Magnetic hydrochar for sustainable wastewater management

Introduction
Effective wastewater management is essential for safeguarding public health and promoting environmental sustainability. Various technologies, including electro/photo-catalysis, adsorption, and advanced oxidation processes (AOPs), have been developed to address pollutants in wastewater1,2. The advancement of these technologies has been closely tied to the development of functional materials3. In addition to material performance, cost is a key factor influencing large-scale applications4,5. As a result, the development of cost-effective functional materials has become a priority6,7,8. Compared with the traditional functional materials, carbon-based functional materials derived from the renewable materials offer an eco-friendly alternative and can be easily modified to enhance their wastewater treatment capacity, making them efficient for a range of pollutants9. Inexpensive bio-feedstocks such as food waste, agricultural by-products, and industrial residues, have been developed for fabricating functional materials. Pyrolysis and hydrothermal heating are common strategies used to process these bio-feedstocks. Biochar, produced through pyrolysis at high temperatures in an oxygen-limited or oxygen-free environment, exhibits a high specific surface area and a well-developed porous structure, leading to a reduced environment impact and effective capabilities for wastewater treatment10,11. Otherwise, hydrothermal carbonization requires less energy due to its relatively low heating value and wet heating environment12,13. This process contributes to the reduction of greenhouse gas emissions and enhances the carbon content of the produced hydrochar, thereby minimizing environmental impact. The resulting hydrochar has attracted significant attention due to its low production cost and abundant surface functional groups14,15,16,17. The mesoporous structure, abundance of functional groups, and favorable chemical properties make hydrochar highly effective for pollutant removal. Furthermore, its tunable surface chemistry enhances its ability to target emerging contaminants12,18.
One challenge associated with hydrochar is its efficient recovery from water systems, particularly with nano-sized particles that pose the risk of secondary pollution. To address this, magnetic hydrochar has been developed as a promising solution for wastewater treatment19,20. By incorporating magnetic compounds into carbon matrices, magnetic hydrochar allows for easy and cost-effective separation using an external magnetic field. This innovation retains hydrochar’s adsorption and catalytic capabilities while optimizing its pore structure and enhancing active sites. Additionally, the carbon layer protects the incorporated inorganic substances from leaching during treatment. Studies demonstrate that magnetic biochar displays minimal differences from pristine biochar, which may be attributed to its larger surface area and smaller pore structure21. The pollutant removal mechanisms of magnetic biochar differ from those of magnetic hydrochar22. Economic analyses have shown that magnetic hydrochar is more cost-effective than traditional commercial activated carbon, offering a sustainable and practical approach to wastewater treatment23. Despite significant progress in the synthesis and application of magnetic hydrochar, a comprehensive review covering its fabrication, properties, and performance is still lacking.
In this review, we aim to fill this gap by exploring magnetic hydrochar’s synthesis from various precursors, examining its physicochemical properties, and analyzing its wide applications in wastewater treatment. The combined benefits of carbon and metal-based materials position magnetic hydrochar as a highly effective and sustainable option for wastewater treatment.
Magnetic hydrochar
Magnetic hydrochar retains many of the advantageous properties of regular hydrochar while incorporating magnetic elements that significantly enhance its functionality, particularly in wastewater treatment. The magnetic components not only enable easy separation from treated water via an external magnetic field but also modify the hydrochar’s physical and chemical characteristics, thereby improving its overall efficiency in pollutant removal. This section discusses the various fabrication methods for producing magnetic hydrochar and explores the factors influencing its development, such as precursor materials and synthesis conditions. Furthermore, the key physicochemical properties of magnetic hydrochar, including porosity, surface structure, and magnetic functionality, are examined to illustrate how these attributes contribute to its superior performance in adsorption and catalysis applications.
Fabrication process
Magnetic hydrochar is synthesized through hydrothermal processing of a variety of renewable organic feedstocks, without specific restrictions on the carbon source. Lignocellulosic biomass, such as woody biomass and agriculture residue24,25,26, has been identified as efficient carbon precursors for hydrochar production27,28. Lignocellulosic materials in organic resources contribute to an increased hydrochar yield29. Non-lignocellulosic substances, including non-woody biomass30, animal manures31, industrial residues32, and pure organic solutions33, underwent carbonization, which also led to the formation of hydrochar. Among these organic materials, iron-enriched organic feedstocks can be directly converted into magnetic hydrochar, whereas other organic feedstocks require the addition of magnetic precursors. Metallic precursors, including iron (Fe)34, cobalt (Co)35, and nickel (Ni) compounds25, were employed to provide the magnetic properties of hydrochar. Most research has concentrated on synthesizing ferromagnetic hydrochar, due to the relatively low cost of these precursors, the strong magnetic characteristics of the resulting hydrochar, and its improved biocompatibility for ecological applications36,37. In these cases, magnetic hydrochar can be synthesized using either a one-step or two-step method. Figure 1 illustrates the different fabrication pathways.
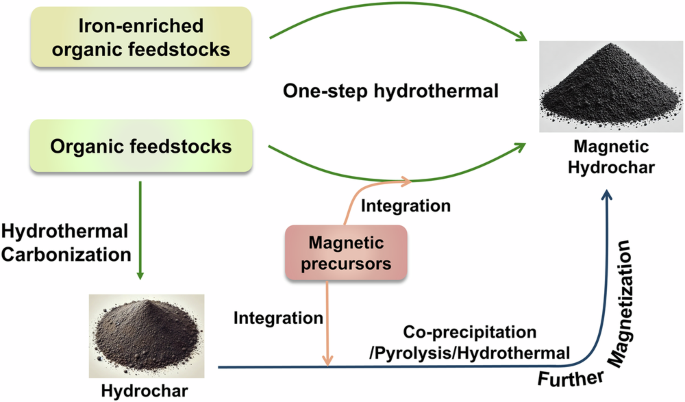
Methods for fabricating magnetic hydrochar.
Iron-rich feedstocks can be transformed into magnetic hydrochar through a one-step hydrothermal carbonization process. During hydrothermal carbonization, the Fe component is reduced by organic matter, leading to the formation of Fe3O4, which enhances both the wastewater treatment efficiency and the magnetic properties of the hydrochar. It has been reported that nanosized magnetite (Fe3O4) particles can disperse onto hydrochar through the hydrothermal carbonization of iron-rich hyperaccumulators38. Additionally, industrial wastes such as Chinese medicine industry waste39, iron-enriched sludge40, and coking sludge41, which are rich in Fe, have been shown to facilitate the formation of magnetic hydrochar via a similar one-step process.
