Hypergravity is more challenging than microgravity for the human sensorimotor system

Introduction
In our daily actions, the amount of force needed to move our limbs to produce the same action often varies, and this may be due to the surrounding force field. For instance, when movements are executed in a moving vehicle, the vehicle’s accelerations or decelerations may temporarily change the gravito-inertial force applied to the body. Altered gravity thus constitutes a specific problem for the central nervous system (CNS) because of the fundamental changes of body dynamics and biomechanical constraints which must be integrated to ensure correct motor performance. Understanding how the CNS is able, or not, to efficiently produce and regulate motor commands in microgravity and hypergravity remains a key issue in the field of human motor control. Such basic understanding is also critical to better prepare for moon or Mars missions.
Pioneer theoretical and computational studies have suggested that in human, the CNS is able to simulate and control sensorimotor behavior based on internal models1,2,3. More recently, the optimal feedback control framework has been proposed to further address the issue of control laws and their flexibility4,5,6. Such framework provides a robust foundation for understanding how the brain adjusts sensorimotor control in response to a changing context. Here, we focused on how sensorimotor control changes in altered gravitational environments.
Previous work suggests that the CNS represents gravity as an inertial force7,8, and a common mechanism may underlie the adjustments of sensorimotor control to different gravitational environments. Based on internal models and state estimation theories3, the CNS could determine, before movement initiation, the characteristics of the environment and their consequences on the motor system to optimize movement execution. However, hypergravity and microgravity have very different consequences on sensorimotor control, and the primary goal of the present study was to determine whether the sensorimotor control of whole-body reaching movements, which requires the coordination of multiple upper-limb, trunk and lower-limb muscles, can be efficiently adjusted in both gravitational environments.
It is well established that the absence of gravitational field results in substantial changes in sensorimotor control9,10,11,12,13. Studying microgravity episodes of parabolic flights has revealed that body unloading is taken into account in the planning and control of whole-body reaching movements14,15. Such reorganization, from the earliest stages of exposure to microgravity during parabolic flights, can result in preserved endpoint performance of reaching movements (i.e., similar as in normogravity; see15,16 for a review).
While motor performance has generally been found to be preserved in microgravity, conflicting findings have been reported regarding motor performance in hypergravity. Some studies reported unaltered motor performance in hypergravity compared to normogravity (e.g., movement accuracy17,18, with evidence of slow or partial sensorimotor reorganization of arm pointing8,19 and grasping20. For instance, Crevecoeur et al. reported that, within 3 trials, upward or downward arm movements were correctly executed in hypergravity (i.e., 1.8 g), as in normogravity17. In contrast, Bock et al. reported systematic errors in hypergravity compared to normogravity19. Although these discrepant observations might be the consequence of the distinct tasks and underlying mechanisms17, the overall findings related to human behavior in hypergravity still appear inconclusive.
Motor performance relies on movement planning and online control which are typically tightly integrated4,21, but in the context of pseudorandom perturbations around movement onset, motor performance is thought to critically rely on the efficiency of fast feedback mechanisms22,23,24. In microgravity, Bringoux et al. found unimpaired, fast visual feedback control mechanisms14 while Bock et al. reported impaired proprioceptive feedback control mechanisms in hypergravity19. In light of these findings, a secondary goal of the present study was to assess online control in altered gravity through the introduction of an unexpected mechanical disturbance concurrent with movement onset.
To sum up, we aimed to determine the effect of microgravity and hypergravity on the neuromuscular control mechanisms underlying whole-body reaching movements. To do so, we performed experiments on human participants in an aircraft with a specific timing during parabolic flights, resulting in experimentally controlled changes in background force level, with alternating periods of hypergravity (1.8 g, nearly twice Earth gravity), normogravity (1 g), and microgravity (nearly 0 g). Considering the rationale developed above, one could reasonably expect specific changes in control laws depending on the gravitational context.
Results
The study was carried out during a 3-day parabolic flight campaign. A programmed sequence of parabolic maneuvers resulted in successive changes of gravitational context (Fig. 1D). In order to study the effect of gravitational context on human motor control, each standing participant was asked at specific times during one of three phases (microgravity, hypergravity or normogravity) to reach toward one of two visual targets by means of upward arm movements (Fig. 1A). These targets were located close (Fig. 1B) and far (Fig. 1C) to study the planning and control of single-joint, shoulder movements and whole-body movements (involving the trunk and lower limbs), respectively. In 20% of trials, an electromagnet unexpectedly generated a mechanical perturbation on the distal part of the forearm at movement onset to specifically assess online control mechanisms. Overall, motor control mechanisms were assessed by means of fingertip and joint kinematics as well as surface electromyography (EMG).
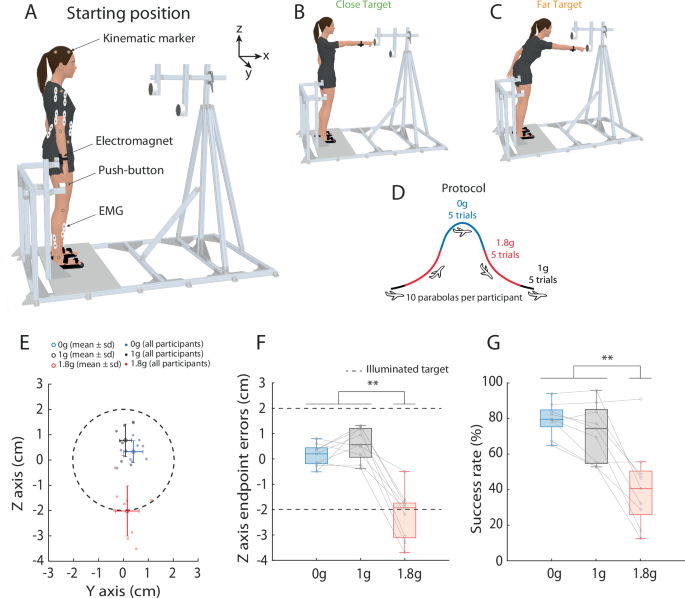
A Global view of the experimental set-up including the kinematic markers, the EMG electrodes, the push-button to standardize the fingertip start position and the electromagnet to unexpectedly perturb reach movement initiation (20% of trials). Reaching movements were performed toward either a (B) close target or (C) far target during parabolic flights, which allowed a modification of the gravitational environment (D). E Endpoint errors as a function of the gravitational environment. The illuminated target is represented as a dotted circle (diameter: 4 cm). Motor performance was impaired in hypergravity (1.8g), as shown by (F) endpoints errors on the Z axis and (G) success rate in each gravitational environment. Error bars represent standard deviation. **P < 0.01.
