Multifunctional injectable microspheres for osteoarthritis therapy via spatiotemporally modulating macrophage polarization and inflammation

Introduction
Osteoarthritis (OA) is the most common joint disease, affecting approximately 500 million people worldwide1. OA first occurs in weight-bearing joints such as the knee and hip, and the deterioration of the disease leads to pain and even disability2,3. It is the leading cause of disability and the source of social costs for the elderly. As the population ages and becomes obese, the syndrome becomes more common than in previous decades4,5. Currently, clinical guidelines only support nondrug treatment or palliative pharmacological care to prevent progression and relieve pain symptoms, but there are still no effective treatments for OA restores lost cartilage now6,7,8. OA treatment should aim to relieve pain, improve function within a reasonable range, and improve the quality of patients’ life. However, there is currently no shortage of clinically effective treatments for OA that can restore lost cartilage6,9,10. At present, only a few biologics, such as platelet-rich plasma (PRP)11, mesenchymal stem cells (MSCs)11, transforming growth factor (TGF-β3)12, Interleukin-1 (IL-1) inhibitors13 have entered the late stage of development and have been investigated. In the future, although these products are non-standardized and have not undergone rigorous research, there is no doubt that they will be more targeted than current palliative drugs and can administered locally, particularly by intra-articular injection. Intra-articular injection of drugs or biological agents is the preferred conservative treatment method for patients for whom unable to take or cannot tolerate oral medication (Patients comorbid gastrointestinal disorders, cardiovascular disease, or debilitating conditions, etc.). The advantages of this treatment include increasing the bioavailability of injections, reducing systemic exposure to subsequently reduce adverse reactions, and reducing the total cost of drugs14. But because cartilage is composed of negatively charged proteoglycan, a dense matrix without blood vessels, the effective concentration of the drug penetrates the cartilage very slowly. Additionally, due to the rapid rate of drug clearance in the joint cavity, prolonging the retention time of drugs in the joint cavity becomes an effective way of treating osteoarthritis15.
Prostaglandin E2 (PGE2) is essential cell growth and regulatory factor and is a metabolite of arachidonic acid cyclooxygenase. PGE2 is a vital PG in inflammation, and researchers have demonstrated that its level significantly increased in OA joints. Cyclooxygenase 2 (COX-2) and microsomal PGE synthase 1 (mPGES1) are the main enzymes involved in PGE2 synthesis during inflammation16,17. PGE2 promotes chronic crippling pain in individuals with arthritis by increasing the sensitivity of peripheral nociceptive primary afferent neurons and central pain-signalling neurons18. However, during an arthritis attack, synovitis causes many signs and symptoms of OA, including pain and swelling, which often induce great pain in patients. Whether administered locally or orally, NSAIDs are the basis of drug therapy and are thus administered to patients to relieve severe pain quickly1. Parecoxib (PXB), a highly selective nonsteroidal anti-inflammatory drug, inhibits progressive cartilage destruction and related synovial inflammation after an intra-articular injection and exerts a strong analgesic effect. It effectively reduces patients’ pain during the inflammatory period19.
However, COX-2 is essential for bone healing, and long-term use of high-dose anti-COX-2 drugs may inhibit cartilage repair, similar to corticosteroids, classic NSAIDs, and low-selective anti-COX-220,21,22. Due to the lack of blood vessels and the low density of chondrocytes, damaged cartilage does not grow or heal spontaneously23,24. Therefore, this paper proposes a treatment strategy combining anti-inflammatory drugs and crucial cytokines involved during cartilage repair to achieve long-term and effective treatment of osteoarthritis3.
In OA patients, synovial tissue involving inflammatory cytokine elevation and inflammatory cell infiltration, especially macrophages, plays an essential role in pathophysiological responses25,26. Activated macrophages, called M1 macrophages, produce a range of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, to maintain and aggravate joint inflammation27. M2 macrophages secrete anti-inflammatory cytokines such as the anti-inflammatory cytokines arginase-1 (Arg-1) and IL-10 to reduce inflammation and secrete osteogenic cytokines such as TGF-β and bone morphogenetic protein 2 (BMP2), which play a crucial role in tissue repair28,29. The polarization from M0 to M2 macrophages in lesions may contribute to the cartilage repair that is damaged in OA30. Therefore, M2 macrophages are considered potential therapeutic targets for relieving OA symptoms.
