Influence of the Covid-19 pandemic on cerebrovascular diseases in the Sao Paulo region of Brazil
Introduction
Cerebrovascular disease (CVD) is one of the leading causes of disability and death globally1. According to Global Stroke Statistics, there are ~12.2 million new cases of CVD and 6.5 million deaths attributed to it each year worldwide2,3. Characterized by the rapid clinical development of signs and symptoms resulting from a focal or global brain disorder, with symptom duration exceeding 24 hours, potentially leading to death1. Several key factors contribute to the development of CVD, including a family history of this condition, Black individuals, age over 55 years, and modifiable clinical conditions such as diabetes and hypertension3,4,5,6,7,8.
Brazil ranks as the third country with the highest number of cases and deaths due to Covid-19 worldwide8,9,10. There are 87,518 deaths attributed to CVD in Brazil from January 1st to October 13th, 2022, an average of 12 deaths per hour or 307 fatalities per day7. São Paulo state, the largest metropolis in South America, records the first fatal case of SARS-CoV-2 infection in Brazil and remains at the top of the ranking for the highest incidence of this disease in the country. By August 12th, 2021, the state had 4,147,665 confirmed cases and 176,465 deaths8.
Given the severity of the pandemic, international initiatives implement contingency plans to expand testing and hospital beds11,12,13. The response to the Covid-19 pandemic in Brazil varies across the country, with different initiatives implemented at both national and local levels14,15. In São Paulo state, for example, sanitary measures and mobility restrictions are in place from March 2020 to December 2021, based on factors such as the availability of hospital beds in each municipality, case incidence, and the emergence of new variants of the SARS-CoV-2 virus8,16.
Previous literature identifies a decrease in hospitalizations for CVD during the Covid-19 pandemic, accompanied by an increase in associated severity and mortality6,17,18,19. The hypotheses suggest that measures to control the transmission of Covid-19, such as mobility restrictions and disruption of healthcare services, may delay medical treatment and monitoring for chronic non-communicable conditions like diabetes and hypertension. Additionally, the immune response triggered by SARS-CoV-2 infection, including inflammatory reactions and coagulation disorders, may contribute to the development of CVD during the pandemic20,21,22,23,24,25.
In light of the global recognition of the necessity to reevaluate clinical decision-making and public policies in developing nations, particularly during public health emergencies like the Covid-19 pandemic, there is an imperative to implement optimal strategies for managing CVD13.
In this context, Brazil, with its vast geographical expanse and notable social disparities, maintains a hospital information system that rigorously documents all admissions and fatalities within the public sector5. This systematic approach, overseen by trained personnel, offers important benefits for gathering epidemiological data and conducting comprehensive assessments of diseases on a broad scale.
Considering the global impact of high mortality and disability from cardiovascular diseases (CVD), it is noteworthy that most national and international studies assessing hospitalizations and deaths from CVD are constrained to the early stages of the Covid-19 pandemic. This study seeks to address this gap by evaluating the influence of the Covid-19 pandemic on hospital admissions and deaths due to CVD in São Paulo, the most populous and principal economic hub in the Southern Hemisphere, spanning the period from 2017 to 2021.
This study reveals a 35% reduction in CVD hospitalizations, particularly in elective admissions and ischemic stroke, but a 6.5% increase in CVD-related deaths. The rise in deaths is especially pronounced among Black and Brown individuals and those aged 20-29 years. Additionally, SARS-CoV-2 infections are inversely correlated with CVD hospitalizations but directly linked to CVD-related deaths, highlighting the pandemic’s impact on vulnerable populations and the need for targeted healthcare strategies.
Methods
This study is an ecological time series analysis conducted on monthly hospitalizations and deaths associated with CVD using data from the Hospital Information System (SIH) recorded in the São Paulo state, Brazil from January 2017 to December 2021.
Eligible CVD admissions and deaths were those coded as I60–I69 according to the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) and classified as hemorrhagic (ICD-I60–I62), ischemic (ICD I63), unspecified (ICD I64), or other types (ICD I65–I69).
To gather a comprehensive profile of hospitalizations and deaths, we extracted the following variables: sex, age group, skin color, and type of admission (elective or emergency). Regarding the time of data collection, the skin color registration was already filled in the dataset through a closed-ended question, with five categories: White, Brown, Black, Yellow, and Indigenous, according to the Brazilian Institute of Geography and Statistics categorizations. The data were collected through the individual’s self-declaration during hospital admission. However, since this data collection could involve individuals who can be unconscious, the individual’s companion may answer this question at the time of medical admission. In cases where no companion is present, the healthcare professional is responsible for classifying the individual’s ethnicity/skin color. SARS-CoV-2 infections reported to the State Data Analysis System Foundation (SEADE) of the state of São Paulo were laboratorial confirmed by molecular (RT-PCR), serological (IgM, IgA, or IgG), and antigen tests10,15.