Magnetic hydrochar has been synthesized from various biowastes combined with external metal salts via a one-step hydrothermal process. For instance, co-precipitation of iron(III) chloride (FeCl3) and iron(II) sulphate (FeSO4) under alkaline conditions, followed by hydrothermal treatment with paunch waste, has been shown to produce magnetic hydrochar embedded with Fe3O4 nanoparticles42. The simultaneous incorporation of all components plays a key role in the successful synthesis of magnetic hydrochar. In another example, magnetic hydrochar was produced from Acacia koa pod covers and FeSO4 in an alkaline solution through the same hydrothermal process43. Incorporating Fe salts in different oxidation states during hydrothermal carbonization of biowaste can result in the formation of magnetic hydrochar44,45. Various additives, such as metal salts with oxidative capacities46, iron-rich waste47, and magnetic solids48, have also been co-hydrothermally treated with organic feedstocks, improving the carbonization and magnetization processes to yield high-quality magnetic hydrochar. However, the mechanism behind magnetic phase formation still requires further investigation.
Magnetic hydrochar can also be fabricated through the magnetization and activation of hydrochar. The choice between one-step and two-step hydrothermal processes plays a crucial role in determining pollutant removal efficiency49. Post-treatment significantly influences the physicochemical properties of magnetic hydrochar. For example, magnetic hydrochar can be synthesized by first producing hydrochar through the hydrothermal processing of glucose, followed by co-precipitation with FeSO4 and FeCl3 in an alkaline solution50. Magnetization through co-precipitation has been shown to increase the specific surface area of the hydrochar51. Additionally, the reduction-precipitation method can be used to create magnetic hydrochar by introducing reducing agents such as sodium sulfite52 or sodium borohydride25 to facilitate the formation of the magnetic phase on the hydrochar. Another approach involves subjecting hydrochar to further hydrothermal treatment with magnetic precursors, providing a straightforward method for functionalizing the material53. Further hydrothermal treatments serve as an efficient means to enhance the properties of magnetic hydrochar54.
Although hydrochar contains abundant functional groups, its relatively low surface area and poor porosity limit its broader environmental applications. To address this, simultaneous magnetization and activation of hydrochar have been explored to optimize its properties. For example, the porous structure of hydrochar has been shown to improve after the pyrolysis of rice husk-based hydrochar in the presence of FeCl3 and zinc chloride (ZnCl2)55. In this process, ZnCl2 acts as an activation agent, creating pores and enhancing the surface area. The production of efficient magnetic hydrochar can be achieved by adjusting activation agents, such as carbon dioxide56, potassium hydroxide30, and various types of iron salts57,58, during the magnetization and activation stages. Furthermore, post-pyrolysis treatment of magnetic hydrochar has been found to improve corrosion resistance in acidic environments and enhance magnetic properties, providing additional benefits for environmental applications59.
Based on the reviewed synthesis methods, magnetic hydrochar can be produced through relatively simple procedures. The fabrication of magnetic hydrochar using a wide range of organic and inorganic feedstocks, along with various synthesis approaches, has been extensively studied. These efforts aim to optimize the process and enhance the performance of magnetic hydrochar for wastewater treatment applications. The key factors influencing the fabrication process will be discussed in the following section.
Properties of magnetic hydrochar
Hydrochar produced through hydrothermal carbonization shows enhanced carbon content and lower H/C and O/C ratios compared to its organic feedstocks. Magnetizing hydrochar tends to increase both carbon and ash content while also raising its aromaticity. The ash in magnetic hydrochar is generally rich in Fe, along with other inorganic elements such as Mn and Co. High levels of carbonization in magnetic hydrochar have been found to significantly improve catalytic efficiency in the Fenton process60. X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) were employed to identify the chemical states of surface elements. The aromatic structure present on the magnetic hydrochar facilitated pollutant adsorption through π-π interactions61. The abundant oxygen-containing functional groups (OFGs) in magnetic hydrochar significantly improved its adsorption and catalytic performance47,62. The point of zero charge (pHPZC) of magnetic hydrochar, influencing electrostatic interactions with pollutants, is shaped by its surface chemical properties. Research has demonstrated that acidic functional groups reduced the pHPZC of magnetic hydrochar from 7.35 to 6.5531. Its surface chemical properties significantly influence both adsorption and catalytic performance.
Magnetic particles can be distributed either on the surface or embedded within the carbon matrix in the magnetic hydrochar, depending on the fabrication methods and variations in magnetic precursors34,63. Fe species in magnetic hydrochar were primarily held by chemical bonds rather than simple physical cohesion64, which enhance electron transfer when functioning as a catalyst. Carboxyl (-COOH) and hydroxyl groups (-OH) on hydrochar interacted with magnetite nanoparticles through coulombic force53. Additionally, it has been suggested that the carbon matrix prevented the agglomeration of metallic nanoparticles, resulting in a high dispersion of magnetic nanoparticles on the surface65. Iron species encased within carbon layers exhibited strong acid resistance, while non-magnetic materials may reduce saturation magnetization.
The stronger magnetism of magnetic hydrochar improves its separation efficiency from the treatment solution. The formation of magnetic inorganic particles, such as ferrites, zero-valent Fe/Ni, and metal oxides, is the primary source of magnetism in hydrochar. Saturation magnetization (Ms), measured by a vibrating sample magnetometer and analyzed through hysteresis loops, was used to indicate the magnetism of hydrochar39. Materials with an Ms > 1 emu/g are considered highly magnetic and suitable for magnetic separation. Variations in the type and concentration of inorganic species led to differences in saturation magnetization31,66. Fe3O4 was a commonly identified component in magnetic hydrochar, as revealed by FTIR and XPS analyses. Fe3O4 possesses high saturation magnetization, although its acid resistance is relatively lower than that of γ-Fe2O363. Uniformly dispersed magnetic particles on hydrochar demonstrate efficient and stable interactions with the carbon matrix, promoting electron transfer and enabling catalyst recovery during wastewater treatment.
Magnetic hydrochar exhibited a range of morphologies, including bulk, layered, spongy, and spherical forms67,68,69. It retained the uneven, porous structure of hydrochar, with enhanced porosity and surface roughness, and was uniformly coated with inorganic particles53. Furthermore, smooth carbon spheres with magnetic particles attached to their surfaces were observed in magnetic hydrochar synthesized from organic wastewater65. The size of the magnetic hydrochar ranges from nanoscale to microscale, with magnetic particles predominantly distributed at the nanoscale. The diverse morphologies and structural characteristics of magnetic hydrochar determine its specific surface areas and pore properties. Figure 2 illustrates the distribution of specific surface areas and pore structures.