We have recently developed a double-layer microsphere preparation, and we have suggested that it not only exerts anti-inflammatory effects but also has the potential to induce cartilage formation in vitro. We used the small-molecule, highly efficient anti-inflammatory and analgesic clinical drug PXB and co-administered it in a single system together with the macrophage polarizing factor IL-4. Anti-inflammatory and repair strategies are “two-pronged” to enhance the therapeutic effect. When used for intra-articular (IA) injections, the combination causes the rapid subsidence of inflammation and pain relief, followed by the regeneration of articular cartilage and sustained release of IL-4 due to the rapid release of PXB (Fig. 1). A rat OA model was established by complete medial meniscus resection (MMx) to generate an OA environment in vivo31,32. The inhibition of COX2 reduced the levels of inflammatory cytokines such as PGE2, IL-1β and TNF-α in synovial fluid in vivo. IL-4 promoted the polarization of macrophages to the M2 phenotype and induced the expression of type II collagen (COL2), glycosaminoglycan (AGGRECAN) and IL-10. In addition, in vivo experiments in a rat knee arthritis model verified its ability to inhibit excessive inflammation caused by joint degeneration, promote the proliferation of chondrocytes, and enhance proteoglycan deposition and cartilage tissue regeneration.
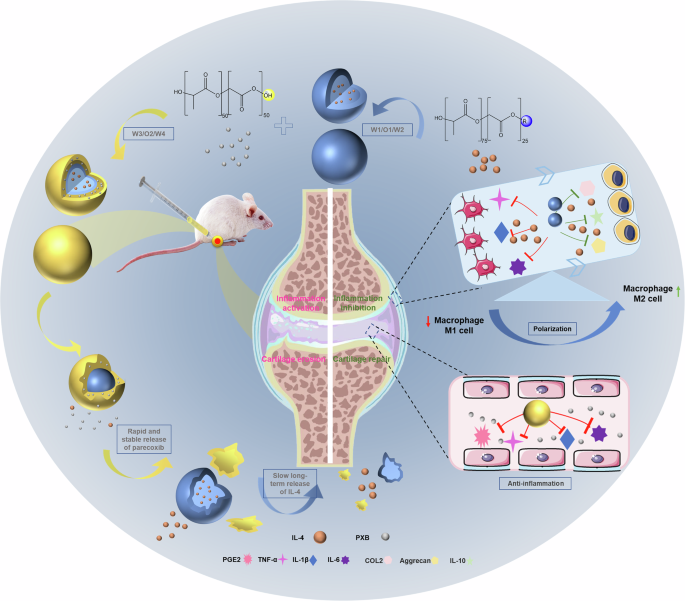
Double-layer microspheres were prepared through the double emulsion method and the spatiotemporal manipulative release of the anti-inflammatory drug (Parecoxib) and macrophage polarization factor (IL-4) achieved by two degradable carrier materials. Parecoxib reduced the levels of inflammatory cytokines (PGE2, IL-1β, and TNF-α) in synovial fluid in vivo, while IL-4 promoted the polarization of macrophages to the M2 phenotype and induced the expression of osteogenic markers (COL2, Aggrecan, and IL-10), and finally improve the long-term prognosis of OA treatment.
Results
Characterization of microspheres
After freeze-drying, the double-layer microspheres prepared using this scheme exhibited a white loose powder appearance with good fluidity, and the suspension was relatively uniform (Fig. 2a). The morphologies of single-layer microspheres (S-MS) and double-layer microspheres co-loaded with IL-4 and PXB (D-MS) were studied using SEM. The average particle size of S-MS was approximately 3–5.5 μm, while the D-MS was approximately 8–10 μm. SEM characterization verified that the prepared microspheres were round and uniform in shape. The encapsulation rate of the drug PXB in the outer layer was 91.31 ± 1.31% and the drug loading was 8.64 ± 1.82%. Besides, the encapsulation rate of the IL-4 in the inner layer was 69.41 ± 3.17% and the drug loading was 4.45 ± 0.22%, while the encapsulation rate of the drug BSA was 70.37 ± 1.76% and the drug loading was 2.64 ± 1.93%.
