants in this study. Note all four variant α-iduronidases are processed similarly to the WT enzyme. C Western blot analysis of various single-cell clones expressing the variant enzymes. Despite differences in enzyme amount within the different clones, the extent of proteolytic processing is comparable. D, E, F Representative blots for different variant-expressing selected cell populations showing variable impact on the proteolytic processing of the enzymes.
p.Leu526Pro α-iduronidase is prone to aggregation
The RSA values for several variants known to be associated with attenuated MPS I indicate that most values lie below the 1–3% range. However, several of the variants that have been identified in attenuated MPS I patients exhibited RSA values that were 10–30% of WT α-iduronidase18. This indicates that the RSA value may not alone be sufficient to assign pathogenicity to certain variants. To address whether other mechanisms contribute to altered α-iduronidase function, we utilized previously isolated single-cell clones to further investigate the p.Leu526Pro VUS, as a model for variants that seem to contradict the ability of the RSA value to predict pathogenicity. This variant has an allele frequency of 3.91 × 10−4 (gnomAD v.4; total allele count of 595) in the general population and an RSA of 15%. This is three times higher than that of the pseudodeficiency variant, p.Ala79Thr, which is clinically benign.
Leu526 is located within a ß-sandwich structure, where it contributes to a series of hydrophobic interactions between two ß-strands near the surface of the enzyme (Fig. 5A). The p.Leu526Pro mutation likely disrupts the beta-sheet structure, and the corresponding disruption exposes hydrophobic residues. We hypothesized this may cause the enzyme to aggregate, impairing its trafficking in vivo. Neither effect would be evident by measuring specific activity of variant enzymes in vitro. Using native gel electrophoresis, we showed the WT and p.Ala79Thr enzyme migrate exclusively as lower MW bands (60–75 kDa), similar to previously shown under denaturing conditions (Fig. 5B). Consistent with aggregated forms of the enzyme the p.Leu526Pro enzyme migrated in two broad heterogenous bands at higher MWs (~125–300 kDa). This aggregation was much weaker for the p.Alal79Thr compared to p.Leu526Pro. To ask if the appearance of high MW bands or aggregates is exacerbated by heat, lysates were incubated at 40 °C for 15 min. As shown in Fig. 5C, with the exception of the WT enzyme, all variant forms of the enzymes tested exhibited increased aggregation when heated. This supports the conclusion that missense variants structurally compromise the enzyme making it prone to aggregation. This aggregation or structural alteration may cause the enzyme to be inefficiently trafficked to the lysosome. Since these variant-containing enzymes retain activity towards the 4-MUI sugar substrate, the aggregation potential caused by these variants is not likely associated with misfolding within the ER. This suggests the enzyme accumulates within other compartments of the secretory pathway. Attempts to localize the variant forms of α-iduronidase using confocal microscopy were not informative, as steady-state analyses of overexpressed enzyme are challenged by the wide enzyme distribution within the cell, even 12-18 hours post-transfection.

A Protein structure of α-iduronidase (PDB: 3W82; RSCB PDB)14,26 inset depicts the location of leucine 526. B Representative blot of WT, p.Ala79Thr and p.Leu526Pro α-iduronidase following native gel electrophoresis. C Western blot showing accumulation of high molecular weight forms of α-iduronidase with certain variants in the presence or absence of heating at 40 °C. D Western blot analysis of WT, p.Leu526Pro, and p.Leu526Met α-iduronidase following denaturing gel electrophoresis. Note that substitution of Leu526 with methionine permits proteolytic processing while proline does not. E Western blot of WT, p.Leu526Pro, and p.Leu526Met α-iduronidase on a native gel with and without prior heating at 40 °C.