Stability of arm reach accuracy across parabolas
How functional is motor performance can be well summarized with final movement accuracy. The accuracy of finger endpoint position relative to the target center was mostly stable across parabolas. Final accuracy was compared between each parabola (from 1 to 10) in the three different environments (microgravity, hypergravity, normogravity). A 10×3 ANOVA performed on endpoint error on the Z axis showed a significant main effect of Environment (F(2, 16) = 48.96; p < 0.001; ηp² = 0.86) and a significant interaction between Environment x Parabola (F(18, 144) = 3.09; p < 0.001). Post-hoc analysis revealed that endpoint accuracy in hypergravity only differed in parabola 1 (mean = -3.7 cm) relative to the other parabolas (mean = -2.1 cm; p < 0.05). This observation, previously reported in hypergravity17, is presumably due to the large initial errors as well as to a rapid, albeit incomplete, adaptation in hypergravity. Overall, a systematic undershooting was observed in hypergravity across the following parabolas compared to microgravity and normogravity (see video in supplementary materials). To focus on the influence of the gravitational level on motor responses and avoid initial exposure effects, data from the first parabola were removed (see Sainburg and Kalakanis24 for a similar method) and will be specifically discussed elsewhere. In the following sections, we dissect motor performance for the last nine parabolas during which movement accuracy did not significantly differ for any given gravitational environment.
Endpoint accuracy and arm angular elevation across gravity conditions
Figure 1E shows that final reach errors were greater in hypergravity than in normogravity and microgravity. A 3x2x2 ANOVA [Environment (Hypergravity, Normogravity, Microgravity) x Target (Close, Far) x Perturbation (On, Off)] performed on endpoint errors on the Z axis showed a significant main effect of Environment (F(2, 16) = 49.77; p < 0.001; ηp² = 0.86). Figure 1F shows that endpoint errors on the Z axis were greater in hypergravity (-2.2 ± 1.2 cm) than in microgravity (0.1 ± 0.6 cm; p < 0.001) and normogravity (0.6 ± 1.0 cm; p < 0.001). No other main effect or interaction was significant (Target: p = 0.61; Perturbation: p = 0.23; Environment × Target: p = 0.1; Environment x Perturbation: p = 0.98; Target x Perturbation: p = 0.81; Environment x Target x Perturbation: p = 0.85). The impaired performance observed in hypergravity was further highlighted by the analysis of the success rate (i.e., the percentage of actual target reaching; Fig. 1G). The ANOVA showed a significant main effect of Environment (F(2, 16) = 24.28; p < 0.001; ηp² = 0.99), and post-hoc analysis revealed that the success rate was lower in hypergravity (41 ± 26%) than in microgravity (79 ± 17%; p < 0.001) and normogravity (71 ± 21%; p < 0.001). To summarize, final accuracy was clearly impaired in hypergravity compared to microgravity and normogravity.
The duration of arm movements was influenced by the gravitational environment and the target position. The ANOVA performed on arm movement duration showed a significant main effect of Environment (F(2, 16) = 42.38; p < 0.001; ηp² = 0.84), Target (F(1, 8) = 126.74; p < 0.001; ηp² = 0.94) and a significant interaction between Environment x Target (F(2, 16) = 12.19; p < 0.001; ηp² = 0.6). Arm movements toward the far target lasted longer (613 ± 90 ms) than those toward the close target (491 ± 60 ms; p < 0.001) whatever the environment. In addition, movement duration was higher in microgravity (613 ± 105 ms) than in normogravity (524 ± 78 ms; p < 0.001) and hypergravity (519 ± 79 ms; p < 0.001), particularly when participants reached toward the far target (697 ± 76 ms; p < 0.001) as compared to the close target (528 ± 448 ms; p < 0.01). No other main effects or interactions were found to be significant (Perturbation: p = 0.82; Environment × Perturbation: p = 0.99; Target x Perturbation: p = 0.18; Environment x Target x Perturbation: p = 0.80). In sum, movement duration was longer in microgravity compared to hypergravity and normogravity.
The temporal organization of arm reaching was also found to be modified in microgravity. The ANOVA performed on the relative duration of the acceleration phase, between movement onset and arm peak velocity, showed a significant main effect of Environment (F(2, 16) = 8.28; p < 0.01; ηp² = 0.51), Target (F(1, 8) = 49.85; p < 0.001; ηp² = 0.86) and Perturbation (F(1, 8) = 5.49; p < 0.05; ηp² = 0.41). Acceleration duration represented a smaller part of movement duration in microgravity (30 ± 3%) than in normogravity (33 ± 5%; p < 0.001) and hypergravity (32 ± 5%; p < 0.001; Fig. 2D). Moreover, the part of acceleration duration was smaller for movements toward the far target (28 ± 4%) than the close target (35 ± 4%; p < 0.001; Fig. 2G). When the electromagnet was switched on, the part of acceleration duration (31 ± 5%) was smaller than when it was off (32 ± 5%; p < 0.05; Fig. 2J). No significant interaction was found between these factors (Environment × Target: p = 0.21; Environment x Perturbation: p = 0.79; Target x Perturbation: p = 0.95; Environment x Target x Perturbation: p = 0.79). The most important finding here may be that movement deceleration represented a larger part of movement execution in microgravity than in normogravity and hypergravity.
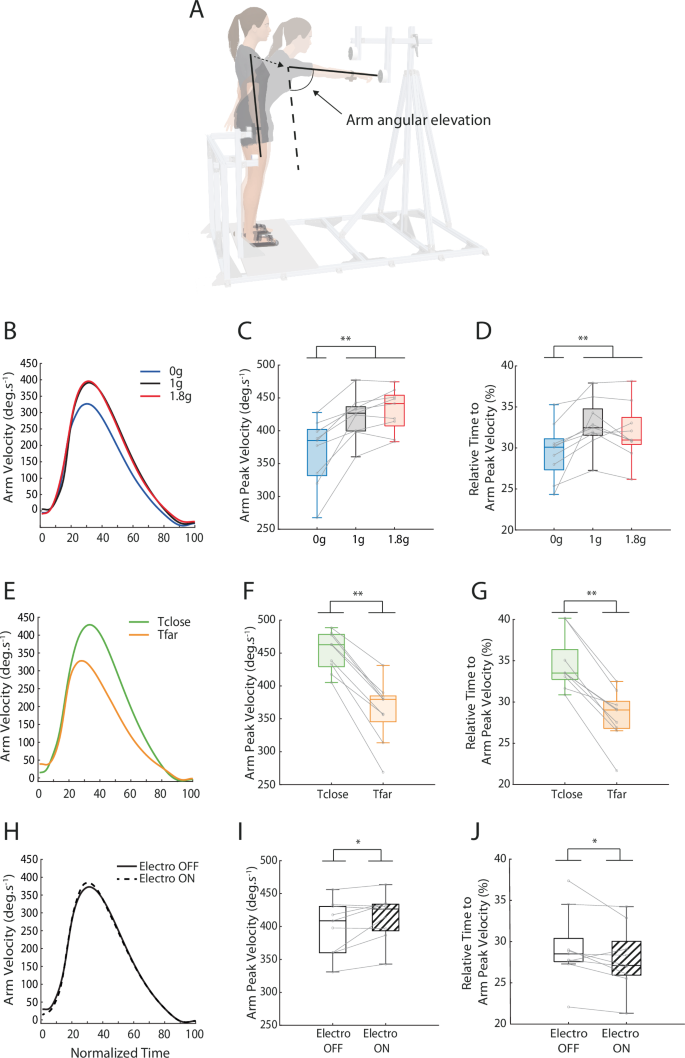
A Illustration of arm angular elevation during reaching toward the far target. (B, E, H) Movement velocity as a function of experimental conditions. (C, F, I) Arm peak velocity, (D, G, J) and relative time to arm peak velocity as a function of (B–D) Environment, (E–G) Target, and (H–J) Perturbation. Environment was microgravity (0g), hypergravity (1.8g) or normogravity (1g). Participants either had to reach toward a close or a far target. On 20% of the trials, an electromagnet was switched on and participants had to adjust motor commands to reach the target. Error bars represent standard deviation. *P < 0.05, **P < 0.01.