To determine the periods of circulation restrictions, we used the monthly social isolation index of the Intelligent Monitoring System of São Paulo (SIMI-SP). This index represents the percentage of displacement between municipalities, with higher values indicating lower population displacement. The desirable social isolation index is considered to be equal to or greater than 45%.
Statistical analysis
The exploratory analysis included the examination of absolute and relative frequencies as well as minimum and maximum values. The data was stratified based on two distinct periods: before the pandemic (January 2017 to December 2019) and during the pandemic (February 2020 to December 2021). To calculate the annual average of hospitalizations or deaths, we considered three years in the pre-pandemic period and two years during the pandemic. The relative difference expressed as a percentage (%), was determined by comparing the average numbers between the pre-pandemic and pandemic periods, with the numerator being the difference and the denominator being the average number in the pre-pandemic period. Zero values indicated no change, negative results indicated a reduction, and positive values indicated an increase in CVD events.
To examine the temporal pattern of CVD hospitalizations and hospital deaths from January 2020 to December 2021, we performed Join Point segmented regression models (SRM) 26 with a negative binomial distribution with offset term. We performed models for the total events and stratified by skin color, age group, and CVD type (ischemic or hemorrhagic). These models allowed us to calculate the annual percentage changes (APC), along with their corresponding 95% confidence intervals (95% CI), of the absolute number of CVD hospitalizations and deaths for each time segment, with the null hypothesis being APC = 0, indicating an absence of variation in hospitalizations and deaths over time. Since month was the regression variable, the APC means a monthly percentage change26.
Considering better visualization/adjustment of the methodology for SRM analysis in skin color stratification, we only considered data from White, Brown, and Black individuals.
During the pandemic period (2020-2021), we built line graphs to visualize the time series of monthly CVD events (hospitalizations or deaths) in relation to the number of SARS-CoV-2 infections. The graphs depicted the onset and conclusion of SARS-CoV-2 waves and the social isolation index. Subsequently, we calculated the Detrended Cross-Correlation (DCC) test coefficient (95% CI)27 to determine the unbiased correlation between the CVD series (hospitalizations or deaths) and the incidence of SARS-CoV-2. The DCC test (two-sided test) yields values ranging from -1 to 1, with zero indicating no correlation, positive values denoting a simultaneous increase in CVD and SARS-CoV-2 events, and negative values indicating an inverse correlation.
We performed the analyses using the libraries ‘tseries’, ‘mgcv’ and ‘segmented_2.1-3′28,29,30 of the free software R, version 4.4.131.
Ethical aspects
This study utilized publicly available anonymous data and did not require ethical approval of the Ethics Committee. The research was registered under number 1698 in the Institutional Research Management System of the Evandro Chagas National Institute of Infectious Diseases at the Oswaldo Cruz Foundation.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Between January 2017 and December 2021, there were 277,272 CVD hospitalizations (monthly median = 4042, minimum = 2083, maximum = 4716) and 42,004 CVD hospital deaths (monthly median = 560, minimum = 453, maximum = 891).
Comparing the average numbers/year of CVD hospitalizations and deaths in the pre-pandemic (2017–2019) and during pandemic (2020–2021) periods, we observed a decrease in the average annual difference for hospitalizations (−34.8%, mean number/pre-pandemic year = 49422.7 and mean number/year during pandemic = 32,251) an increase in the average annual number of hospital deaths (6.5%, mean number/year pre-pandemic = 8371 and mean number/year during pandemic = 8917).
Table 1 displays the distribution of CVD hospitalizations and deaths categorized by demographic and clinical characteristics, as well as the pre and during pandemic periods. The data reveals a higher prevalence of men, individuals with White skin color, those over 50 years of age, emergency admissions, and unspecified types of CVD.
When comparing the two periods, it was evident that the average annual number of hospitalizations decreased across all characteristics, with high reductions observed in skin color not informed (−43.9%), ischemic CVD type (−50.3%), and elective hospitalizations (−85.5%). Conversely, there was a proportional increase in the average number of deaths in nearly all analyzed subgroups, particularly among Black or Brown individuals (15.9% and 14.4%, respectively), as well as individuals aged 20–29 years and 70–79 years (14.2% and 10.3%, respectively). Notably, ischemic-type CVD showed an increase in deaths during the pandemic (10.8%), despite a decrease in hospitalizations.
