Hypothesis on the outflow of optic nerve cerebrospinal fluid in spaceflight associated neuro ocular syndrome

Introduction
In 1961, the first human travelled into space, marking the beginning of the field of space medicine. Our understanding of how the human body responds to short- and long-duration spaceflights (LDSF) has improved over time. Yet studies on astronaut health have shown that the micro-gravity environment, radiation, and carbon dioxide affect nearly every organ system, including the eyes, muscles, bones, kidneys, and brain1,2,3,4,5,6.
In 2011, Mader et al.7 first reported the findings of spaceflight-associated neuro-ocular syndrome (SANS), including abnormalities of optic nerve sheath (ONS) distention, optic disc edema (ODE), choroidal folds, globe flattening, cotton wool spots, and hyperopic shifts in astronauts after LDSF. Many of the abnormalities and pathological changes are probably adaptations to micro-gravity, but long term space residual may produce secondary changes or injury. There is concern regarding the prolonged persistence of ODE from SANS following return to Earth, with some cases lasting for several months before complete resolution8. The refractive error, choroidal folds, and posterior globe flattening associated with SANS may persist for years after re-entry and could potentially become permanent for certain individuals8,9. It is already well known that various risk factors, including the duration of the mission, genetic predisposition, radiation exposure, and the environmental conditions within the spacecraft, contribute to the complexity of the situation. The potential countermeasures such as Lower body negative pressure (LBNP)10,11,12, nutritional supplementation13, and centrifugation14 in mitigating the physiological effects of microgravity have garnered considerable attention in research and exploration.
However, with the effectiveness of one promising countermeasure, LBNP, has been questioned recently15, the exact mechanism underlying these ophthalmic abnormalities which remains baffling16, is more paramount to be explored. In the weightless micro-gravity environment, there is reported a loss of hydrostatic pressure which may lead to venous stasis and can cause a fluid shift towards the head and neck17,18,19, namely, leading to increased intracranial pressure (ICP)7. Nevertheless, it is still not definitively established whether ICP is the primary cause or a secondary manifestation of an underlying condition20,21. In magnetic resonance imaging (MRI), Mader et al.7 noted that ONS distension is consistent with congestion seen at the level of the optic disc. The recent identification of meningeal lymphatic vessels (MLVs) and a glymphatic pathway in the brain and the optic nerve (ON)22, combined with new findings regarding spaceflight-associated alterations in the brain and ocular lymphatic system, may shed more light on the pathophysiology of SANS and providing a novel direction on research and developing more countermeasures.
Due to the inability to recurrence of LDSF, it poses a challenge in formulating a precise aetiology hypothesis through traditional analytical methods like cohort or case-control studies. However, the scientific hypothesis is a seed from which the entire research work sprouts, offering the necessary groundwork for advancing and maturing academic theory. In this perspective, we generate a novel hypothesis that the SANS may be associated with the impaired outflow of cerebrospinal fluid (CSF) and optic nerve cerebrospinal fluid (ON-CSF) anatomically, by investigating existing scientific evidence and theories, reasoning, and formulating a hypothesis.
Methods
For investigating existing scientific evidence, we conducted a comprehensive search, and this perspective focuses on the potential relationship between the impaired out-flow of the optic nerve cerebrospinal fluid and SANS, and this means that other studies (for example cardiovascular changes) were excluded. Studies included were identified by advance searches of Medline, ISI, NASA, and Taikonaut from their inception dates to June 10, 2024. Keywords and Mesh terms searched included ‘Spaceflight associated neuro-ocular syndrome’, ‘space flight-induced visual impairment’, and ‘SANS’ for defining patient type; ‘space-flight’, ‘long-duration space-flight’, ‘LDSF’, and ‘micro-gravity’ for defining intervention. When it comes to analyzing specific aetiology, we used ‘cerebrospinal fluid’, or ‘optic nerve cerebrospinal fluid’ for defining anatomical structures that may indicate potential aetiology.
Then, we demonstrated three premises for reasoning and drew conclusions, and at last, we generated a novel hypothesis.
Hypothesis generating
Study screening
With defining patient type and intervention, these comprehensive searches revealed 376 studies. Co-authors reviewed the findings and iteratively suggested additional relevant articles. The entire team of authors identified key articles. Of these studies, 74 were duplications and 147 were excluded after reviewing the titles and abstracts. In total, 155 studies were identified for full analysis.