Participants modified the spatiotemporal organization of arm movements in microgravity but not in hypergravity with respect to normogravity. The ANOVA performed on arm peak velocity showed a significant main effect of Environment (F(2, 16) = 30.61; p < 0.001; ηp² = 0.79), Target (F(1, 8) = 81,2; p < 0.001; ηp² = 0.91) and Perturbation (F(1, 8) = 8.3; p < 0.05; ηp² = 0.51). Arm peak velocity was lower in microgravity (369 ± 73 deg.s-1) than in normogravity (424 ± 61 deg.s-1; p < 0.001) and hypergravity (433 ± 63 deg.s-1; p < 0.001; Fig. 2B-C). We also observed that arm peak velocity was greater for movements toward the close target (454 ± 48 deg.s-1) than the far target (363 ± 61 deg.s-1; p < 0.001; Fig. 2F). Arm peak velocity was greater when the mechanical perturbation was unexpectedly switched on (418 ± 71 deg.s-1) than when it was off (400 ± 71 deg.s-1; p < 0.05; Fig. 2I). No significant interaction was found between these factors (Environment × Target: p = 0.26; Environment x Perturbation: p = 0.21; Target x Perturbation: p = 0.61; Environment x Target x Perturbation: p = 0.66). These findings highlight the fact that movements were slower in microgravity compared to normogravity and hypergravity.
Whole-body kinematics
While we asked participants to perform movements toward the close target in order to study motor control when there is no requirement to change the body posture to reach the target goal, movements toward the far target were used to study interactions between arm movement and body posture control mechanisms. Indeed, Fig. 3A shows that during whole-body reaching, changes in body posture have to be coordinated with the arm movement in order to accurately and rapidly reach a target. Analysis of body orientation was thus performed only for movements toward the far target. The analysis revealed that for these movements, whole-body reorganizations were present in microgravity but not in hypergravity compared to normogravity. Indeed, the ANOVA on whole-body tilt showed a significant main effect of Environment (F(2, 16) = 27.77; p < 0.001; ηp² = 0.78; Fig. 3B). When participants reached toward the far target, whole-body tilt in microgravity (13 ± 3 deg) differed from that in normogravity (9 ± 2 deg; p < 0.001) and hypergravity (10 ± 2 deg; p < 0.001). No other significant main effect or interaction was found with the other factors (Perturbation: p = 0.31; Environment x Perturbation: p = 0.26). This indicates that a different whole-body coordination strategy was used in microgravity compared to normogravity and hypergravity.
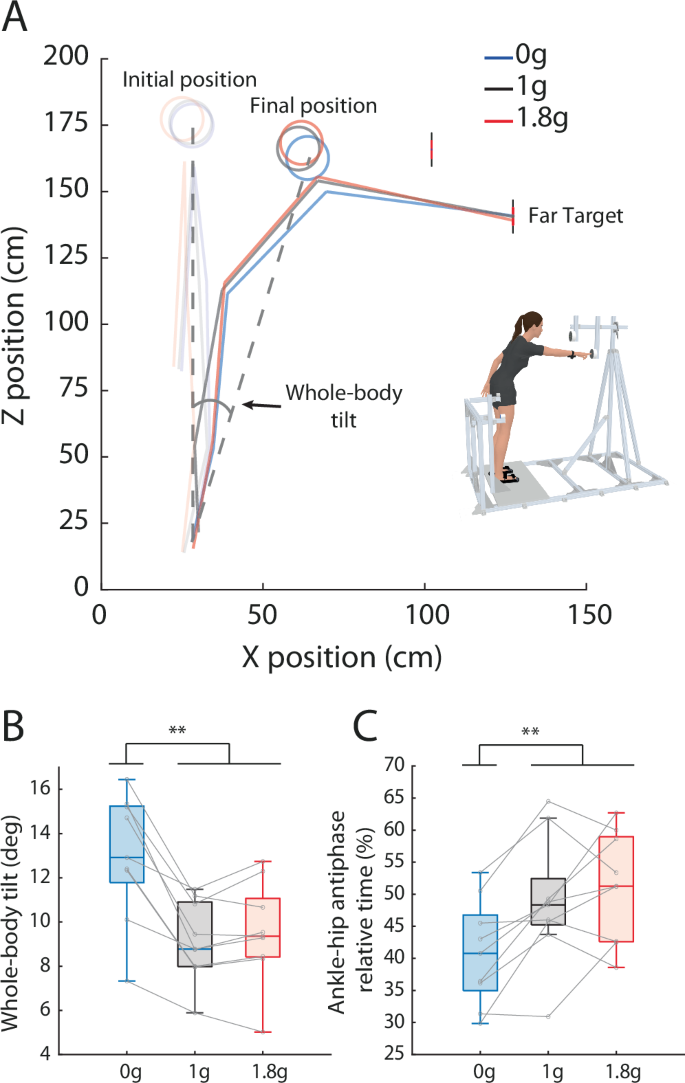
A Representative whole-body kinematics during reaching toward the far target for a typical participant in microgravity (0 g), hypergravity (1.8 g) and normogravity (1 g). B Mean forward whole-body tilt (orientation of the ankle-head segment relative to vertical) as a function of the Environment. C Mean time spent using a hip strategy as a function of the Environment. Whole-body reorganization was only significant in microgravity (compared to normogravity and hypergravity). Error bars represent standard deviation. **P < 0.01.