Figure 1 illustrates the monthly percentage change in CVD hospitalizations (Fig. 1A) and deaths (Fig. 1B) from January 2020 to December 2021. We could observe a significant increase in the monthly percentage change from early 2021, specifically February 2021 for hospitalizations (APC = 2.5; CI = 1.43|3.62) and March 2021 for hospital deaths(APC = 3.7, CI = 1.50|6.08), followed by a decline from July/2021 for deaths (APC = –4.5, CI = –6.06|−3.09).
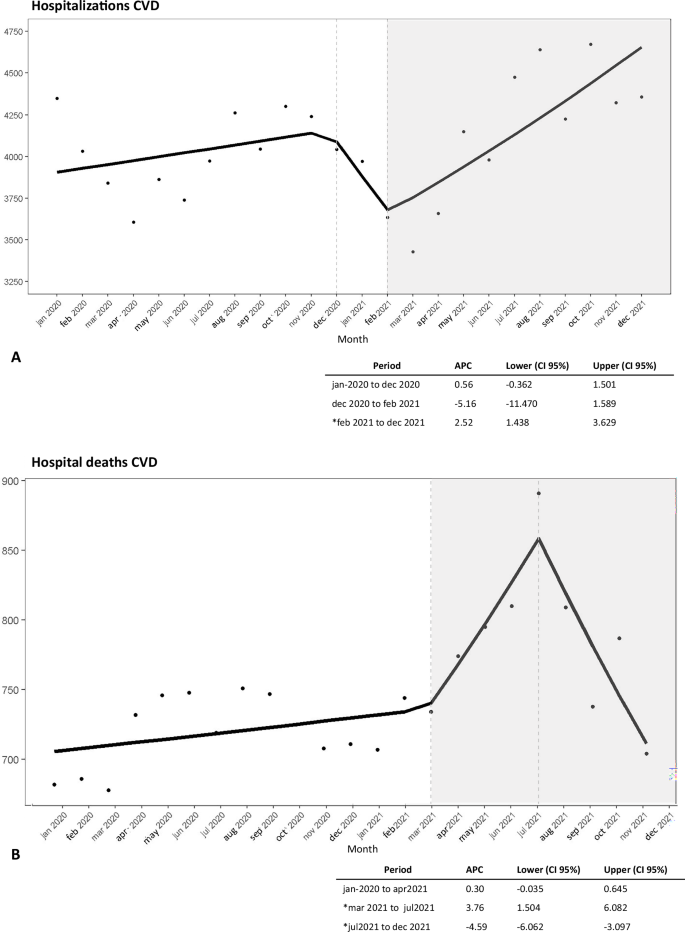
A Time series of CVD hospitalizations. B Time series of CVD hospital deaths. Note: gray area = significant APC values; Dataset: Supplementary Data 1.
We observed significant variations in all periods for hospitalizations and deaths in the age group over 60 years (Fig. 2). This age group exhibited a reduction in the absolute number of hospitalizations until March 2021, followed by an increasing trend of hospitalizations between March and October 2021 (APC = 2.8, CI = 1.56|4.20), and a subsequent decline from October 2021 (APC = −34.2, CI = −40.34|−27.44). In contrast, for individuals aged under 60 years, the decline in hospitalizations began at a lower level and earlier, in October 2020 (APC = 1.47, CI = −2.84|−0.07) (Fig. 2A).

A Time series of CVD hospitalizations by age group. B Time series of CVD hospital deaths by age group. Note: gray area = Significant APC values; dataset: supplementary data 2–5; Script analysis: Supplementary Data 6.
Regarding deaths (Fig. 2B), similar patterns to the hospitalization series were observed (Fig. 2A), with a decline starting from August 2021 for those over 60 years old (APC = −20.3, CI = −22.45|−18.10) and from October 2021 for those under 60 years old (APC = −47.97, CI = -56.41|−37.89).