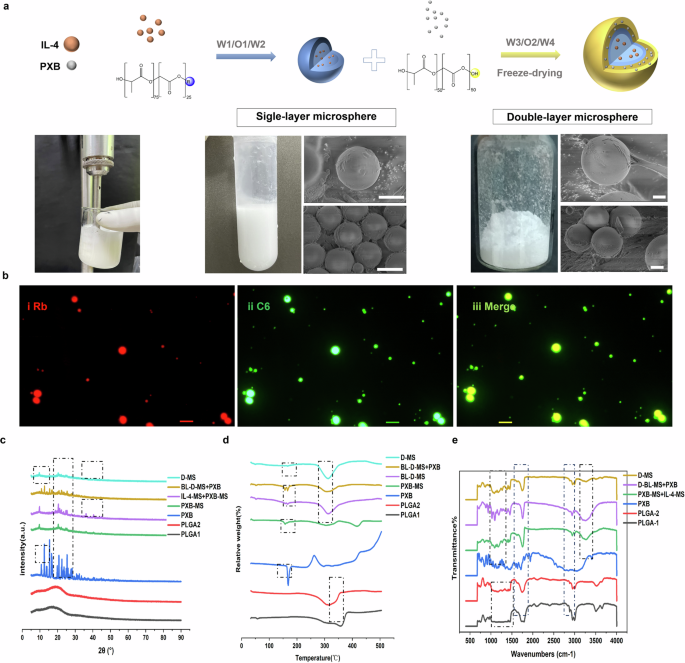
a Scheme of synthesis process of double-layer microspheres. Scanning electron micrographs of the double-layered microspheres prepared by the double-emulsification method twice, and the single-layered microspheres formed by the first double-emulsification and the finally prepared double-layered microspheres. Scale bar, 2.5 μm. b Fluorescence microscope images of single and double-layered microspheres. Scale bar, 10 μm. c–e The XRD spectra, TGA curves and the FTIR spectra of the two carrier materials of microspheres, PXB, the physical mixture of blank double-layer microspheres and PXB powder, and the double-layer microspheres co-loading two drugs.
As shown in Fig. 1b, the red fluorescence was concentrated in the inner part of the microsphere, while the green fluorescence surrounded the outer part. The merge plot (Fig. 2b-iii) shows that the inner part of the microsphere is yellow, indicating that the two dyes were successfully loaded into the inner and outer carriers of the microsphere, respectively.
The structure of double-layer microspheres is further clarified by the analytical characterizations (Fig. 2c–e). XRD analysis was conducted to assess any possible changes in the crystallinity due to drug–polymer interaction. The absence of peaks in the XRD pattern for PLGA material and PXB encapsulated in microspheres confirmed its amorphous nature, while pure PXB powder showed abundant typical peaks between 8 and 15° and between 20 and 30°, indicating that it was in a crystalline state (Fig. 2c). The amorphization of PXB in PLGA1 may have facilitated its rapid release. The IL-4-MS also showed more intense peaks around 2θ = 35–45°. And these characteristic peaks disappeared in the double-layer microspheres, suggesting that their crystalline shape was changed, which indicates that IL-4 was completely encapsulated inside the D-MS. No remarkable changes in either the number or the intensity of peaks were observed in physical mixture of IL-4-MS + PXB-MS and D-MS, compared to XRD spectrum of PXB-MS. These results suggest the absence of any incompatibility issues between inner and outer microspheres.