To further explore the mechanism of aggregation and impaired lysosomal delivery, we engineered another variant at position 526 (p.Leu526Met) predicted to have a less severe impact on the secondary structure of the protein. The p.Leu526Met variant IDUA was introduced into the IDUA KO HEK293 cells and selected pool of cells was obtained. The relative specific activity of this variant enzyme was considerably higher (74%) than seen with the corresponding proline substitution (15%), indicating a less severe impact on enzymatic function (Fig. 5D). Comparison of the electrophoretic mobility (on native and denaturing gels) and lysosomal processing of p.Leu526Pro and p.Leu526Met enzymes demonstrated clear differences in the characteristics of the two variant proteins (Fig. 5E). Unlike p.Leu526Pro which shows little or no processing, the p.Leu526Met variant α-iduronidase was processed similar to the WT enzyme. Most noteworthy was the lack of aggregation observed with the p.Leu526Met enzyme. This supports the hypothesis that the proline substitution more substantially alters enzyme structure increasing the aggregation potential.
Despite having an RSA value that falls within the benign range, our results support the conditional pathogenicity of the p.Leu526Pro variant under conditions that promote enzyme aggregation and impaired lysosomal delivery. Any variant enzyme that fails to reach the lysosomal compartment will not be available to effectively catabolize GAGs despite having sufficient residual catalytic function to degrade substrates. To address this possibility, we performed GAG analysis on single-cell clones expressing different variant forms of α-iduronidase and assayed which of the variant enzymes prevented lysosomal GAG storage (Fig. 6). A two- to three-fold elevation in GAG abundance was observed in the HEK293 IDUA null cells compared to the parental WT HEK293 cells. The elevation in total GAG abundance was effectively eliminated in both the WT IDUA-expressing clone and p.Leu526Pro-expressing cells, indicating that despite the poor apparent lysosomal delivery and processing in the latter, there is sufficient enzyme function to degrade GAGs. We estimate that the α-iduronidase is overexpressed roughly 500 times in the single cell clones compared to the parental WT HEK293 cells so with the impaired lysosomal delivery caused by the variant, enough enzyme still reaches the lysosome. It is unclear whether this would be true under endogenous expression levels.

Graph depicting abundance of total GAGs (dermatan and heparan sulfate) in WT parental and IDUA-KO HEK293 cells, and IDUA-KO HEK293 cells-expressing either WT or p.Leu526Pro IDUA. Measurements were made in four independent replicates. Error bars represent the standard error of the mean. Statistical analyses were performed using a Student’s t-test; *** denotes P < 0.001, n.s. denotes not significant.
Lastly, we explored the impact of the p.Leu526Pro variant further by performing the semi-quantitative analysis of endogenous non-reducing end (NRE) GAGs, specifically UA-HNAc(1S) that is elevated in MPS I patients, in urine from patients bearing this variant. A summary of the findings is shown in Fig. 7. In all cases where p.Leu526Pro is present in trans with a pathogenic or likely pathogenic variant, urine UA-HNAc(1S), while elevated compared to normal controls (2.6–48 apparent µmol/mol creatinine versus <0.50), is much lower than levels observed in patients with a confirmed clinical diagnosis of MPS I (906–5326 apparent µmol/mol creatinine). While the urine GAG elevation is not in the range expected for patients with MPSI, there is a measurable increase that does support the accumulation of some GAG storage when this variant enzyme is expressed in trans with other loss-of-function alleles. More samples from patients with atypical attenuated phenotypes, such as the patient described by Asumda and colleagues18, which had mostly a cardiac phenotype with retinopathy, will help clarify the clinical significance of mild urine NRE elevations, both in patients with the p.Leu526Pro variant and in those with other variants of uncertain clinical significance.

Graph depicted NRE levels in different individuals as a function of age. Samples from two patients carrying the p.Leu526Pro were analyzed. Levels of the MPS I NRE marker UA-HNAc(1S) are expressed in apparent μmoles/mole of creatinine. Patient 1 is 27 years and 10 months old, with a genotype of p.Trp402Ter (pathogenic)/p.Leu526Pro (VUS). This patient had 48 apparent μmoles/mole of creatinine. Patient 2 (same genotype as patient 1) is 7 month-old male with 57 apparent μmoles/mole of creatinine. Their levels of UA-HNAc(1S) early are above the normal range (≤0.5 apparent μmoles/mole of creatinine), however they are not in the affected range seen for patients with MPS I (range: 906–5326 apparent μmoles/mole of creatinine).