Throughout the analyzed period (Fig. 3), the White group consistently displayed higher number of hospitalizations and deaths compared to Black and Brown individuals. Between March and October 2021 (Fig. 3A), both populations experienced similar increases in hospitalizations (APC White = 2.5, CI 1.19|4.01; APC Black and Brown = 2.3, CI = 0.99|3.63), followed by a decline from October 2021 (APC White = −33.3, CI = −40.04|−25.92; APC Black and Brown = −38.85, CI = −44.68|−32.40). However, the increase in deaths among Black and Brown people began earlier and persisted longer than in White individuals, spanning from March 2020 to September 2021 (APC = 0.91, CI = 0.53|1.28). Notably, the decline in deaths for the Black and Brown group commenced 1 month after the decline observed in the White group, September 2021 for Black and Brown(APC = -16.9 CI −20.28|−13.51), and August 2021 for White (APC = −18.4, CI = −20.72|−16.01) (Fig. 3B).
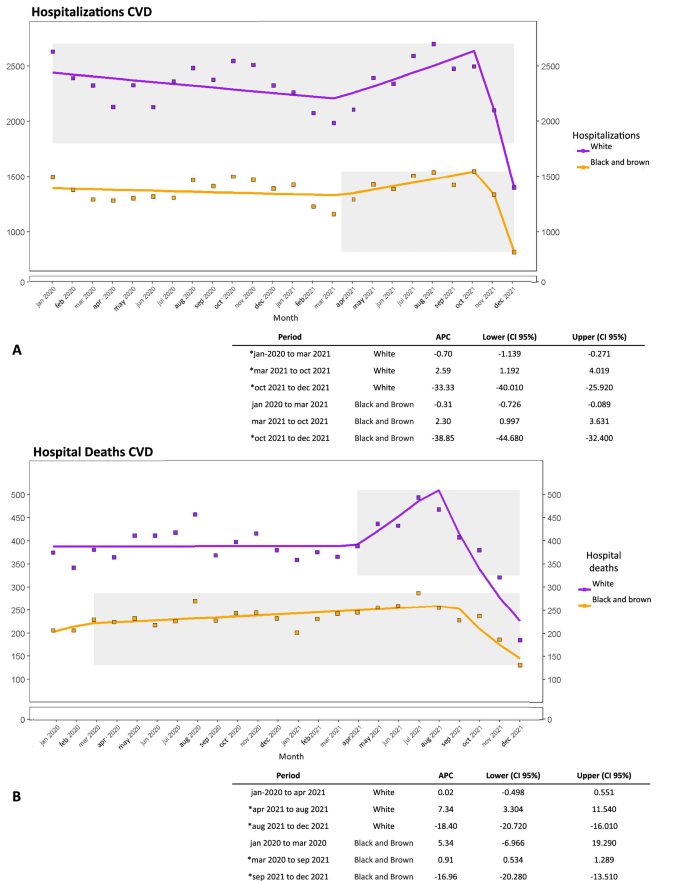
A Time series of CVD hospitalizations by skin color group. B Time series of CVD hospital deaths by skin color group. Note: gray area = significant APC values; dataset: supplementary data 7–10; script analysis: supplementary data 11.
Regarding the type of CVD (Fig. 4), a significant decline in hospitalizations of the ischemic type was observed in the initial months of the Covid-19 pandemic until March 2020 (APC = −23.5, CI = −31.49|−14.68), followed by a significant decline between August 2021 and December 2021 for ischemic (APC −4.5, CI = −8.02|−1.02) and hemorrhagic (APC = −15.0, CI = −17.99|−11.90) hospitalizations (Fig. 4A).

A Time series of CVD hospitalizations by classification type. B Time series of CVD hospital deaths by classification type. Note: Gray area = Significant APC values; dataset: supplementary data 12–15; script analysis: supplementary data 16.
For deaths, we observed significant declines between August 2021 and December 2021 in hemorrhagic classification (APC = −14.9, CI = −20.08|−9.44) and September 2021 and December 2021 for ischemic classification (APC = −25.0|CI = −38.77, −8.26) (Fig. 4B).
Figures 5 and 6 illustrate the monthly time series of SARS-CoV-2 infections, CVD hospitalizations, and hospital deaths during the pandemic (February 2020 to December 2021). A negative cross-correlation was observed between the hospitalization series and SARS-CoV-2 (p value = 0.02; correlation = −0.54; 95%CI −0.820|−0.071) and a positive correlation between the hospital deaths series and SARS-CoV-2 (p value = 0.05; correlation=0.40; 95%CI 0.025|0.691), especially evident during the second wave of the pandemic (Jan to Aug/2021).

Black circle: period of social isolation greater than 45%. Blue area: first wave of SARS-CoV-2 infections. Orange area: second wave of SARS-CoV-2 infections. Blue line: hospital deaths due to cerebrovascular disease (CVD). Orange line: cases of SARS-CoV-2 infection. Green triangle: start date of COVID-19 vaccination. Note: p value = 0.05; correlation coefficient = 0.40; Confidence interval = 0.025|0.691; dataset: supplementary data 1.