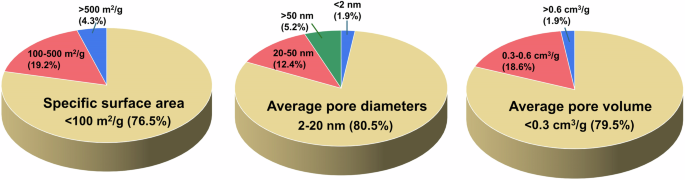
Structural properties of magnetic hydrochar (Data collected from Google Scholar up to November 2024; sample sizes: 187 for specific surface area, 154 for average pore diameter, and 156 for average pore volume).
The specific surface area of most magnetic hydrochar is generally below 100 m2/g, likely due to the constraints of low hydrothermal temperatures and solution conditions during synthesis. Therefore, further high-temperature pyrolysis can result in a specific surface area exceeding 500 m2/g, and in some cases, reaching up to 973.56 m2/g30. The pore structure of magnetic hydrochar predominantly fell within the mesopore range (2 nm to 20 nm), while both micropores (pore diameter < 2 nm) and macropores (pore diameter > 50 nm) are also present in smaller quantities70,71. The average pore volume remains below 0.3 cm3/g. Specific surface areas and pore volumes affect the capacity for pollutant treatment, while pore diameters influence pollutant entry and diffusion, impacting both adsorption and catalysis.
Various carbon-based materials have been applied in wastewater treatment, each with distinct characteristics. Biochar, known for its substantial specific surface area, has proven to be a promising material72. Hydrochar possesses numerous functional groups73, while magnetic hydrochar features a multifunctional surface, a mesoporous structure, and integration with inorganic components, offering significant potential for wastewater treatment.
Influencing factors by the fabrication parameters
The production of functional carbon materials largely depends on the properties of the precursor, activation conditions, and processing parameters74. Likewise, the fabrication of magnetic hydrochar is significantly influenced by the choice of organic feedstock, the selection of Fe precursors, and specific process conditions. Each of these factors plays a critical role in determining the chemical and structural characteristics of the resulting magnetic hydrochar.
Feedstocks
The physicochemical structure of hydrochar is largely influenced by the chemical properties of the feedstock75. For example, a study comparing magnetic hydrochar derived from sunflower husk and orange juice residue found that the magnetic hydrochar from orange juice residue (MHCOR) exhibited a higher BET surface area and smaller pore diameter compared to that from sunflower husk (MHCSFH)76. Additionally, the chemical structure was altered due to the differences in biomass feedstocks, with MHCOR demonstrating a higher capacity for removing malachite green. The distinct components in organic feedstocks optimized both the carbon and magnetic inorganic structures of the resulting materials. Magnetic hydrochar was synthesized by co-precipitating iron precursors with hydrochar derived from egg white and sucrose77. Sucrose played a key role in forming the hydrochar framework, where higher sucrose content led to a morphological transition from layered to spherical structures.
It has also been reported that reducing sugars, such as glucose in the feedstock, facilitate the formation of magnetic Fe3O4 rather than Fe2O3 during one-step synthesis78. Fe3O4 exhibits strong ferromagnetic properties, ensuring sufficient magnetic separation capability for hydrochar. This was further supported by findings where glucose reacted with Fe(III) during a one-step hydrothermal process, leading to the formation of Fe3O479. The concentration of glucose significantly influenced the morphology and size of the magnetic hydrochar nanocomposites. A synergistic effect between carbohydrates and proteins was also observed during the hydrothermal treatment of sewage sludge, which enhanced the formation of magnetic Fe3O480. Moving forward, more attention should be given to the co-existing components and the proportion of specific elements in organic feedstocks to design more efficient magnetic hydrochar.
Magnetic precursors
The magnetic properties of most magnetic hydrochar originate from the addition of inorganic compounds. The formation of magnetic Fe3O4 depends on the specific types of iron salts used. Studies have indicated that Fe(III) plays a critical role in the synthesis of iron carbide, while the combined addition of Fe(III) and Fe(II) favors the formation of magnetic Fe3O466. The presence of Fe(II) promotes the formation of highly magnetic Fe3O4. Co-hydrothermal treatment with Fe2(SO4)3, sewage sludge, and glucose produced magnetic hydrochar with strong magnetic separation capability, enhanced by the reducing properties of glucose78. The use of high FeCl3 concentrations was shown to facilitate the hydrolysis of organic substances, resulting in increased saturation magnetization81.
The introduction of inorganic substances during the hydrothermal process significantly also alters the carbonization pathways of the organic material, thereby influencing the chemical composition and pore structure of the resulting magnetic carbon82. For instance, the addition of ferric sludge during the hydrothermal carbonization of biological sludge has been shown to inhibit pore development, leading to a reduction in pore size60. The use of strong oxidizing agents like K2FeO4 resulted in magnetic hydrochar with a high specific surface area, larger pore volume, and an increased presence of oxygen-containing functional groups following hydrothermal processing with organic feedstocks24. Additionally, studies comparing various iron salts in the synthesis of magnetic hydrochar via a two-step process revealed that FeCl3 is particularly effective in producing high-porosity, acid-resistant magnetic hydrochar from sawdust. Other iron salts, such as FeC2O4, Fe(C4H5O7), and Fe2(SO4)3, were found to hinder pore structure development due to reduced carbonization63. Magnetic hydrochar synthesized with high Fe concentrations via co-precipitation demonstrated improved adsorption capacity and stability, underscoring the importance of iron salt selection in optimizing the performance of magnetic hydrochar83.
Additional functionalization agents
Further activation and modification present promising methods for fabricating functional materials that contribute to sustainable wastewater management. Alkalis are commonly employed for activating hydrochar84. In one study, the etching of pharmaceutical industry waste under alkaline hydrothermal conditions demonstrated that alkali treatment not only removed organic matter and ash from the hydrochar but also enhanced its porosity and surface area32, while introducing oxygen-containing groups and Fe3O4 into the magnetic hydrochar. Acid-assisted hydrothermal treatment facilitates the reduction of Fe(III) and modifies the surface functional groups on magnetic hydrochar, thereby enhancing its applicability85.
The incorporation of additional surface functional groups can significantly improve the adsorption capacity of magnetic hydrochar for specific pollutants. Nitrogen-containing substances, such as urea, diethylenetriamine, and ethylenediamine, have been used to modify magnetic hydrochar, further enhancing its functionality33,86,87. Similarly, sulfur-doped magnetic hydrochar has been produced by introducing sulfur compounds like chlorosulfonic acid and carbon disulfide, which improve pollutant adsorption69,88. Grafting organic substances with specific functional groups onto magnetic hydrochar has also been shown to enhance its pollutant removal capabilities. For example, magnetic hydrochar functionalized with silymarin, chitosan, and β-cyclodextrin has demonstrated improved adsorption and catalytic performance in removing pollutants89,90,91. Additionally, metal oxides such as manganese dioxide (MnO2) and calcium oxide (CaO) have been anchored onto magnetic hydrochar, resulting in structural changes that influence its adsorption capacity92,93. The combination of magnetic hydrochar with other metal oxides has also been found to significantly enhance its catalytic activity, further expanding its potential for environmental applications94,95.