TGA analysis was performed to further verify the successful loading of PXB and IL-4 in the Fig. 2d. In the TGA thermograms of PLGA1 and PLGA2, broad endothermic peaks were observed at 350 °C and 300 °C, respectively, indicating that the melting points were 350 °C and 300 °C, respectively. A narrow endothermic peak was observed in the TGA thermogram of PXB at 169 °C, and continuous exothermic peaks appeared at temperatures above 400 °C. In the single-layer microspheres loaded with PXB, the endothermic peak at 169 °C decreased due to the endothermic transition, and some endothermic peaks were observed at 307 and 416 °C. The curve of the blank double-layer microspheres showed a small endothermic peak at 520 °C and a broad endothermic peak at 312 °C, which was approximately the same position as the endothermic peak of the outer carrier material PLGA2. On the other hand, all endothermic peaks of the blank double-layer microspheres and the broad endothermic peak of PXB powder appeared in the thermogram of the physical mixture. The curve of the double-layered microspheres loaded with the two drugs showed a broad endothermic peak at 312 °C, and an insignificant small absorption peak appeared at approximately 165 °C, possibly because a small amount of PXB was loaded on the outer layer of the carrier. As shown in the Fig. 2d, the double-layer microspheres loaded with the two drugs have similar glass transition temperatures to the outer carrier material PLGA2, representing that the two drugs are basically completely encapsulated inside the double-layer microspheres.
In order to obtain further insight into possible interactions between PXB, IL-4 and PLGA materials, FTIR spectral analysis was performed and the resulting spectra were analyzed for any changes in spectra peaks. As described in Fig. 1e, PXB has characteristic absorption peaks in the range of 500–2000 cm−1, 3000–3200 cm−1 and 3500 cm−1. PLGA1 and PLGA2 carrier materials have characteristic peaks with strong absorption at 1800 cm−1 and existing absorption peaks at 3000 and 3500 cm−1. The characteristic absorption peaks of PXB in the range around 3200 cm−1 are still present in the peak plots of the physical mixture (PXB-MS + IL-4-MS, BL-D-MS + PXB), only with reduced intensity. In contrast, the characteristic strong absorption peak of PLGA1 carrier at 3000 cm−1 almost disappeared and exhibited a weak absorption of PLGA2, which may indicate that PLGA1 carrier material was completely encapsulated inside the microspheres. The double-layer microspheres exhibited weak absorption of PLGA2 carrier material only at 1800 and 3000 cm−1, which further verified that it encapsulated both drugs and the inner carrier material inside.
In vitro release behaviour studies and safety evaluation of double-layer microspheres
Studies on in vitro release of drugs can simulate the in vivo release behavior of preparations. PXB and IL-4 release from D-MS were studied in vitro, respectively. The release curves of PXB and the BSA model protein from double-layer microspheres are shown in Fig. 3a, b. The release rates of PXB and BSA from PLGA microspheres were significantly accelerated with decreasing pH. The time of 100% PXB release in the pH 6.8 release system was 9 days, while it was approximately 40% in the pH 7.4 release system on the 9th day. However, both the PXB and PXB-MS exhibited a sudden release behavior, and both showed a large amount of release on the first day. Since BSA is encapsulated in the inner and outer layers of the carrier material, the release rate of the two drugs at physiological pH (pH 7.4) was gradual and slow compared with the release system at inflammatory acid environment (pH 6.8), the duration of release was 1–17 days for PXB and more than 60 days for BSA. The reason for the sudden release may be the diffusion-mediated release of the fat-soluble drug PXB embedded in the surface of the microspheres, and the slower and late release of the drug encapsulated in the outer material. PLGA polymers are the most extensively investigated for controlled release technologies, partly due to the ease of altering drug release rates over several days to months33,34. The rate and mode of drug release from the PLGA matrix depend not only on drug diffusion through the matrix but also on the different end groups, molecular weight and monomer ratio of the carrier material PLGA35. Protein released faster in the weak acid release system of pH 6.8, indicating that the acidic environment is not conducive to long-term sustained release of protein and peptide drugs.