Black circle: period of social isolation greater than 45%. Blue area: first wave of SARS-CoV-2 infections. Orange area: second wave of SARS-CoV-2 infections. Blue line: hospital deaths due to cerebrovascular disease (CVD). Orange line: cases of SARS-CoV-2 infection. Green triangle: start date of COVID-19 vaccination. Note: p value = 0.02; correlation coefficient = −0.54; confidence interval: −0.820|−0.071; dataset: supplementary data 1.
Discussion
The present study revealed an important reduction of ~35% in CVD hospitalizations during the pandemic period (2020–2021) in São Paulo state, primarily observed in elective, ischemic, and unspecified CVD. Conversely, there was an approximate 6% increase in hospital deaths, particularly among Black and Brown individuals, youth and elderly, and those with ischemic CVD. Furthermore, as the number of SARS-CoV-2 infections increased, there was a tendency for a decrease in CVD hospitalizations.
The reduction observed in elective hospitalizations and cases of ischemic CVD, coupled with the simultaneous increase in deaths, can be attributed to challenges faced in accessing healthcare services during the pandemic. In 2021, the disease control coordination of the state of São Paulo published a report highlighting the disruption in the provision of care for chronic non-communicable diseases (CNCD) during the Covid-19 pandemic. According to the report, there was an alarming increase in the premature mortality rate from CNCDs, rising from 35.2 to 50.8 per 100,000 inhabitants in the state of São Paulo. This increase was primarily due to a 72% reduction in outpatient services as per the state’s guidelines, leading to an impact on healthcare access and continuity of care for CNCDs32.
Supporting this hypothesis, Siegler et al.24 attributed the reduction in CVD diagnosis during the pandemic to potential delays in initial care caused by circulation restrictions and strain on the healthcare system, including the limited bed availability and emergency care capacity. Consequently, the increase in CVD severity and mortality at the onset of the pandemic has been extensively discussed in the literature, prompting calls for further investigations into the long-term impact6,19.
This discourse gains additional support from the findings presented by Rocha et al.33, which highlight the increase in the number of intensive care beds across various municipalities following the onset of the pandemic. However, despite this augmentation, a decline in CVD hospitalizations was observed. The rise in CVD-related deaths occurring at home and other locations, as suggested by Orellana et al.34,35, Brant et al.36 and the Mortality Information System (MIS, 2021), may indicate potential delays in initial care due to circulation restrictions and strain on the healthcare system. This underscores the importance of incorporating this variable into future studies examining the location of CVD deaths during the Covid-19 pandemic.
Additionally, during the first year of the pandemic (2020), internal migration may have impacted hospitalizations and deaths related to COVID-19 and CVD. However, Lorenz et al.37 highlighted that internal migration within São Paulo state primarily involved a minority of high-income individuals. This underscores the importance of incorporating these variables into future studies examining the location of CVD deaths and the impact of migration during the Covid-19 pandemic.
Despite a similar reduction in CVD hospitalizations across all ethnic groups, there was a notable increase in hospital deaths among Black or Brown individuals during the pandemic compared to the White group. These results are in accordance with previous Brazilian studies that have consistently found higher CVD mortality rates among Black and Brown individuals4,38. These findings align with similar studies conducted in North America, which examined the effects of social resources on stroke survivors in New York and racial disparities in ischemic stroke subtypes in Los Angeles39,40. The more considerable increase in the average number of deaths among Black and brown individuals, as observed in our study, may reflect the heightened severity of the disease, potentially stemming from socioeconomic disadvantages and limited access to healthcare services, which might have been exacerbated during the Covid-19 pandemic6,19,41.
The observed increase in the average number of CVD deaths among the elderly aligns with previous findings that indicate higher mortality rates with advancing age7. In contrast, we observed a 14% increase in deaths among young individuals aged 20 to 29 years, particularly in the ischemic subtype. This unpredicted finding could potentially be attributed to the thromboembolic effects associated with Covid-196,42,43,44.
Conversely, Lorenz et al.37 associated the start of the vaccination campaign for the elderly (January 2021) and the relaxation of circulation restriction measures with an increase in Covid-19 deaths in the younger population (aged 20 to 59 years). In this context, Freitas et al.45 analyzed that different variants could alter the pattern of Covid-19 mortality among age groups, as well as change its profile of pathogenicity and virulence.