Temperature
Fabrication conditions, particularly temperature, heating methods, and the choice of synthesis methods, influence the structural and chemical properties of magnetic hydrochar, determining its adsorption and catalytic capabilities. Compared to hydrothermal duration and initial solution pH96, the hydrothermal temperature has a more pronounced effect on the transformation of organic and inorganic precursors during synthesis97, resulting different physicochemical structure. It has been found that high temperatures accelerated the carbonization of organic feedstock, producing higher carbon content and an abundance of aromatic functional groups64. Correspondingly, the surface morphology of magnetic hydrochar may be altered, contributing to a higher specific surface area and larger pore volume. Excessive carbonization at higher temperatures reduced the specific surface area and pore volume due to coke formation and the accumulation of ash. The bond strength between iron and other elements improved with increasing temperature, enhancing the stability of the magnetic hydrochar. Elevated temperatures promoted the conversion of Fe-containing precursors into magnetic Fe3O4 during the synthesis41. The functional groups and iron oxides formed on magnetic hydrochar, which are influenced by the hydrothermal temperature, result in varying adsorption and activation properties35,46. Furthermore, high temperatures enhance the crystallinity of magnetic species and reduce the impact of organic substances on magnetic properties, leading to increased saturation magnetization. In a two-step hydrothermal process, it was demonstrated that maintaining a low hydrothermal temperature is essential for producing magnetic hydrochar composites with high porosity, minimized Fe leaching, and suppressed graphitization. These temperature-controlled conditions play a key role in optimizing the structural and functional characteristics of magnetic hydrochar for enhanced performance in environmental applications98.
Heating technology
Microwave-assisted heating has been employed during hydrothermal processing to reduce energy consumption while impacting the structural properties of the resulting material99. For instance, the use of microwave-assisted hydrothermal synthesis was found to reduce the yield of magnetic hydrochar compared to traditional methods, as observed in one-step microwave hydrothermal synthesis100. However, microwave assistance enhanced the disintegration of organic components, leading to a highly porous structure and the formation of distinct functional groups on the magnetic hydrochar. This method has shown improved efficiency in removing metal ions and organic pollutants, making it a promising approach for fabricating functional magnetic hydrochar for environmental applications101.
Synthesis methods
The selection of synthesis procedures significantly influences the properties of magnetic hydrochar, dictating the associated energy consumption and environmental impact102,103. The one-step hydrothermal carbonization method requires fewer operational steps and lower energy input. Moreover, inorganic substances act as catalysts in the hydrothermal carbonization of organic matter, facilitating the formation of well-structured magnetic hydrochar optimized for wastewater treatment64. This method resulted in higher magnetic particle loading on hydrochar compared to the two-step synthesis method, yielding uniformly dispersed spherical magnetic particles within a carbon matrix that prevents Fe leaching34. In contrast, the two-step process, which combines hydrothermal carbonization with post-impregnation, led to lower Fe loading and surface aggregation of Fe particles.
Magnetic hydrochar produced by the one-step method demonstrates superior catalytic activity and stability in wastewater treatment, with reduced environmental impact. While the two-step synthesis requires more energy, it allows for tailored functional properties through precise control of various operating parameters83,103. For instance, magnetic hydrochar synthesized by hydrothermal carbonization followed by post-pyrolysis exhibited a large specific surface area, leading to a high adsorption capacity for As(V) in a single adsorption cycle31.
The complex and heterogeneous nature of feedstocks, combined with the inherent variability of hydrothermal reactions, results in magnetic hydrochar with diverse properties and performance. This variability presents challenges in achieving precise control over the characteristics of magnetic hydrochar. To address this, further research is needed to comprehensively understand the effects of fabrication conditions. Despite these challenges, the flexibility of magnetic hydrochar synthesis allows for its application in treating a wide range of complex wastewater, making it a versatile solution for environmental management.
Applications in wastewater management
The combination of abundant functional groups and the porous nature of magnetic hydrochar enhances its effectiveness in pollutant adsorption and redox processes during wastewater treatment. Pollutant removal efficiency may vary based on the specific properties of magnetic hydrochar and the nature of pollutants present, as different removal mechanisms are involved. Clarifying the pollutant removal mechanism of magnetic hydrochar and identifying the key role of its properties are crucial for optimizing pollutant removal efficiency104. Figure 3 provides the specific adsorption mechanism for pollutant removal by magnetic hydrochar.
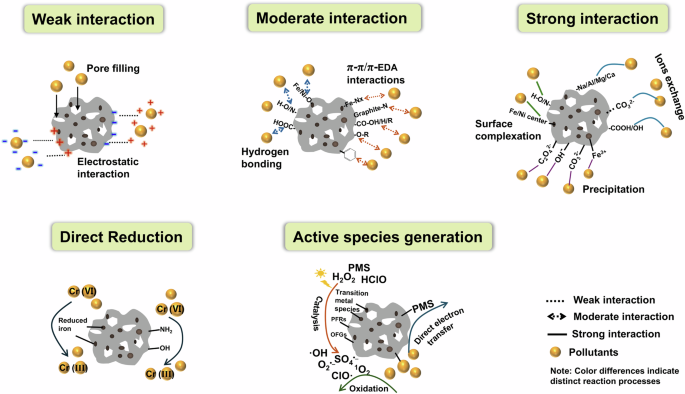
Pollutant removal mechanism of magnetic hydrochar.
Pollutant adsorption mechanism on magnetic hydrochar
Organic and metal ion pollutants can be efficiently removed by adsorption onto magnetic hydrochar. Mechanisms of adsorption include pore filling, electrostatic forces, hydrogen bonding, π-π stacking, surface complexation, ion exchange, and precipitation, each contributing with different levels of interaction strength.
The porous structure of magnetic materials enhances pollutant adsorption by the pore-filling effect. A decrease in surface area and pore volume following the adsorption of pollutants like tetracycline underscored the significance of pore filling in this process57. Additionally, a positive correlation has been noted between the maximum adsorption capacity of magnetic materials and their surface area and micropore volume105. Magnetic hydrochar, with its abundant mesoporous structure, provides internal sites for pollutant adsorption via this pore-filling mechanism.