Existing scientific evidence
To clarify the current and significant knowledge about SANS, especially in exploring the aetiology of ICP and the glymphatic system, we created a timeline of important research in this field (Fig. 1).
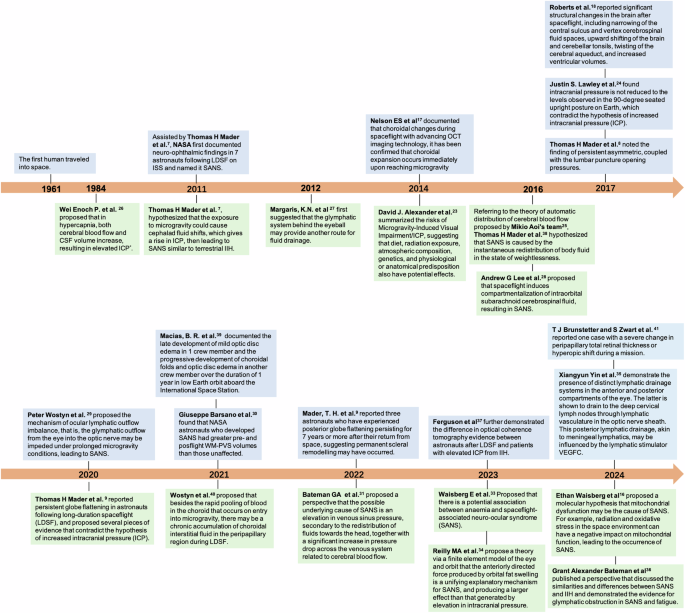
The green parts highlight hypothetical works and the blue parts highlight reported evidence. *The partial pressure of carbon dioxide in the ISS environment is ~20 times that at sea level. LDSF long-duration spaceflight, ISS International Space Station, OCT optical coherence tomography, WM-PVS white matter perivascular space, VEGFC Serum vascular endothelial growth factor C.
Premises
Premise I. The brain and meninges contain lymphatic vessels
Previously, scientists believed that CSF existed only in the subarachnoid space. However, Iliff et al.23 used in vivo imaging technology in 2012 to discover how CSF and interstitial fluid (ISF) exchange substances, and they named this exchange the glymphatic system. This system is mainly composed of three parts: the para-arterial CSF influx channel, the paravenous ISF efflux channel, and the aquaporin-4 (AQP4) water channel in astrocytes connecting these two channels. Briefly, CSF flows into the brain through the para-arterial space and exchanges with ISF via AQP4, which can drive metabolites and ISF into the paravenous space and then into CSF circulation, or directly through the lymphatic capillaries into the cervical lymphatics.
The presence of lymphatic vessels within the meningeal vasculature has been a subject of much debate since the 18th century24,25, and the old dogma is that the brain performs no immune functions. In 2015, Louveau et al.26 confirmed the existence of MLVs (Fig. 2) by staining the entire meninges; using tracer technology in the same year, Aspelund et al.27 reported the distribution of lymphatic vessels in detail and found that CSF and adjacent ISF were absorbed by MLVs and transported to cervical lymph nodes by MLVs. Another study26 found that although the structure of MLVs was similar to peripheral lymphatic vessels to a certain extent, MLVs structure uniquely demonstrated: (1) a lack of smooth muscle cells and valve structure; (2) a smaller diameter than the peripheral lymphatic vessels; and (3) functional migration of T-lymphoid immune cells. Since then, researchers have confirmed the presence of MLVs in fish, rats, and non-primates28. Furthermore, Lohrberg and Wilting29 found MLVs in the dura mater and dural septae entering deeper parts of the brain. These additional MLVs may participate in CSF drainage or represent potential routes of ocular tumour dissemination.