The unexpected findings of increased mortality from CVD in young individuals warrant further investigation into the pathophysiology of CVD in this specific age group, considering its subtype, risk factors, and the particularities of COVID-19 in this population.
Additionally, socioeconomic factors may have played a role, as many young individuals lost their jobs during the pandemic, with this age group often being considered economically active46,47. The impact of deaths among economically active individuals in the most populous state of Brazil could compromise the workforce of a region that functions as the country’s largest economic center.
When examining the monthly percentage changes in CVD hospitalizations and hospital deaths during the Covid-19 pandemic, we identified an increase in both outcomes among individuals aged 60 years or more, a reduction in hospitalizations among the White population, and an increase in deaths among the Black and Brown populations during the corresponding period in 2020. Previous literature emphasized the importance of equitable access to information and healthcare resources for vulnerable populations during the pandemic. Santos et al.48 highlighted the impact of Covid-19 containment efforts in the context of socioeconomic and racial inequality, particularly in the first pandemic year.
In 2021, there was a significant overall increase in monthly percentage change in CVD hospitalizations, albeit with delays in increments among different age strata and Black and Brown individuals. Several contributing factors could be considered for the global increase, such as the reorganization of the healthcare system after the first year of the pandemic with more available hospital beds48,49, the use of telemedicine for clinical management of covid-19 mild cases50,51, flexibilization in circulation restriction measures52,53, and the potential impact of new virus variants and immunization against Covid-19 on the clinical manifestation of CVD54,55,56,57.
The association between SARS-CoV-2 infection and CVD hospitalizations and deaths appears to exist, predominantly observed during periods of higher incidence of SARS-Cov-2 infection, such as the second wave in the beginning of 2021. The available literature is scarce regarding CVD trends throughout 2020 and 2021. However, there were descriptions from the early periods of 2020 that support our findings, emphasizing the importance of continued observation over time23,25,58,59.
In this scenario, there is an international acknowledgment of the need to reassess clinical decision-making and public policies in developing countries, aiming to implement best practices in managing CVD when dealing with public health crises, such as the covid-19 pandemic13,46,59. It is crucial to investigate socioeconomic indicators, regional and ethnic disparities in healthcare coverage and access, and the continuity of care for chronic non transmissible diseases (CNCD) during pandemic situations, closely linked to CVD incidence and mortality.
It is important to acknowledge the limitations of this study. Firstly, the hospital information system, primarily designed for financial purposes, may have been prone to underreporting. This potential underreporting, along with delays in notifications, could explain sudden drops observed in the stratified pattern during the last quarter of 2021 for all evaluated outcomes. Secondly, our results might be affected by completeness failures due to the large volume of data during the pandemic.
The elevated number of events attributed to unspecified CVD could be a result of this scenario. However, São Paulo boasts a high-quality standard of routinely-collected data, such as the hospital information system. The study’s sample size and time series surpassed that of previous studies, most of which were conducted in the early stages of the pandemic or at local healthcare units.
The third limitation concerns the use of raw data stratified by age instead of age-standardized rates in the descriptive analyses. From 2015 to 2020, population estimates and censuses in the state of São Paulo showed a proportional increase in the population aged 60 years and older, from 13.2% to 15.3%, respectively60,61. Despite the results not being presented by standardized rates, we found a proportional reduction in the raw values of hospitalizations and hospital deaths across all age groups.
Furthermore, there is acknowledgment in the literature of the need to investigate CVDs during the Covid-19 pandemic. However, studies have focused on the early stages of the pandemic17,20 or on local healthcare services with smaller populations in comparison to the state of São Paulo21,23. Our study makes a valuable contribution by taking these challenges in a wider and more densely populated setting.
Based on our findings, the Covid-19 pandemic may have influenced a reduction in hospitalizations for CVD, with an increase in hospital deaths over the study period. In 2021, there were improvements in healthcare due to vaccination, restructuring of the health system, and follow-up of CNCDs. Despite this improvement in 2021, we can still identify the most affected subgroups and highlight disparities in deaths based on skin color, as well as a higher occurrence among youth and the elderly. The elevated proportion of unspecified CVD also suggests a vulnerability in accessing health resources, exacerbated by the Covid-19 pandemic.
These findings enrich the existing knowledge base, especially for the most influential state in Latin America, and can provide valuable insights for future actions in management of CVDs, which is particularly relevant considering the potential emergence of new outbreaks of Covid-19 and other pandemics.

Responses