Electrostatic attraction also plays a crucial role in the physical adsorption process of magnetic hydrochar. The surface charge of magnetic hydrochar is closely related to its surface functional groups and the pH of the solution, as indicated by its point of pHPZC value. When the aqueous pH exceeds the pHPZC, deprotonation of oxygen-containing functional groups creates a negatively charged surface that enhances the adsorption of cations. Conversely, at pH values below the pHPZC, the surface becomes positively charged, enhancing electrostatic repulsion and reducing the adsorption of cationic pollutants. Qiu’s research demonstrated the significance of electrostatic interactions, showing that anionic surfactants compete with anionic Congo red for adsorption on magnetic hydrochar106.
Chemical adsorption emerges as the dominant mechanism for pollutant removal, as indicated by the adsorption kinetics analysis of pollutants on magnetic hydrochar. This process includes hydrogen bonding, π-π interactions, surface complexation, ions exchange, and precipitation, suggesting moderate to strong interactions between pollutants and hydrochar. Chemical adsorption mechanisms were explored by examining significant alterations in the chemical states of the adsorbent before and after adsorption, providing insights into the nature of these interactions.
Hydrogen bonding and π-π interactions have been identified as key mechanisms for moderately binding pollutants to magnetic hydrochar. Hydrogen bonds are formed due to the oxygen (O)/nitrogen (N)-containing groups on the magnetic hydrochar, as indicated by changes in the FTIR peaks associated with O-H/N-H groups, and shifts in O1s/N1s peaks observed in the XPS analysis54,92. Additionally, Fe-O and Ni-O groups on the magnetic hydrochar act as active sites for hydrogen bond formation, further facilitating pollutant adsorption54. π-π and π-electron donor-acceptor (EDA) interactions are also confirmed as important adsorption mechanisms, evidenced by shifts in the binding energy of C 1s peaks and alterations in the stretching vibrations of aromatic C = C bonds54,107. These interactions involve electron transfer between pollutants and the magnetic hydrochar. Density functional theory (DFT) calculations have supported the presence of strong π-π interactions between dye molecules and magnetic hydrochar, based on analyses of equilibrium configurations and partial density of states (PDOS)58. Aromatic groups, along with functional groups such as carboxyl, carbonyl (C = O), ether groups (C-O-C), can participate in conjugated adsorption reactions with pollutants through π-π or π-EDA interactions108. Graphitic N and Fe-NX structures further support pollutant adsorption by intensifying π-π interactions. This effect stems from a reduction in electron cloud density and an increase in π-electron acceptor capacity in the carbon framework109.
Magnetic hydrochar exhibits strong interactions with adsorbed pollutants, primarily through mechanisms such as surface complexation, ion exchange, and precipitation. The coexistence of inorganic and organic components on magnetic hydrochar allows for surface complexation with organic or metal ion pollutants during wastewater treatment. Competition for adsorption sites can arise, as observed in cases where organic molecules like ethylenediaminetetraacetic acid (EDTA) and tetracycline competed for binding to metal centers on the adsorbent, triggering complexation reactions57. The functional groups in organic pollutants, acting as soft and hard bases, can form complexes with metal ions such as Ni(III) (borderline acid) and Fe(III) (hard acid) through acid-base interactions or strong coordination bonds54. For the removal of inorganic ions in wastewater, functional groups containing oxygen and nitrogen readily form complexes with heavy metals101,110. Additionally, the strong affinity of Fe oxides in magnetic hydrochar enhances metal ion complexation during the adsorption process111. The lone pair electrons in oxygenous/nitrogenous groups facilitate coordination with metal ions112.
Ion exchange and co-precipitation have been widely identified in the adsorption of ionic pollutants. During the adsorption process, functional groups such as -OH and -COOH, along with protonated iron oxides, facilitate cation exchange with pollutants like protonated tetracycline46. However, the inorganic components of magnetic hydrochar, such as carbonate ions (CO32−) and metal ions like aluminum (III), calcium (II), magnesium (II), and sodium (I), are primarily responsible for driving the ion exchange process106,113. Xu et al. investigated that magnetic hydrochar derived from red mud engaged in ion exchange during the adsorption of pollutants, while magnetic hydrochar synthesized with FeCl3 does not exhibit ion exchange in the removal of chromium ions(Cr(III))114. The presence of aluminum (III) in red mud promoted ion exchange with Cr(III) due to their similar spatial structure and ionic size. X-ray diffraction revealed newly adsorbed compounds on the magnetic hydrochar70, while ions exchanged from the material were found in the aqueous solution115. In addition to ion exchange, metal cation pollutants can be precipitated through reactions with selected compounds on magnetic hydrochar. The Fe(III) present in the magnetic hydrochar is capable of binding with lead ions (Pb(II)) and Cr(III), leading to the formation of stable precipitates101,109. Furthermore, precipitates such as lead(II) oxalate ((PbC2O4), cadmium carbonate (CdCO3) or cadmium hydroxide (Cd(OH)2) have been identified in the utilized magnetic hydrochar, caused by the associated anions on its surface110. These reactions contribute significantly to the pollutant removal efficiency of magnetic hydrochar in wastewater treatment.
In the adsorption process, multiple mechanisms often work together to achieve effective pollutant removal, with the dominant mechanism depending on the specific characteristics of the pollutants and the conditions of the wastewater. To optimize adsorption capacity and enable selective removal of pollutants, the physicochemical structure of magnetic hydrochar should be precisely engineered based on the specific application. This precise engineering ensures that the material’s surface structure, pore size, functional groups, and overall composition are optimized to target specific contaminants efficiently.
Adsorption performance of various pollutants
Investigations into the adsorption mechanism indicate that the adsorption capability of magnetic hydrochar is primarily determined by its surface functional groups rather than its specific surface area. Magnetic hydrochar has been observed to achieve an adsorption efficiency of over 90%, significantly outperforming activated carbon (below 78%) for pesticide removal, even though activated carbon features a larger specific surface area116. Magnetic elements and functional groups in the hydrochar improve its adsorption capacity117. However, the overall effectiveness remains dependent on the specific characteristics of target pollutants. Table 1 summarizes the adsorption performance for different pollutants.
Magnetic hydrochar demonstrates high adsorption efficiency and ease of recovery in dye wastewater treatment21. While it effectively removes a range of dyes, the adsorption process may vary under different conditions92,118. Qiu et al. studied the selective adsorption of Congo Red (CR) with magnetic hydrochar in the presence of other pollutants106. The larger molecular structure of coexisting pollutants hindered CR adsorption due to spatial limitations. The impact of organic pollutant structure and size on adsorption performance has also been discussed in other studies54,105. Importantly, synergy and competition in adsorption were observed between CR and coexisting organic substances, influenced by their surface charge in solution. For ionic pollutants, their surface charge in aqueous solutions dictates their adsorption performance on magnetic hydrochar. Cationic pollutants enhance the adsorption of anionic CR due to their strengthened electrostatic interactions. In contrast, anionic pollutants significantly inhibit CR adsorption competitive adsorption for positively charged active sites. Studies also indicate that modified magnetic hydrochar can achieve selective adsorption of oppositely charged ionic pollutants, with pH adjustments playing a key role in this process87. Pollutants with similar adsorption mechanisms compete for adsorption sites on the magnetic hydrochar, reducing the adsorption capacity for target pollutants.