The analysis of joint coordination confirmed that the whole-body strategy in microgravity differed from normogravity and hypergravity. An ANOVA performed on the relative time participants used a “hip strategy” (ankle-hip antiphase relative time) showed a significant main effect of Environment (F(2, 16) = 8.61; p < 0.01; ηp² = 0.52; Fig. 3C). The ankle-hip antiphase relative time was indeed lower in microgravity (41 ± 8 %) compared to normogravity (49 ± 10 %; p < 0.01) and hypergravity (51 ± 9 %; p < 0.01). No other significant main effect or interaction was found with the other factors (Perturbation: p = 0.47; Environment x Perturbation: p = 0.26). Overall, participants used less a “hip strategy” (and more an “ankle strategy”) in microgravity compared tonormogravity, as well as hypergravity.
Muscular synergies
In order to identify synergies between the eight main muscles activated during whole-body reaching, a non-negative matrix factorization was used (NNMF: see methods and26 for more details). The NNMF decomposed the electromyographic (EMG) signals in a few synergies to describe the contribution of each muscle (from 0 to 1) and the temporal activation pattern of the synergy over time (from 0 to 1). The NNMF computed on the 8 muscles revealed that 3 main synergies were used to control whole-body reaching movements toward the far target (Fig. 4). Synergy 1 mainly involved the tibialis anterior and the rectus abdominis around movement onset to prepare the forward body displacement necessary to reach the far target (Fig. 4C). Synergy 2 mainly involved the deltoid anterior and the biceps brachii to produce both forward body displacement and arm elevation. Synergy 3 mainly involved the deltoid posterior and triceps brachial, mostly around movement offset to slow down and stop arm elevation. In parallel, leg and trunk antagonist muscles (soleus, rectus abdominis and erector spinae) were activated to maintain final position while the body was tilted forward.
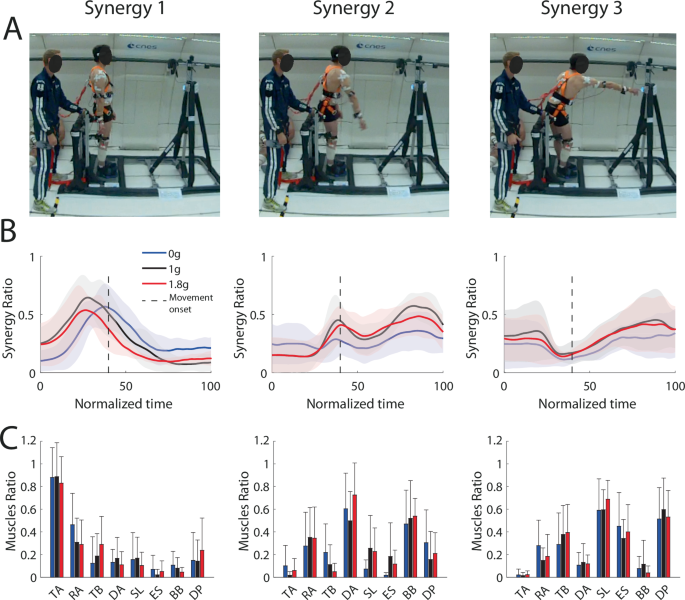
A Illustration of three synergies corresponding to three main movement phases in microgravity (0g), normogravity (1g) and hypergravity (1.8g). B Mean and standard deviation of muscular synergy ratio and (C) mean of muscle ratio for each environment. Synergy 1 mainly corresponded to an activation of Tibialis Anterior (TA) and Rectus Abdominis (RA) early in the movement. Synergy 2 mainly involved the agonist muscles (Deltoid Anterior, Rectus Abdominis, Biceps Brachii) which allowed forward body tilt and arm elevation. Synergy 3 was mainly present at the end of movement where participants slowed down arm elevation by activating antagonist arm muscles (Triceps Brachii and Deltoid Posterior) and maintained final position by activating leg and trunk muscles (Soleus, Rectus Abdominis and Erector Supinae). Error bars represent standard deviation.
To determine whether muscular synergies changed as a function of the gravitational context, a repeated-measure ANOVA was conducted with statistical parametric mapping (SPM) analysis and revealed a significant effect of Environment (F(2, 16) = 8.164) only for synergy 1. However, post hoc analyses with Bonferroni correction showed no significant difference (p > 0.02). Overall, this analysis revealed that motor control relied on a similar set of few synergies in the normogravity, hypergravity and microgravity environments that we studied.
Level of muscle activation
The previous synergy analysis mainly focused on the temporal characteristics of muscle activity. In a second EMG analysis, we aimed at quantifying the level of muscle activity during the planning stage of motor commands to focus on the ability of the nervous system to anticipate the consequences of the gravity environment. We used a 200 ms window from EMG activity onset (see materials and methods for more details) and, because antagonist muscles are mostly inhibited around movement onset, only the four agonist muscles (tibialis anterior, rectus abdominis, deltoid anterior and biceps brachii) were analyzed.
The ANOVA performed on tibialis anterior RMS showed a significant main effect of Environment (F(2, 16) = 8.32; p < 0.01; ηp² = 0.51; Fig. 5A-B). Muscular activity of tibialis anterior was lower in microgravity (196.47 a.u. ± 4.67) than in normogravity (316.25 a.u. ± 76.96; p < 0.01) and hypergravity (271.32 a.u. ± 91.04; p < 0.05). No other significant main effect or interaction was found (Perturbation: p = 0.47; Environment x Perturbation: p = 0.23).
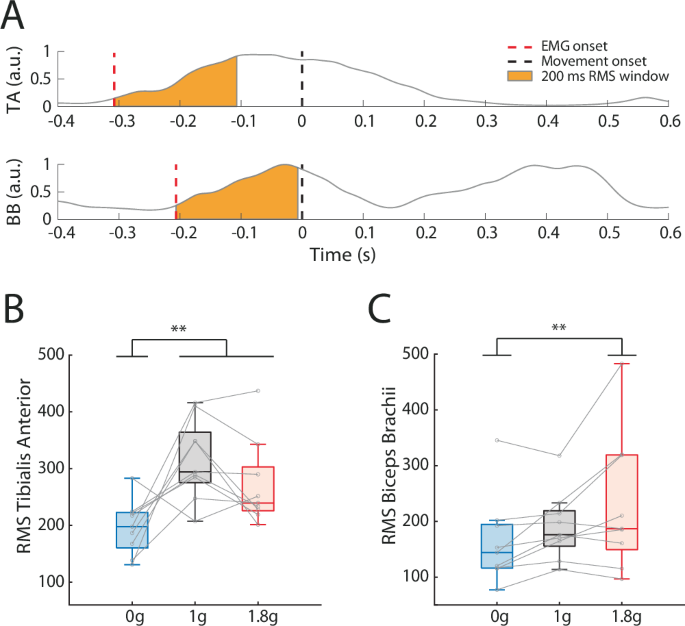
A Mean normalized EMG activity of Tibialis Anterior (TA) and Biceps Brachii (BB) during reaching toward the far target for a typical participant in normogravity. The orange areas represent the temporal windows during which Root Mean Square (RMS) was computed. Mean RMS of (B) Tibialis Anterior and (C) Biceps Brachii as a function of Environment. Activity of Tibialis Anterior was lower in microgravity (0 g) than in normogravity (1 g) and hypergravity (1.8 g), and activity of Biceps Brachii was lower in microgravity than in hypergravity. Error bars represent standard deviation. **P < 0.01.