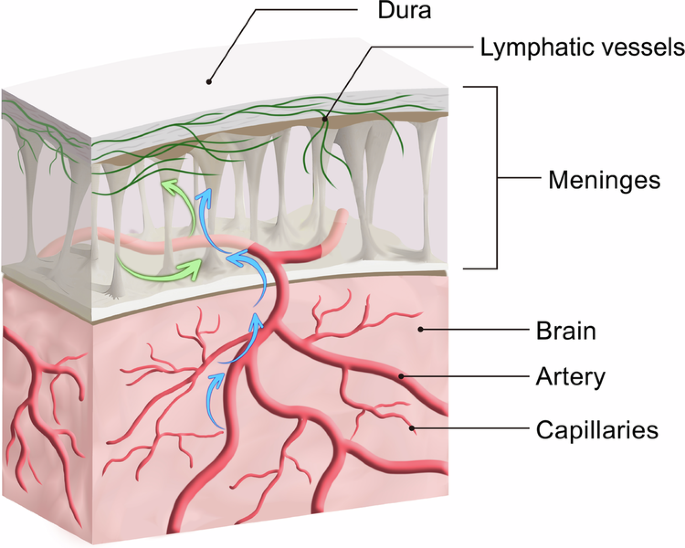
The green arrow represents the cerebrospinal fluid (CSF) flow, and its direction indicates the pathway through which CSF moves within the dura and towards meningeal lymphatic vessels. The blue arrow represents the interstitial fluid (ISF), and its direction indicates the movement in which ISF interacts with the Virchow–Robin space (VRS) and dura, then together with CSF, into meningeal lymphatic vessels. (This figure is created by an adaptation of Fig. 2 from Yang Xu et al.30).
In addition to providing an anatomical basis for brain immunity, the discovery of MLVs provides theoretical support for new metabolic excretion pathways. Previous findings30,31,32 suggest that the ability of the glymphatic system to clear brain metabolites depends, to a certain extent, on whether the structure and function of MLVs are normal. For example, in a transgenic mouse model with hypoplasia of MLVs, transport of CSF through MLVs to the cervical lymph nodes was impaired, blocking the excretion of macromolecular metabolites27.
Since MLVs are closely related to the glymphatic system in structure and function, they may also play a key role in CSF efflux. There is a special architectural feature known as the Virchow–Robin space (VRS), which is regarded as perivascular spaces containing perforating arteries extending into the brain parenchyma33,34,35, basically allowing the bi-directional exchange between CSF and ISF (Fig. 2). The VRS is characterized by the presence of endothelial, pial, and glial cell layers, each separated by their distinct basement membranes33. Other than the glymphatic system, solutes and fluid may be drained from the brain interstitium via the VRS into MLVs, then lead to cervical lymphatics33.
Premise II. The optic nerve and its sheaths contain lymphatic vessels
The ON is an extension of the central nervous system in the orbit, and the fibrous wrappings that ensheath ON are continuous with the meninges32. Mesenchymal cells infiltrate the surrounding tissue to create the vascular septa of ON, and myelin sheaths form the brain periphery to ON, reaching the lamina cribrosa at birth. The lamina cribrosa and peripapillary sclera separate the intraocular chamber from the subarachnoid space of ON, which can result in a pressure gradient between the intraocular fluid and CSF in ON. Given the anatomical commonalities, do the ON and its sheaths contain lymphatic vessels and a glymphatic system?
The presence of lymphatic vessels was not clear21 until 1999 when Killer et al.36 used electron microscopy and immunohistochemistry to identify lymphatic vessels in ONS, and an Indian ink tracer indicated that these lymphatic vessels could drain CSF. Aspelund et al.27 and Ma et al.37 also used CSF tracing technology and found that MLVs gathered around the ON and followed it as it exited the skull. To confirm the presence of lymphatic vessels in ONS, we previously conducted a series of experiments using lymphatic-specific molecular markers which demonstrated that lymphatic vessels are present in ONS of rats, rabbits and human (unpublished).
Regarding a glymphatic system, studies have revealed that CSF tracers injected into the subarachnoid space drained into periorbital tissues and cervical lymph nodes38. The relationship between ON and the glymphatic system has also attracted attention. In a 2017 study39, CSF was labelled with contrast agents of different molecular weights and immunofluorescence was used to detect the presence of a glymphatic system in the ON of mice; markers below 70 kDa were found to enter the ON parenchyma through the glymphatic system. This suggests that the glymphatic system plays a role in CSF circulation and substance transport in ON, and the transport is related to the molecular weight of substances. In the same year, Wostyn et al.40 used Indian ink as a CSF tracer in the subarachnoid space of ON, and while observing the cross-section of ON with a light microscope, they found that Indian ink accumulated in the perivascular space of ON. This result revealed a perivascular space in the human ON that had not been previously observed. However, its function and significance require further elucidation. Furthermore, it is reported that there is a perivascular transport system allowing CSF-ISF exchaging between ON subarachnoid space and ON parenchyma22,41. CSF can be actively transported out of ON into the ISF through those cells with aquaporin-4 (AQP4; water channel proteins)42, which can also be considered a part of the glymphatic system in ON8.