Organic pollutants containing π-structures, such as TC, interact with magnetic hydrochar primarily through π-π or π-EDA interactions. In this mechanism, TC serves as both an electron donor and acceptor108. For methylene blue (MB) and methyl orange (MO) adsorption, MB acts as an electron acceptor, while MO acts as an electron donor. Magnetic hydrochar shows stronger electron transfer and reactivity with MB than with MO58. Consequently, organic pollutants with varying physicochemical properties could be selectively removed from the co-existence solution.
In addition to its ability to adsorb organic pollutants, magnetic hydrochar has also been extensively used in the treatment of metal ions. Differences in the physical and chemical properties of pollutants result in distinct adsorption processes on magnetic hydrochar113,119. Both metal ions and organic pollutants are typically adsorbed through weak interactions. Organic pollutants are mainly adsorbed via hydrogen bonding, π-π/EDA interactions, and surface complexation, while metal ions are primarily adsorbed through electrostatic interactions, surface complexation, ion exchange, and precipitation. Research on the removal of cadmium (Cd) ions and chlortetracycline (TCT) in a binary system found that at lower Cd(II) concentrations, TCT removal was enhanced due to reduced electrostatic repulsion, facilitated by the coordination of Cd(II) with TCT111. However, as Cd(II) concentrations increased, competition for complexation sites intensified, reducing the removal efficiency for both TCT and Cd(II). Zhang et al. developed magnetic hydrochar for the simultaneous adsorption of Cd(II) and anthracene, as their distinct adsorption mechanisms prevented competitive interactions120. Optimizing the specific adsorption mechanisms for each pollutant can improve selective removal in treatment processes.
The adsorption of metal ions on magnetic hydrochar involves various mechanisms. Factors such as ionic radius, electronegativity, and hydration energy influence the adsorption behavior of metal ions121. Meanwhile, the adsorption efficiency of metal ions depends on their affinity for the functional groups on magnetic hydrochar110. For example, the adsorption of As(V) occurs through multiple synergistic mechanisms, including pore filling, electrostatic attraction, hydrogen bonding, and complexation31. Magnetic hydrochar, containing lone pair electrons, facilitates interactions with aqueous arsenic (As) anions. A recent study has shown that iron oxide species, rather than organic oxygen-containing groups, played the primary role in coordinating with As(V) anions96. Further surface functionalization of magnetic hydrochar has been demonstrated to enhance As(V) adsorption via complexation mechanisms122.
The removal of lead (Pb) and cadmium (Cd) ions by magnetic hydrochar is highly efficient, owing to its mesoporous structure and surface functionalization, with ion exchange, surface complexation, and precipitation being the dominant processes involved38,123. Studies on Pb(II) removal have highlighted that surface complexation was the primary mechanism of aminated magnetic hydrochar, while ion exchange dominated in systems enriched with inorganic compounds70,115. The adsorption behavior of metal ions can be tuned through surface-functionalized magnetic hydrochar, offering the potential for selective pollutant adsorption. Sulfur-modified magnetic hydrochar derived from sludge selectively adsorbed Pb(II) from a mixture containing Cd(II), Cu(II), and zinc ion (Zn(II)), primarily due to Pb(II)’s higher affinity for xanthate functional groups88. Similar selective adsorption of Pb(II) was also observed on sulfide-modified magnetic hydrochar produced from pinecones124.
Investigations into the adsorption of various pollutants on magnetic hydrochar reveal that the unique physicochemical properties of pollutants lead to different adsorption behaviors. Customizing magnetic hydrochar to align with the specific attributes of target pollutants can significantly boost the efficiency and sustainability of wastewater treatment. Nevertheless, overcoming the challenge of competitive adsorption among pollutants with similar properties remains a major obstacle. Furthermore, research on the regeneration and disposal of spent magnetic hydrochar remains insufficient, potentially increasing environmental risks and energy costs.
Redox of pollutants mediated by magnetic hydrochar
In addition to adsorbing pollutants from wastewater, magnetic hydrochar also facilitates the chemical transformation of toxic pollutants into less harmful components. The high efficiency of magnetic hydrochar in pollutant degradation reduces the risk of secondary pollution during subsequent treatments involving spent materials. A notable example is the treatment of Cr(VI), where magnetic hydrochar participates in redox reactions, facilitating the reduction of Cr(VI) to the less toxic Cr(III)23. This reduction process is a crucial aspect of its pollutant remediation capabilities. Table 2 illustrates various redox transformations of pollutants using magnetic hydrochar. Understanding the redox mechanisms of pollutants is essential for advancing the design of more efficient functional materials. As shown in Fig. 3, magnetic hydrochar can directly participate in redox reactions with pollutants or act as a catalyst, generating reactive species that promote degradation indirectly. These functions are largely attributed to the specific functional groups present on the magnetic hydrochar surface.
Surface electron-donating groups, including low-valence iron, oxygen-containing functional groups (OFGs), and amino groups (-NH2), directly participate in the reduction of metal ions, thereby aiding in the remediation of metal ion-contaminated wastewater. Low-valent metals in the hydrochar primarily act as reducing agents in the metal ion removal process114,125. A detailed examination showed that the decrease in Cr valence was accompanied by an increase in Fe valence26. Scavenging of reductive Fe(II) by 1,10-phenanthroline notably hindered the conversion of Cr(VI) to Cr(III). Furthermore, OFGs contributed to Cr(VI) reduction or acted as electron transfer carriers, facilitating electron movement between the reducing agent and metal ions in solution. The introduction of electron-donating groups, such as amino groups, boosted the reducing ability of magnetic hydrochar. In contrast, quaternary amine groups, which are electron-accepting, improved Cr(VI) adsorption but impeded its reduction to a lower oxidation state33. Therefore, functionalizing magnetic hydrochar provides greater flexibility, allowing for the customization of its properties to suit various wastewater treatment applications.
Pollutants can undergo indirect oxidation during AOPs, which catalyzes oxidants such as hypochlorite (HClO), hydrogen peroxide (H2O2), and peroxymonosulfate (PMS), leading to the generation of highly reactive oxygen species126,127. Extensive research has explored the catalytic function of magnetic hydrochar in removing metal ions and organic pollutants.