The ANOVA performed on biceps brachii RMS showed a significant main effect of Environment (F(2, 16) = 6.45; p < 0.01; ηp² = 0.45; Fig. 5C). Biceps brachii RMS was higher in hypergravity (230.79 a.u. ± 143.5) than in microgravity (162.92 a.u. ± 93.82; p < 0.01) and almost significantly than in normogravity (190.78 a.u. ± 60.92; p = 0.051). No other significant main effect or interaction was found (Perturbation: p = 0.36; Environment x Perturbation: p = 0.31). Furthermore, there was no other significant effect on the other recorded agonist muscles
(rectus abdominis and deltoid anterior).
Principal Component Analysis
A Principal Component Analysis was performed on the recorded kinematic and electromyographic data to further study and compare the coordination patterns in normogravity, microgravity and hypergravity. For all gravity environments, scree plots in Fig. 6A-C show that two Principal Components (PCs) could explain more than 50% of the variance: for the sake of clarity, we focused our analysis on PC1 and PC2. The projection of variables of these two PCs were displayed for each environment in Fig. 6D-F. These panels revealed that in normogravity and microgravity, success rate resulted from a complex interaction of multiple factors. In hypergravity, the PCA revealed an unexpected relationship between arm peak velocity and success rate, as these variables were in the same quadrant and had a similar direction. This indicated that in hypergravity, the higher arm peak velocity, the higher success rate. This may be counter-intuitive at first sight considering the well-known speed-accuracy tradeoff and the expected overshoot in faster movements, but given that hypergravity was typically associated with undershoot, faster movements were actually associated with a greater success rate (Fig. 6F). At last, it is worth mentioning that success rate was seen to be directed opposite to endpoint error, which makes sense only because endpoint errors were negative (thus, success rate increased as the negative values of endpoint errors increased toward zero).
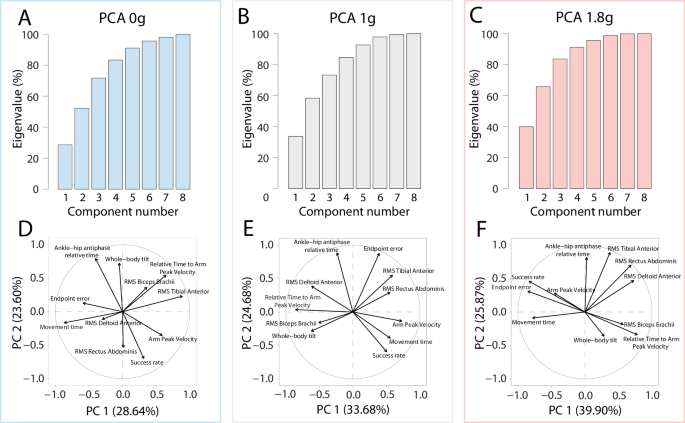
A–C Scree plot of eigenvalues for each environment. D–F PCA loading plot of mean kinematic and muscular activity variables for each environment. PC1 and PC2 together explain at least 50% of the variance. The distribution of kinematic and EMG variables varies along these components according to the environment.
Discussion
The present study tested the hypothesis that hypergravity and microgravity have distinct effects on the performance of a complex motor skill compared to normogravity. We investigated the influence of gravity level on the neuromuscular control of whole-body reaching movements, which requires the fine coordination of multiple finger, hand, arm, trunk and leg muscles. The net output of the underlying control mechanisms was assessed with endpoint accuracy, which showed little effect of microgravity compared to normogravity, while undershooting was systematically found across participants in hypergravity. Analysis of arm and whole-body kinematics, as well as muscle activity, revealed a reorganization of motor planning in microgravity compared to normogravity, but little reorganization in hypergravity. This supports the view that microgravity can rapidly and efficiently be taken into account by the nervous system to maintain functional motor performance, while hypergravity is a more challenging environment. Despite the fact that online motor control, assessed with responses to unexpected mechanical perturbations, was found to be functional in hypergravity, it was not sufficient to minimize reach errors to the level seen in microgravity and normogravity. The singularity of parabolic flight environments and experimental set-up necessarily limits the generalization of our conclusions on adaptive mechanisms to changing force fields (e.g., aeronautics or automotive research) which may take place in everyday motor behavior. Nevertheless, our results may constitute a clear basis for better understanding how different reorganizations may be at work in different gravitational contexts.
Microgravity did not affect whole-body reaching performance in terms of final spatial accuracy. This is consistent with previous work15,25,27,28 and further suggests that the optimal integration of novel body and arm dynamics in microgravity allows the central nervous system to produce adapted motor commands. The complete behavioral reorganization observed in microgravity could rely on rapid, vestibular-based mechanisms which may allow a rapid updating of the internal model of limb dynamics16,29,30,31. This updating was observed on the spatiotemporal organization of arm movement as, in line with a previous study15, arm peak velocity was observed earlier during the movement in microgravity. This corresponded to a larger deceleration phase relative to movement duration, which may facilitate sensory feedback control in microgravity to maintain endpoint accuracy as in normogravity32,33. Consistent with this idea and previous findings20, the present study also revealed a lower arm peak velocity and a longer movement duration in microgravity. In line with these observations, a PCA confirmed the relationship between final movement accuracy and kinematic variables, supporting the idea that a longer relative deceleration time may be an efficient strategy to benefit more from feedback control. However, this strategy prioritizing accuracy is sufficient when facing microgravity34,35 but not hypergravity.
The major finding of the present study may be that motor performance was largely altered in hypergravity. The analysis of spatial accuracy revealed a systematic undershooting in hypergravity, where about half of the trials were off target. This suggests that, in hypergravity, motor commands are produced according to a normogravity standard and consequently, the force produced to reach the target is not sufficient to reach the target36,37. In the present study, we found mostly similar muscle activations before movement onset (except for the biceps brachii) in hypergravity and normogravity. The insufficient changes of motor commands in hypergravity compared to normogravity, as observed with muscle synergies and principal component analyses, appears to be directly related to the observed reaching errors, suggesting that, overall, hypergravity represents a greater challenge for the human nervous system than microgravity. We speculate that whole-body loading is harder to deal with than unloading for the sensorimotor system because, for instance, higher gravitational torques and thus additional energy must be engaged to compensate for the supplementary mechanical constraints exerted at numerous body joints19,38,39. In this sense, perceived changes in limb inertia and joint torque requirements in hypergravity may affect motor control strategies and provide an additional explanation for the decrease in performance.