Discussion
In this study, we developed a functional platform that can be used to stratify IDUA variants identified by newborn screening in part based on their relative specific activity (RSA). Relative to the wild-type enzyme, this value is a measure of a variant enzyme’s activity towards a simple sugar substrate normalized to its amount within the same cellular lysate. It is important to note that this RSA value represents a single readout of enzymatic function, and that other factors, such as expression level, trafficking, processing, and stability are also relevant components to consider to accurately determine the pathogenicity of a given variant within the context of a cell or tissue. This platform, which accurately assigned the status of several known benign and pathogenic IDUA variants, was used to characterize thirty-five other IDUA variants, including several pseudodeficiency alleles and VUS. We observed a remarkable range of RSA values across these variants, highlighting the diversity of effects caused by the different missense variants. The strengths and limitations of the platform, and the new insights gained into how variants in this enzyme affect its function, are considered below.
The most variable component of the RSA value is the measurement of enzyme amount by western blot, which is semi-quantitative in nature. We observed highly consistent enzyme activity values between replicates of a single cell clone or selected population. The number of processed bands for some variant enzymes create a challenge for accurate densitometry because the sensitivity of anti-α-iduronidase antibody toward the different bands may be variable. In this report, however, we presumed that all three major bands (at 75, 70 and 62 kDa) have the same reactivity toward the antibody we used. Nonetheless, it is important to note that the western blot analysis in the platform is not just providing enzyme amount but provides highly useful information regarding the extent of proteolytic processing. As observed, several variant forms of α-iduronidase show no sign of proteolytic processing, with the exception of what is likely removal of the signal peptide in the ER. While we hypothesize the lack of proteolytic processing may indicate that the enzyme is poorly transported to the lysosome, it is also possible that such lysosomal delivery and processing does occur but that the lower molecular weight forms (70 or 62 kDa) are unstable and rapidly degraded. This would result in low or undetectable steady-state levels of the processed form of α-iduronidase. For example, p.Leu526Pro did not show the processed low molecular bands in most western blots. However, we observed some faint processed bands with longer primary antibody incubation.
Determining RSA values allowed us to estimate whether the inherent enzymatic function of the enzyme is maintained (despite low overall stability or expression) or compromised. It also permits the analysis of certain variant α-iduronidase enzymes with very low specific activity values (e.g., active site or misfolding variants) that still produce a stable enzyme. We estimate that for most variants, the resulting enzyme is overexpressed roughly 500-fold times higher than the abundance of α-iduronidase in parental, non-transfected HEK293 cells. This is beneficial in some sense as it makes it possible to reliably detect and quantify relative specific activity for variant α-iduronidase enzymes with very low residual activity (e.g., pathogenic variants). We could measure RSA values reliably down to 0.01%. Two variants (p.Ala75Thr or Thr364Lys) showed RSA values < 0.01%, which we noted approaches the level of background in our enzymatic assay. As an important limitation of the system, the overexpression of the enzyme did not allow for accurate localization within the secretory pathway, nor were we able to look at the clearance of endogenous GAG storage since it appears that some overexpressed variant enzymes may still reach the lysosome in sufficient abundance to remove any accumulated total GAGs. Future efforts will be directed at developing inducible expression systems for certain variants to better assess their trafficking within the secretory pathway as well as ability to clear lysosomal GAG fragments, and ask whether the phenotypes noted in our platform are consistent at endogenous levels of expression. Another limitation of the system relates to the possibility that certain single-nucleotide variants that create a missense change instead exert their effect at the level of splicing. Measuring RSA values using constructs that only reflect the missense change (like we did for p.Arg100Lys and p.Arg100Gly) will not give an accurate picture of pathogenicity and instead require independent analysis with SpliceAI or other tools to predict the possible splicing effects.