Premise III. SANS cannot be fully unexplained by intracranial hypertension, and new experimental phenomena indicate the lymphatic vessels may participate or contribute to SANS
In 2011, Mader et al.7 first reported the symptoms of SANS to include ODE, choroidal folds, globe flattening, cotton wool spots, and hyperopic shifts in astronauts after LDSF. After returning to Earth, SANS-related ODE may persist for months before complete resolution9, and refractive errors, choroidal folds, and posterior globe flattening may persist for years, even becoming permanent in some astronauts9,43. Those changes can not be fully explained by adaptations to micro-gravity as the changes should be resolved soon after returning to Earth.
Given that the brain is unable to be unloaded by simply standing up periodically, it is suggested a mild, but chronic, elevation of the average 24-h ICP takes place in space compared to Earth7. So, SANS findings were initially hypothesized to occur as a result of increased ICP and chronic venous hypertension similar to terrestrial idiopathic intracranial hypertension (IIH) 7,17,44,45. However, studies suggested that the chronic, mildly elevated ICP in the weightless micro-gravity environment, is primarily attributed to the reduction of hydrostatic pressures and hemodynamic changes in the circulation of the CSF system within the cranium43,46. And with an increasing number of studies21,47,48,49,50 have challenged the hypothesis of intracranial hypertension, suggesting that other mechanisms may explain SANS better, including impaired cerebral venous outflow, ONS compartment syndrome, translaminar cribrosa pressure gradient changes, other obstacles to CSF and lymphatic drainage, mitochondrial dysfunction, and radiation, it is still not definitively established whether ICP is the primary cause or a secondary manifestation of an underlying condition20,21,51.
The mechanisms underlying SANS development and ON injury and their relationship to ICP are not fully understood7,47,52. Lawley et al.53 provided significant evidence regarding ICP: (1) Normal changes in posture cause very large changes in ICP in humans; (2) Complete removal of gravitational gradients (such as the zero-gravity of space) does not pathologically elevate ICP but instead prevents the normal lowering of ICP when standing; and (3) Despite the duration of zero-gravity during parabolic flight, there was no evidence of a progressive increase in ICP from cephalad fluid shifts with prolonged simulated micro-gravity in the head-down tilt position.
Using a head-down bed rest model (simulating body fluid transfer to the head under micro-gravity) in 2020, Rasmussen et al.54 found that intracranial CSF flowed through deep cervical lymphatic vessels to the obstructed deep cervical lymph nodes in humans. Gashev et al.55 discovered that the pumping function of the deep cervical lymphatic vessels in rats was significantly inhibited in a rat tail suspension model (simulating the effect of micro-gravity on the ground) and that these deep cervical lymphatic vessels were the only route to the deep cervical lymph nodes.
Recent research suggests a close correlation between the obstruction of meningeal lymphatic drainage of CSF and neurophysiological changes in the brains of astronauts56. Moreover, head-down and micro-gravity studies have documented that the cerebral arterial diameter and blood flow velocity are autoregulated and do not change significantly during space flight57. However, micro-gravity fluid shifts can lead to jugular vein distension and mild thickening of the optic nerve’s retinal nerve fiber layer, as measured by optical coherence tomography (OCT)30.
Conclusions
Conclusion I. The outflow of cerebrospinal fluid through the optic nerve sheath may be impaired under micro-gravity
Among the theories to explain SANS, the theory that CSF and lymphatic reflux become blocked has garnered special attention, especially since the discovery of MLVs26 and the revelation that these vessels can drain CSF26,58 Based on Premises II and III, we know that there is an ISF-CSF exchange between the ON and the subarachnoid space and the flow of those two into the lymphatic vessels8,34,41 (Fig. 3), then we posit that the flow of CSF from ON can be absorbed by lymphatic vessel, then through the deep cervical lymphatic vessels to the deep cervical lymph nodes becomes obstructed under micro-gravity. Therefore, it is speculated that the outflow of cerebrospinal fluid through the optic nerve sheath may be impaired in microgravity.