While inertial factors remain unchanged regardless of the gravitational context (as arm mass does not change), the increased or decreased gravitational torque (in hypergravity or microgravity, respectively) alters body dynamics. Bringoux et al. showed that in microgravity, maintaining reach accuracy requires a reduction in the joint torque generated by the shoulder muscles14 as without such neuromuscular adjustment, a systematic overshooting would be observed. In hypergravity, the combination of increased gravitational torque and maintained limb inertia could complicate motor control. Studies of object manipulation reported that an increase in the weight of the arm and the object being manipulated can be perceived as an increase in mass, and therefore lead to an overestimation of the inertial load20,29,40. In the case of whole-body reaching, such overestimation could lead to excessive deceleration of the mobilized limb, resulting in an undershooting of the intended final position of the end-effector. The fact that the acceleration phase of the movements was similar in normo- and hypergravity supports the latter idea. At a more theoretical level, future work is needed to clarify whether undershooting in hypergravity is due to an impaired internal model of limb dynamics.
The optimal control theory4,41 presents an interesting framework to explain motor performance in altered gravity. Optimality rules usually consist in minimizing a parameter (e.g., energy cost or error) by optimizing muscular activations42) while maximizing task success43. For instance, motor performance in normogravity was associated with a more homogeneous distribution of muscular activity variables (as shown by the PCA) than in micro- or hypergravity. In normogravity too, a positive correlation between success rate and movement time clearly appeared: the longer the movement time, the higher the success rate, reflecting a classic speed-accuracy tradeoff, which has been widely studied on Earth, but also in modified gravity conditions44. The absence of gravitational constraints in microgravity implies a decrease of required muscular activity that favors the minimization of energy costs and that typically does not alter task success. On the other hand, the higher gravitational constraints in hypergravity did not result in a change in control policy to optimize the tradeoff between task success and energy cost. Therefore, it seems that hypergravity constitutes a significant challenge that cannot be easily solved by the sensorimotor system.
Consistent with previous studies, we found that microgravity resulted in a reorganization of coordination patterns across the whole body to maintain the performance of reaching arm movements toward the far target. The muscle synergies and principal component analyses were consistent with the idea that whole-body postural reorganization is critical for arm motor control in a microgravity environment. For instance, a larger body displacement was found in microgravity compared to normogravity15,45. Moreover, the analysis of hip and ankle joint coordination during movement execution revealed a simultaneous flexion, which corresponds to a preference for an “ankle strategy”46 in microgravity which differed from the strategy typically observed in normogravity47,48. Because gravitational constraints acting upon the control of stance were absent in microgravity, participants in our study appeared to exploit the possibility of exceeding the usual limits of support surface and showed a larger forward body tilt than in normogravity.
In hypergravity, whole-body movement control appeared to be similar to that in normogravity. More specifically, forward body displacement, hip and ankle coordination, and muscle activations were similar in hypergravity compared to normogravity. While this still allowed participants to maintain balance in hypergravity, we hypothesize that the lack of reorganization at the control level made it difficult to maintain an accurate reach performance.
We analyzed muscular synergies to investigate the possible dimensional reduction operated by the central nervous system to produce complex movements in various conditions49. This also allowed us to provide a global description of temporal and spatial muscular activity. Interestingly, the set of synergies which best explained all muscular activations remained similar despite the great differences between gravitational contexts. This is consistent with a study of Botzheim et al. who investigated the effects of gravity on an arm cycling task by comparing two cycling conditions (i.e., sitting and supine) and found the same set of synergies in both conditions despite considerable biomechanical differences50. Instead of changing muscular synergies for distinct gravitational constraints, the central nervous system sometimes appears to maintain a general structure of muscle activations, as seen for upper limb movement coordination51. The similar synergies despite different gravitational contexts could reveal a fundamental building block of whole-body reaching performance. This finding is in contrast with studies on motor primitives and modularity which suggested that synergies may be flexibly adjusted to meet task demands49,51 as recently shown in microgravity52. While our statistical results were likely influenced by the limited sample size of our study, the conservation of a normogravity pattern during hypergravity may reflect a failure to respond to task demands, which could explain the low success rate observed in hypergravity. Nevertheless, there have been numerous debates on the issue of fixed or flexible muscle synergies53,54 and further work is necessary to clarify this issue in hypergravity.
Although synergies were not found to change ‘qualitatively’ across gravitational contexts, we found subtle adjustments of muscle activations as a function of gravity level. PCAs revealed smaller vector lengths of muscle RMS in the micro- and normogravity conditions compared to the hypergravity condition, suggesting that the activity of the recorded muscles explained less of the variance of the performance observed in normal or microgravity conditions. Indeed, arm and whole-body kinematics adjustments observed in microgravity compared to normogravity and hypergravity appeared directly linked to context-specific muscular changes. In microgravity, unloading reduces the necessity to produce a high torque at a given joint during whole-body reaching movements14. Here, we observed in microgravity a decrease of tibialis anterior activation at the onset of muscle activation, which may reflect the lower gravitational constraints at the ankle joint during forward body tilt. In hypergravity, only arm muscular involvement was affected, as revealed by the increased biceps brachii activity around movement onset, although insufficiently to counteract the greater gravitational acceleration and maintain final accuracy as in normogravity. These findings support the idea that microgravity constraints are taken into account by the nervous system, contrasting here with hypergravity. A complementary hypothesis could be attributed to direct biomechanical responses55, as changes in muscle activity do not necessarily reflect high-level sensorimotor loops. While gravity conditions may also affect sensory receptors’ activation10, the activation changes observed in the biceps brachii or tibialis anterior might reflect the straightforward biomechanical effect of gravitational change, without necessarily being the result of a complex CNS reorganization.
Online motor corrections following an unexpected mechanical perturbation were still effective in microgravity and hypergravity to maintain final reach accuracy with respect to movements achieved without perturbation, as classically observed in normogravity. The perturbation on the forearm around movement onset was associated with an increased velocity of arm elevation, which likely reflects the compensatory response. In microgravity, which required participants to deal with the absence of external forces, an unexpected mechanical perturbation was not an obstacle to motor performance. Online motor control was thus efficient, and our findings support and extend those obtained on a similar task by Bringoux et al. who reported efficient feedback responses in microgravity when the target was unexpectedly displaced at movement onset14. In the present study, participants mostly looked at the illuminated target and considering that the forearm was essentially out of sight at the time when it was perturbed, we believe that compensatory responses mostly reflect proprioceptive feedback loops, which were efficient enough to maintain final accuracy at the same level for perturbed and unperturbed movements in all three force fields. This is consistent with findings observed with robotic force fields56,57,58. Overall, the nervous system has an impressive ability across various conditions to maintain efficient feedback responses, which is likely linked to the functional interplay between adaptive and fast feedback control mechanisms21,59,60,61.