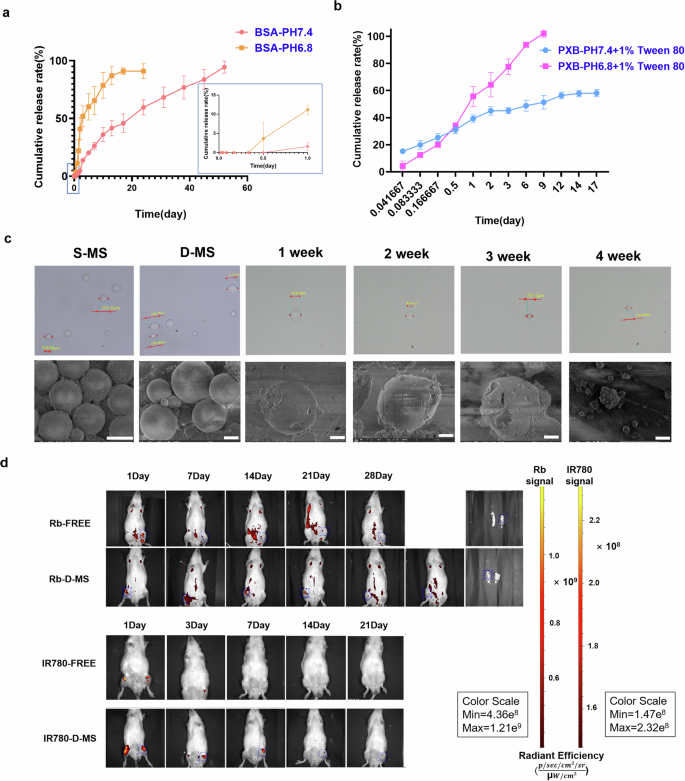
a, b Cumulative release curve of BSA and PXB at pH 6.8 and pH 7.4 of the double-layer microsphere preparation. Data are mean ± SD, n = 3 per time point. c SEM images of single-layer microspheres and double-layer microspheres, and SEM images of morphological changes during the release process. Scale bar, 2.5 μm. d In vivo imaging of the retention of the double-layer microspheres containing Rhodamine b and IR-780 and two free fluorescent dyes in the joints of rats.
The microspheres in the release system were removed every other week, and the morphology was characterized using and super depth of field microscopy and SEM, as shown in the Fig. 3c. With a prolonged release time, the morphology of microspheres changed from a round and uniform sphere to a gradually collapsed structure that ruptured and contained carrier debris; finally, the solution in the release system became clarified. The drug release process of microspheres is accompanied by morphological changes, and eventually, all of the carrier degrades.
The degradability of the PLGA material is the reason for the excellent safety demonstrated by the microspheres in cytotoxicity tests. The safety of nondrug-loaded blank microspheres was evaluated in vitro. The viability of rat chondrocytes was measured after treatment with increasing concentrations (0, 6.25, 12.5, 62.5, 625, and 1250 μg/ml) (Supplementary Fig. 1a) in the absence of double-layer microspheres containing PXB and IL-4. The viability of rat chondrocytes treated with microspheres at concentrations up to 625 μg/mL was >90%. Only chondrocytes incubated with high concentrations (1250 μg/mL) were covered with a layer of microsphere precipitation. It may hinder the normal gas exchange and nutrient absorption of cells, leading to substantial cell death. Interestingly, the addition of blank microspheres at 6.25 to 62.5 μg/mL seemed to promote chondrocyte proliferation.
When the culture environment is known to be acidic, as shown in Supplementary Fig. 1b, the growth status of the chondrocytes and RAW264.7 is slightly affected. Compared with the culture condition of pH 7.4, the cell viability of RAW264.7 after 24 h and 48 h of culture reduced by 13.0% and 13.3%, while rat chondrocytes were reduced by 15.4% and 19.2%, respectively.
Microspheres retention in the rat joints
To test in vivo degradation, we injected intra-articular Rb-only, IR780-only, Rb-loaded D-MS or IR780-loaded D-MS into the right knee joint space of healthy rat models. Figure 3d shows the retention of double-layer microspheres in vivo. The signal obtained around the forepaw and lower abdomen was attributed to spontaneous fluorescence.