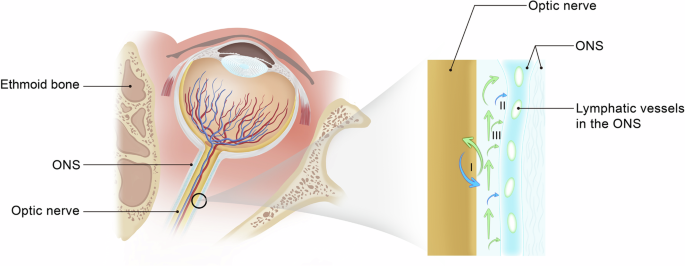
The green arrow represents the cerebrospinal fluid (CSF) flow, and the blue arrow represents the ISF. (I) illustrates the exchange of CSF and interstitial fluid (ISF) within the optic nerve sheath. (II) illustrates the CSF absorption by lymphatic vessels. (III) illustrates the adjacent ISF absorption by lymphatic vessels. ONS= optic nerve sheath. (The axial diagram of the right eye is created from an adaptation of Fig. 1–20 from Paul Riordan-Eva et al.73, and the fluid dynamics diagram is originally designed.).
Conclusion II. The impaired outflow may cause optic nerve sheath distension in SANS
Based on Premises I, II, III and Conclusion I, we posit that both the CSF and ON-CSF share an efflux mechanism; therefore, when they are obstructed, the CSF will accumulate between ONS, which leads to sheath distension or dilation in astronauts with SANS, which are usually found and reflected by MRI.
Conclusion III. The retained fluid may cause optic disc edema
Based on Premise III and Conclusion I, we posit that when the outflows of CSF and ON-CSF efflux and the corresponding lymphatic vessels are impaired, the body cannot compensate and the function of CSF-ISF exchange in the glymphatic system will also be impaired. If so, two results may occur:
-
1.
Protein-containing ISF and metabolites may accumulate in the interstitial space and cause lymphedema.
-
2.
Pressure-induced ischemia and hypoxia may cause hydropic cellular degeneration. (In this case, beta-trace protein may be a new noninvasive immunological marker of impaired blood-brain barrier integrity31).
In our previous study (unpublished), we demonstrated that CSF can flow through the lymphatic vessels of ONS and into the deep cervical lymph nodes. Before submitting the manuscript of that study, came as a surprise when Song Eric’s research group at Yale University published their findings in Nature on February 28, 2024. Their study not only reaffirmed the presence of lymphatic vessels within ONS but also discovered the passage of cerebrospinal fluid through this structure, leading to lymphatic drainage into the deep cervical lymph nodes22.
However, these lymphatic vessels can become obstructed in reduced gravity. Therefore, our team proposes that in micro-gravity, the obstruction of CSF lymph drainage through the sheath lymphatic vessels of ON leads to the expansion of ONS in astronauts, and the subsequent retention of CSF impairs the exchange of CSF and ISF within ON. Ultimately, the barrier to cerebrospinal fluid renewal may result in damage to the structure and function of ON and the development of SANS, such as increased ICP, elevated post-flight CSF pressure, optic disc oedema, and choroidal folds which were reported7,17,50,59,60. The detailed hypothetical reasoning process is shown in Fig. 4.
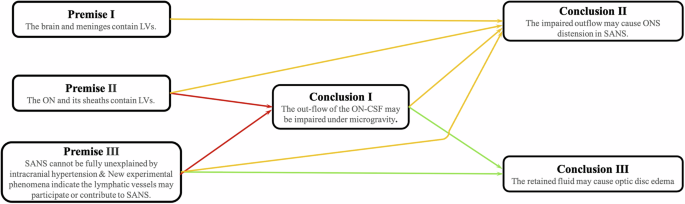
ON optic nerve, SANS spaceflight-associated neuro-ocular syndrome, ON-CSF optic nerve cerebrospinal fluid, CSF cerebrospinal fluid, ODE optical disc edema, LVs lymphatic vessels.
Hypothesis
Our team hypothesizes that in the micro-gravity environment of space, the obstruction of CSF drainage through the sheath lymphatic vessels of ON in astronauts may lead to the expansion of ONS, the retention of ON-CSF, and ultimately the formation of SANS due to impaired CSF renewal, resulting in damage to the structure and function of ON.
Our hypothesis may explain why ONS distension and nerve fibre layer thickening is the most common ophthalmic changes reported in astronauts with SANS (observed in 86%), followed by optic disc edema (observed in 71%), choroidal folds (observed in 57%), and elevated post-flight CSF pressure, an indicator of increased ICP (observed in 57%)7,17,49,59,60. It may also explain why previous studies did not find headaches or other symptoms commonly associated with increased ICP in SANS patients47,53. Based on our hypothesis, more effective countermeasures may be developed to promote drainage of the ON-CSF through MLVs and to protect the structure and function of ON, besides several countermeasures including LBNP10,11,12,15, nutritional supplementation13,centrifugation14, and pre-flight risk assessments.


Responses