As parabolic flights involve significant constraints which restrict the number of participants, the lack of a significant difference in some parameters (e.g. muscular synergies at 0 g) may be due to the limited statistical power associated with the small sample size in this study. Also, such a small number of participants was less than ideal to further study inter-subject variability: it would thus be interesting that additional work characterizes the variety of compensatory strategies which may be used in microgravity and hypergravity. While surface EMG provides some insights, other neurophysiological recordings would provide additional clarifications in the underlying sensorimotor control mechanisms.
Despite the limitations listed above, the present study provides new insights on motor performance in altered gravity. We studied hyper- and microgravity environments to study the influence of gravity. We found that hypergravity represents an interesting test of the conclusions which may be reached by studying microgravity, as the asymmetry of our findings in hyper- and microgravity suggests that what has been found in microgravity may not systematically be generalized to hypergravity. Errors in whole-body reaching, as well as the general lack of sensorimotor reorganization observed in hypergravity, indicate that hypergravity represents a greater challenge than microgravity for human motor control.
Among the numerous challenges inherent to space exploration62, our findings suggest that the weightlessness conditions which may be encountered during lunar or Martian missions may not present an insurmountable problem for human motor skills. However, the transition phases during space travel (e.g., take-off and landing) may be associated with impaired sensorimotor interactions (such as take-over). More broadly, the understanding gained from the study of sensorimotor control processes in such extreme environments may be transferred to the field of health and rehabilitation. The challenge there is to improve the treatments of sensorimotor impairments, for which loading or unloading protocols may not produce the same effects.
Materials and methods
Participants
Nine right-handed volunteers (mean age = 30.8 ± 8.5 years, 4 female) participated in the study. They had no prior experience of parabolic flight and were naïve to the purpose of the study. None of the participants reported any neuromuscular or sensory impairments, as confirmed by a prior medical examination, and all had normal or corrected-to-normal vision. Participants gave their signed informed consent prior to the study in accordance with the Helsinki Convention and gave a written consent to publish the details, images, or videos. Before the parabolic flight, participants were given comfort medication (scopolamine) to avoid motion sickness. This medication has been shown to not alter sensorimotor control (e.g., reflex, neuromuscular control, balance performance and force-generating capacity63). The study was authorized by the French National Agency for Biomedical Security (ANSM) and approved by the National Ethic Committee (CPP # 2015-A01231-48).
Apparatus
Participants stood upright and their feet were fastened to the aircraft floor with foot-straps (Fig. 1A). A push-button located alongside the right side of their body at arm length distance from the shoulder was used to standardize the starting fingertip position. In front of each participant, two circular targets (external diameter: 10 cm; illuminated dotted circle diameter: 4 cm; Fig. 1B-C) were located relative to participant’s anthropometry. A close target was set at shoulder’s height at a distance corresponding to the arm length, and a far target was positioned 25 cm away and 20 cm below the close target. The positions of the close and far targets were designed to investigate the neuromuscular control of single-joint shoulder arm movement and whole-body movements (i.e., involving movements of the trunk and lower-limb joints), respectively. Participants wore a metal surface bracelet at the forearm, which had to be positioned against an electromagnet located above the push-button (Fig. 1A) before movement onset. When activated, the electromagnet could generate a mechanical brake (pullout force: 60 N) at reach initiation. Target illumination and electromagnet activation were controlled with a homemade software (Docometre©) and a real-time acquisition and control system (ADwin-Gold©, Jäger, Lorsch, Germany).
Infra-red active markers were installed on the head, shoulder, hip, knee, ankle, and index fingertip. Their coordinates were recorded at 100 Hz with an optical motion capture system (Codamotion CXS and ActiveHub; Charnwood Dynamics, Leicestershire, UK) for offline analysis of both arm and whole-body displacement.
Surface electromyography (EMG) was recorded at 2000 Hz (BIOPAC Systems, Inc., Santa Barbara, CA) from 4 agonist muscles (Tibialis Anterior, TA; Rectus Abdominis, RA; Deltoid Anterior, DA; lateral head of Biceps Brachii, BB) and 4 antagonist muscles (Soleus, SL; Erector Spinae, ES; Deltoid Posterior, DP; short head of Triceps Brachii, TB) involved in the neuromuscular control of whole-body reaching movements. Participants’ skin was cleaned with alcohol and rubbed with an abrasive paper before affixing the surface electrodes (Ag-AgCl; diameter 1 cm, spacing 2 cm) along a line parallel to their fiber orientation to increase the signal-to-noise ratio (according to the SENIAM recommendations)64,65,66.
Procedure
The study was carried out during the 3-day parabolic flight campaign #142 of the French National Space Research Center (CNES). The micro- and hypergravity contexts were produced by a series of parabolic maneuvers in an A-310 ZERO-G aircraft chartered by CNES and Novespace. For each participant, a flight involved successive changes of gravitational context that could be decomposed in 24 s of hypergravity (1.8 g), 22 s of microgravity (0 g) and 22 s of hypergravity (1.8 g) followed by normogravity (1 g) during 1 min between each parabola (Fig. 1D). Each participant performed 15 trials successively (5 trials in 0 g, 5 trials in 1.8 g and 5 trials in normogravity) for each of the 10 parabolas. Procedural constraints hardly enabled randomization or counterbalancing of exposure to different gravitational conditions. The 1.8 g condition was tested only during the pull-down phase as in Crevecoeur et al17. and we acknowledge that this specific order constitutes a limitation with regard to the influence of gravity on our results. The total of 150 trials was composed of 75 trials toward the close target and 75 trials toward the far target. In 20% of trials, the electromagnet generated a mechanical brake applied to the foream while the hand was in start position. Participants were informed of the possible occurrence of these perturbed trials but had no information regarding their sequencing. Target and Perturbation (i.e., mechanical brake) conditions were presented in a pseudorandom order and counterbalanced between participants.
The task consisted in reaching toward the target with the outstretched arm, as fast and as accurately as possible when the target was switched on (see Supplementary Video). Participants had to maintain the final finger position until target extinction (3 s after movement onset) before returning to the starting position (i.e., upright standing, with the right index finger pressing the start push-button). The experimental session lasted about 20 min.