The synergy between magnetic transition metals and specific functional groups results in remarkable catalytic performance of magnetic hydrochar, enabling efficient pollutants oxidation128. Iron-enriched magnetic hydrochar was investigated as a catalyst in Fenton-like oxidation processes, involving mechanisms driven by hydroxide radicals (•OH), superoxide radicals (O2•−), and nonradical singlet oxygen (1O2) mechanisms40. Hydroxyl radicals (•OH), primarily produced through iron-activated H2O2, accounted for 80.3% of total radical generation.
Magnetic hydrochar with higher Fe content exhibited enhanced performance in the Fenton-like reaction, where Fe(II) played a critical role in stimulating the generation of reactive species85. A series of comparative experiments demonstrated that the synergistic interactions between iron species and the carbon matrix significantly enhanced the Fenton-like catalytic reaction129. The carbon matrix shows limited ability to activate H2O2 for generating reactive species, mainly enhancing iron-catalyzed reactions and accumulating pollutants near active sites. The carbon matrix prevents the aggregation of nano Fe3O4 particles, allowing for better distribution of active sites and improved catalytic efficiency130. Persistent free radicals (PFRs) and electron-donating surface groups, such as amino, hydroxyl, and carboxyl groups, complex with iron species to promote the Fe(III)/Fe(II) cycle, sustaining continuous •OH generation. Additionally, functionalizing magnetic hydrochar with silymarin enhanced Fe(III) reduction, increasing Fe(II) levels and boosting •OH production for TC degradation89. PFRs also contributed to the formation of O2•−. Active sites responsible for 1O2 formation were likely associated with hydrochar defects or •OH transformation.
Magnetic hydrochar with both transition metals and oxygen-containing functional groups exhibits the capability to activate PMS131, generating sulfate radicals (SO4•−) with strong pollutant degradation potential, along with •OH. In addition to radical pathways, non-radical degradation mechanisms, such as the production of 1O2, high-valence metal, and direct electron transfer, also play a significant role during PMS activation. Distinct degradation mechanisms of magnetic hydrochar were observed for monochlorobenzene (MCB) and p-chloroaniline (PCA)35, with radical-based degradation dominating MCB removal, while PCA degradation, driven by non-radical pathways, showed less susceptibility to interference from co-existing pollutants.
Both transition metals and carbon-based materials demonstrate high efficiency in persulfate activation94. Cobalt (Co) and iron (Fe) were incorporated into magnetic hydrochar to enhance its potential for PMS activation, promoting the generation of radicals, singlet oxygen, and high-valent metals. Further modification of Fe/Co elements on magnetic hydrochar increased the availability of Co active sites, enhancing the removal efficiency of antibiotics through both radical and non-radical mechanisms68,95. Electron exchange between Fe and Co could promote the Co(II)/Co(III) cycle, ensuring stable catalytic activity. Similarly, surface-reducible functional groups like C-OH supported the transition metal redox cycle. Furthermore, C-OH contributes to PMS activation and the generation of radical. Carbonyl groups (C = O) likely facilitate electron transfer from nucleophilic PMS, leading to the generation 1O2. Carbon components within magnetic hydrochar can activate PMS, facilitating pollutant degradation via direct electron transfer.
Magnetic hydrochar displays photosensitivity under light exposure and in acidic environments, generating highly reactive species, such as such as photogenerated holes (h+), superoxide radicals (O2•−), and •OH132, which were crucial for the pollutants degradation. In addition to typical photosensitive catalysts like bismuth oxybromide and titanium dioxide, the incorporation of hydrochar and Fe3O4 can accelerate the generation of reactive oxygen species during photocatalysis133. Hydrochar facilitates electron transfer under light irradiation, reducing the recombination of photogenerated electrons and holes134. Moreover, the surface oxygen-containing groups are responsible for the absorption of solar light, leading to the production of H2O2 and •OH from oxygen molecules. The narrow-bandgap Fe3O4 can also produce h+ and electron (e−) under UV irradiation, contributing to organic pollutant breakdown. The photogenerated h+ and e− interacted with molecular oxygen, hydroxyl ions, and water, forming superoxide O2•−, and •OH radicals. However, rapid recombination of electron-hole pairs in Fe3O4 can limit photocatalytic efficiency. To overcome this, citric acid (CA) was introduced to form a complex with Fe(III) on the hydrochar surface, enhancing electron transfer and photocatalytic performance61.
Magnetic inorganic substances contribute to the catalytic properties, while functional groups facilitate electron transfer, facilitating the generation of reactive species. As a result, magnetic hydrochar modified with both transition metals and functional groups offers improved catalytic performance, enabling more efficient and sustainable wastewater treatment. Otherwise, further investigations are required to explore and expand the potential catalytic applications of functionalized magnetic hydrochar. In advanced oxidation processes, additional chemical or energy input, along with the design and operation of complex equipment, must be carefully considered to ensure sustainable wastewater treatment.
Sustainability of magnetic hydrochar for wastewater treatment
Magnetic hydrochar has demonstrated strong potential in contaminant adsorption and catalytic oxidation. However, its practical application still faces challenges, particularly regarding cost-effectiveness and environmental sustainability. In this section, the economic feasibility and environmental impact of magnetic hydrochar are discussed.
The fabrication cost and environmental impact of magnetic hydrochar are critical factors for scaling up its practical applications. The cost of feedstock and pre-processing has been reported as economically favorable114. Economic analysis estimates the cost of magnetic hydrochar at 12.6 USD/kg, making it more affordable than commercial activated carbon (20 USD/Kg)23. The stability of hydrochar post-production is crucial for its subsequent applications, as it undergoes physicochemical changes during storage and pre-treatment stages135. Magnetic hydrochar exhibits enhanced stability due to the presence of inorganic components, reducing storage and transportation costs while minimizing environmental risks59,136. Removal efficiency of pollutants by magnetic hydrochar remained consistently high after 30 days of storage92.
Prior to the application of magnetic hydrochar, water washing is frequently used as a pretreatment step until the rinse water reaches a neutral pH, followed by ethanol washing to remove unstable impurities86. Additionally, sodium hydroxide and hydrogen peroxide are employed to activate magnetic hydrochar by modifying surface functional groups87,137. Consideration of chemical costs and non-hazardous treatment of residual solutions is essential for a comprehensive economic and environmental risk assessment138. Additionally dry conditions such as oven or freeze drying were found to affect its catalytic efficiency139. Freeze drying preserves the structure of catalytic components, supporting the stability of magnetic hydrochar. Therefore, enhancing the stability of magnetic hydrochar is vital for improving economic benefits and reducing environmental risks. Studies have reported that hydrochar stability can be increased by optimizing fabrication and pretreatment procedures, including fabrication parameters140,141, storage conditions135, and the use of washing chemicals142. Future studies should prioritize the stability of magnetic hydrochar.