The inclusion of several established benign and pathogenic variants was necessary to validate this platform and demonstrate its utility for use in the formal classification of novel variants identified in IDUA with PS3 ACMG variant classification criteria. Of note, we included several variants commonly identified in abnormal newborn screening cases that have been associated with pseudodeficiency (low enzyme activity but not disease-causing)4,19,20. These variants exhibited an unexpected range of relative specific activity values (from ~5 to 70%), highlighting that an RSA value as low as 5% can be associated with benign variants. The p.Ala79Thr variant, a well-documented pseudodeficiency allele, was an example of a poorly processed enzyme with a low RSA value. Nonetheless, this variant has not been associated with severe or attenuated MPS I disease. The known pseudodeficiency variants in this study have allele frequency values between 0.0016 and 0.0051 (gnomAD v.4). We also noted that as expected, pseudodeficiency variants do not lead to elevated levels of NRE GAGs (Fig. 7).
The VUS p.His449Asn, which has been identified in numerous patients with an abnormal newborn screen for MPS I, has a comparable allele frequency (0.0024) to other pseudodeficiency variants. Together with its high, but not wild-type level, RSA (70%) and proper proteolytic processing, this suggests that p.His449Asn is also a pseudodeficiency variant. Additionally, p.Arg263Trp, which has been documented as another benign pseudodeficiency variant showed 31% of RSA and a very good processing score akin to WT-IDUA21. In contrast, p.Gly253Cys (RSA value of 31%) or p.Ser569Leu (RSA of 26%), showed no processing. Thus, interpretation of these variants is more challenging even though the RSA values are within the range not expected to cause clinical disease. Of note, p.Gly253Cys has been found in two previous MPS I patients (Hurler/Scheie or Scheie)22. Looking at protein structure, p.Gly253Cys does not seem to cause severe constraint since the Cys253 side chain would protrude to outside of enzyme surface. Interestingly, the Cys241 amino acid residue is found within 5 Å distance from Gly253 residue, providing a chance to make new cysteine bond (Supplementary Figure 2; the Gly253Cys variant structure was generated using DynaMut)23, possibly disrupting its structure significantly. These examples highlight the need to carefully interpret other factors when assessing variant pathogenicity.
Despite the observation that many of the variant enzymes fold and exit the ER, a surprising number exhibited defects in proteolytic processing, suggesting an impairment in reaching their final destination in the lysosome. We hypothesize that at least some of these variant α-iduronidases are prone to aggregation and entrapment within the post-ER secretory apparatus. This is consistent with the early observations made by Brooks and Hopwood on a more limited set of variants that were functionally characterized in CHO cells17,24. Our attempts to visualize the localization of the overexpressed enzyme were not successful, however, and other approaches to define the mechanisms that lead to possible post-ER aggregation are being explored. Collectively, our biochemical studies on the p.Leu526Pro variant do suggest that the structural changes caused by this and other variants make the enzyme more prone to aggregation. The cellular factors that dictate where this aggregation takes place within the secretory pathway, if any, are still unknown but of interest to understand as their manipulation may represent a therapeutic strategic to allow more enzyme to reach the lysosome.
Considerable effort was directed towards understanding the impact of the p.Leu526Pro variant on α-iduronidase function. This variant has been identified in patients with very attenuated MPS I4,18,19 but exhibited an RSA value within the non-disease-causing range, presenting a challenge for the current platform and highlighting the need for other functional studies to fully characterize its impact. We believe caution is warranted for certain variants when proteolytic processing of the variant enzyme is poor but the RSA value falls above the ~5% value, as it is possible some of these variant enzymes fail to efficiently traffic to the lysosome and can lead to the development of intralysosomal GAG storage (and ultimately, disease) despite substantial residual catalytic function. Numerous patients with p.Leu526Pro in trans with pathogenic variants exhibit only mild elevations of the UA-HNAc(1S) NRE fragment in urine, significantly lower than the elevations observed in MPS I patients with classically defined attenuated or severe phenotypes. Variants like p.Leu526Pro may also exert distinct overall clinical impacts on patients depending on the identity of the second variant, presence of multiple variants on the same allele, or their genomic background.
In summary, we present a new functional platform for the characterization of IDUA variants identified by newborn screening and uncover new features on how these variants impact enzyme function. The guidance provided by this functional work can assist genetics providers in making informed medical management decisions following positive newborn screens.

Responses