Data processing
Data were analyzed using Matlab (Mathworks, Natick, MA). Raw positional data of kinematic markers were low-pass filtered with a dual-pass Butterworth (cut-off frequency: 10 Hz; order: 3). Movement duration was defined as the time between movement onset and offset, which were determined when the tangential velocity of index finger exceeded and fell below 2% of arm peak velocity, respectively. The spatial accuracy of reaching movements was analyzed by computing the signed deviation along the longitudinal axis (z-axis, perpendicular to the floor) of the right index fingertip at movement offset with respect to the target center. Positive and negative longitudinal endpoint error corresponded to overshooting and undershooting, respectively. The success rate was also calculated, corresponding to the percentage of trials where the index fingertip actually reached the target (i.e., within the 4 cm diameter circle).
To analyze arm kinematics during reaching movement, arm elevation (i.e., shoulder-fingertip angle in the sagittal plane with respect to its initial orientation; Fig. 2A) was computed across time14,15,67. From these values, the arm peak velocity and its relative time of occurrence expressed in percentage of movement duration were computed.
Whole-body movement kinematics and multi-joint coordination patterns were also analyzed. The body displacement at movement offset was computed as the ankle-head angle relative to the z axis (perpendicular to the aircraft floor, in the sagittal plane (Fig. 3A). The coordination between the ankle and hip joints was examined, using the Continuous Relative Phase analysis (CRP)68,69. For each trial, ankle and hip phase angles were expressed through a “parametric phase plot” where the normalized angular positions (relative to the maximum value of each trial) were plotted relative to the normalized arm velocity. Then, the calculated CRP corresponded to: ({CRP}left(tright)={varphi }_{1}left(tright)-,{varphi }_{2}(t)) where ({varphi }_{1}left(tright)) and ({varphi }_{2}(t)) are the normalized phase angles for ankle and hip, respectively. Finally, the CRP was scaled from 0° to 180° where a CRP(t) < 90° indicates a preferential ‘ankle strategy’ (ankle and hip moving in-phase) while a CRP(t) > 90° indicates a ‘hip strategy’ (ankle and hip moving in anti-phase). We extracted a single variable from this analysis by computing for each trial the relative time spent by participants using hip strategy (ankle-hip antiphase relative time; expressed in percentage of total movement duration).
Muscular synergies were analyzed, using a non-negative matrix factorization (NNMF;26). Studies comparing and validating different factorization methods conclude that NNMF performs as well as or better than other methods70,71,72. NNMF has already been applied to both cyclic52 and discrete motion73 but, like any factorization method, NNMF has some limitations due to the assumption of their time invariance as well as to the choice of the number of synergies, such that we need to be cautious in the interpretation. Raw EMG data were band-pass filtered with a Butterworth-type filter (cut-off frequency: 20-400 Hz; order: 4) centered around the mean and rectified. Then, a low-pass Butterworth filter (cut-off frequency: 10 Hz; order: 374) was applied twice (forward and backward to remove phase shift) and the signal was normalized relative to the maximum value of each trial and time was normalized (in % on a time window from 400 ms before movement onset to movement offset). NNMF was computed to identify synergies between the eight recorded muscles. Briefly, the EMG signals were combined into an M × T matrix, where M represents the number of muscles (8 in this study) and T the number of EMG data points (2000) obtained in each trial. The NNMF decomposed the EMG signals in a few synergies (n < M). Each synergy could thus be described as a synergy vector W representing the contribution of each muscle (from 0 to 1) and a temporal activation pattern H over time (from 0 to 1). The difference between reconstructed and original EMGs was computed using the total variance (tVAF). The maximum iteration of NNMF algorithm was scaled at 1000 times for each number of synergies from 1 to 7 (number of muscles -1), until tVAF increase was < 0.05%. Finally, the number of synergies selected corresponded to a tVAF >90%, that is, the number of synergies allowing the reconstruction of 90% of the original EMG signal.
To identify the quantitative changes of muscle activity, root mean square (RMS) of EMG signals was computed for the four agonist muscles (tibialis anterior, rectus abdominis, deltoid anterior and biceps brachial as typically, antagonist muscles are mostly inhibited around movement onset). First, raw EMG data were band-pass filtered with a dual Butterworth (cut-off frequency: 20-400 Hz; order: 4) centered around the mean and rectified. Then, a low-pass Butterworth filter (cut-off frequency: 3 Hz; order: 3) was applied twice (forward and backward to remove phase shift) to create an envelope of the EMG signal74. The activity of each muscle was normalized and expressed as a percentage of the maximum activity observed in far-target trials in normogravity (without mechanical perturbation). The onset of EMG activity was identified when the EMG signal exceeded 10% of its peak in the trial. From this onset time, the RMS was computed on a 200 ms window.
To further characterize the potential effects of gravity on the coordination patterns underlying the whole-body reaching task, a principal component analysis (PCA) was conducted on kinematic (endpoint error, success rate, arm peak velocity, relative time to arm peak velocity, whole-body tilt and ankle-hip antiphase relative time) and EMG data (RMS tibialis anterior, RMS rectus abdominis, RMS deltoid anterior and RMS biceps brachii). The number of principal components (PC) was identified in accordance with the scree plot ( > 15%; Fig. 6A-C).
Statistical analyses
To determine the statistical effect of repeated exposure to gravitational changes across the successive parabolas, repeated-measure analyses of variance (ANOVAs) were used to compare the longitudinal endpoint error between each parabola (from 1 to 10) in the different environments (0 g, 1.8 g, 1 g). Subsequently, repeated-measure ANOVAs including 3 Environments (0 g, 1.8 g, 1 g) x 2 Targets (close, far) x 2 Perturbation conditions (Electro ON, Electro OFF) were performed on arm and whole-body kinematics and RMS EMG variables. Repeated-measure ANOVAs were performed using Statistica software (StatSoft, Inc.). The normal distribution of data for each variable was confirmed by Kolmogorov–Smirnov tests. Posthoc analyses were carried out using Newman–Keuls tests and significance threshold was set at p < 0.05.
To quantify whether muscular synergies changed as a function of the gravitational context, a SPM analysis allowing a statistical comparison on the whole time-series of each synergy was used. A repeated-measure ANOVA was conducted on each synergy (1 to 3) for the 3 Environments (0 g, 1.8 g and 1 g). SPM analyses were computed in Matlab (MathWorks Inc., Natick, MA) using the open-source software package spm1D (version: M.0.4.7; www.spm1d.org). Significance threshold was set at p < 0.05. Posthoc analyses were performed using SPM paired t-test (0 g vs. 1.8 g, 0 g vs. 1 g, 1 g vs. 1.8 g). Bonferroni correction was used to reduce the statistical risk caused by the multiple tests across these three pairs (significant threshold was p < 0.02). PCA analyses were computed in R (version 4.3.0) using the open-source package (FactoMineR).




Responses