Reusability tests were conducted to evaluate the stability of magnetic hydrochar, providing insights into its economic cost and environmental impact. Typically, these tests involved 3 to 10 cycles of adsorption or catalytic experiments with repeated use of magnetic hydrochar. The pollutant removal efficiency of magnetic hydrochar across multiple cycles is presented in Table 1 and Table 2. Magnetic hydrochar consistently demonstrated high removal efficiency throughout consecutive treatment cycles, which contributes to a significant reduction in overall production costs.
The reusability of the adsorbent was assessed over multiple adsorption-desorption cycles, typically employing various desorbing agents, including acids, alkalis, organic solvents, and chelating agents70,119,143. The smallest chloride ions demonstrated higher desorption capacity for Pb(II) compared to nitrate and sulfate ions123. Alkaline solutions were particularly effective for desorbing organic pollutants, with sodium hydroxide showing the highest regeneration efficiency for toxic organophosphorus insecticides, followed by ammonium hydroxide and potassium hydroxide116. Sequential desorption with various agents has been used to achieve stepwise removal of different pollutants120, enhancing the effective regeneration of magnetic hydrochar. Selecting appropriate desorbing agents and optimizing the desorption process can enhance the stability of magnetic hydrochar during long-term experiments, thereby reducing production and operational investments. In catalytic applications, magnetic hydrochar enables effective pollutant degradation, reducing the need for frequent regeneration. The continuous flow studies also have demonstrated the stability of magnetic hydrochar68. Previous studies have shown that the removal efficiency of magnetic hydrochar decreased by only 3.51% after long-term experiments144.
Additionally, the inevitable leaching of magnetic hydrochar during multiple reuses should be considered, as it impacts reusability and poses potential environmental risks. The surface of magnetic hydrochar is also susceptible to partial oxidation by reactive oxygen species129. However, magnetic hydrochar maintains stable pollutant removal efficiency with minimal leaching of active components, resulting in cost-effectiveness and low environmental impact133. A solution pH exceeding 2.0 in catalytic reactions has little impact on iron leaching from magnetic hydrochar61,105. Moreover, iron leaching remained below the detection limit of atomic absorption spectroscopy when 0.1 M nitric acid was used as the desorption agent119. Furthermore, spent magnetic hydrochar shows minimal heavy metal leaching after treatment with heavy metal solutions, thereby reducing the risk of secondary pollution88. Therefore, the leaching from magnetic hydrochar has a negligible impact on its environmental footprint, demonstrating potential in sustainable wastewater treatment applications.
The stability of magnetic properties was evaluated following long-term experimental procedures, as this factor is critical in assessing the economic viability and environmental impact of magnetic hydrochar. A minor decline in magnetic separation efficiency was observed in reusability tests, though the magnetic hydrochar remained adequately recoverable for further applications115. Yan et al. reported that 85% of the spent magnetic hydrochar was still recoverable under an external magnetic field, emphasizing its practicality for wastewater treatment applications145.
Throughout prolonged reuse studies, the accumulation of contaminants and degradation by-products on active sites leads to the progressive degradation of magnetic hydrochar, ultimately causing catalyst deactivation. Currently, few studies have reported the end-of-life management of spent magnetic hydrochar. Final disposal methods for magnetic hydrochar require careful consideration to support its commercial-scale production146. Common disposal options include incineration and landfilling, both of which are simple and relatively safe. Alternatively, reusing magnetic hydrochar as a catalyst, soil amendment, or construction material presents a potentially cost-effective approach. However, the long-term economic, social, and safety implications of these options must be rigorously evaluated.
Life cycle assessment revealed that biochar has a higher energy consumption compared to hydrochar, highlighting the sustainability hydrochar as an electrode catalyst147. A comprehensive life cycle assessment (LCA) should be conducted to evaluate the economic, social, and environmental impacts of magnetic hydrochar across all stages of wastewater treatment, promoting sustainable application in this field148,149.
Conclusions and outlook
In conclusion, magnetic hydrochar stands out as a highly promising material for sustainable wastewater management, offering an impressive combination of adsorption efficiency, catalytic potential, and easy magnetic separation. Despite these advantages, there are critical challenges that must be addressed to fully realize its potential. Beyond conventional pollutant adsorption, the removal of emerging contaminants and microplastics remains underexplored. Coupling it with advanced water treatment technologies, such as electrocatalytic oxidation, could drastically expand its role in environmental remediation, offering a multi-functional approach to removing complex pollutants. To fully exploit the potential of magnetic hydrochar, further innovation is needed, particularly in integrating it with other functional materials. This includes modifying specific functional groups, adding high-performance catalytic components, and incorporating nanomaterials.
Refining its synthesis, particularly in co-hydrothermal processes with complex organic precursors, is a pressing priority, as these reactions are often unpredictable and can significantly affect the material’s properties. A more in-depth investigation into the relationship between precursor composition, experimental conditions, and magnetic hydrochar’s final characteristics is essential to ensure consistency and performance. Employing advanced analytical tools like machine learning can further optimize synthesis, allowing for precise control over its structure and surface functionalities. For large-scale production of magnetic hydrochar, exploring efficient synthesis methods is essential. Integrating hydrothermal carbonization with advanced techniques such as humification and falsification enables the development of multifunctional materials with reduced production costs and minimized environmental impact.
Additionally, significant challenges remain in achieving scalability for magnetic hydrochar, with few examples of large-scale implementations involving magnetic hydrochar. Therefore, assessing the efficiency and reusability of magnetic hydrochar for a long-term is critical to determining its viability for actual wastewater application. Cost-effectiveness and environmental risks during long-term operation, including the regeneration of magnetic hydrochar, should be carefully evaluated. The toxicity of by-products formed during pollutant degradation must be evaluated, along with the potential for leaching of organic or metal components, to prevent secondary pollution.
High-flow, energy-efficient magnetic separation systems should also be designed to match the specific properties of the adsorbent and the characteristics of the target wastewater. Such customization will help achieve optimal separation performance and support the scalability of magnetic separation technologies in wastewater treatment. Additionally, proper management of spent magnetic hydrochar is essential to minimize energy consumption and environmental footprint. A comprehensive life cycle assessment (LCA) will be crucial to determining its environmental impact and to optimizing its cost-effectiveness for scalable, sustainable wastewater treatment. Addressing these challenges head-on will position magnetic hydrochar as a key material in the fight against water pollution, ensuring a cleaner, more sustainable future.


Responses