IVIS analysis of IA Rhodamine b encapsulated in D-MS showed a distinct signal only at the site of injection, contrary to the aqueous suspension of the free fluorescent dye which quickly redistributed from the knee throughout the whole body within the first 24 h. The retention time of Rb-free in the joint cavity was less than one week. Besides, the signal of free IR780 also disappeared rapidly in approximately 3 days, due to the rapid metabolism in the joint after diffusion to other parts of the body. The potential explanation is that free lipid-soluble drugs are more easily degraded after transport through the phospholipid bilayer structure of the cell membrane. Clear signals of dyes-loaded D-MS were observed at the knee joint after intra-articularly injection without re-distribution to other parts of the body. Finally, Rb-D-MS accumulated and were retained in the joint for more than 4 weeks, and IR780-D-MS was retained for more than 2 weeks. According to previous studies, IR780 maintained for up to 70 days in the carrier, and the PLGA material used in this study can maintain it for at least 2 weeks36. Thus, the signal loss should be attributed to metabolism after the release of fluorescent dye rather than quenching to make the signal disappear. In contrast, the metabolism of free dye disappears after rapid blood circulation to the whole body. The controlled release of the microspheres was confirmed by the slower disappearance of the fluorescence signal in vivo compared to the same amount of free dye, which is possible due to the carrier material enhances its retention in the joint.
In vivo therapeutic effect on osteoarthritis
To determine its therapeutic effects in vivo, a rat model of OA was used to evaluate the anti-osteoarthritic effects of D-MS (Fig. 4a). In week 6 after treatment, as shown in Fig. 4d, groups with less frequent dosing, such as the PXB (free) group and the D-MS group, showed a lower degree of inhibition of swelling of the joints. Especially in the D-MS group with the extension of treatment time, and the joint circumference of the two feet was similar at week 12. Extending the retention of the drug in the joint, resulting in its continued action throughout the study period, significantly reduced joint swelling.
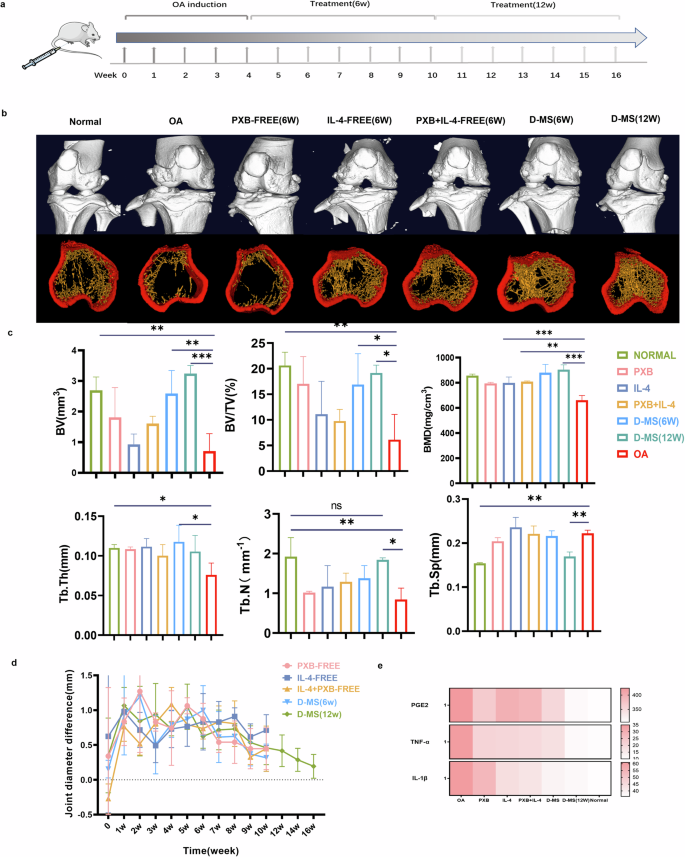
a Timeline of administration of rat osteoarthritis. b 3D micro-CT of rat bone and joint reconstruction in the double-layer microsphere group containing two drugs and all control groups and separated trabecular bone images. c In the 6th and 12th weeks of treatment, the quantified data of the micro-CT analysis. d Tracking and monitoring of the difference in the circumference of the bilateral joints of the rats in each treatment group during the treatment, n = 3. e Analysis of the content of cytokines PGE2, TNF-α and IL-1β in the extracted synovial fluid. Data are presented as means ± SD in (c, d).
Micro-CT analysis
To evaluate bone formation and anti-inflammatory effects of different treatments after 1 and 2 months of injection, we analyzed micro-architectural properties of knees by micro-CT. The results (Fig. 4b) showed that the D-MS group exhibited significantly reduced erosion and damage to subchondral bone. Compared with the sham operation group, the joint space width showed an increasing trend, the joint surface was smoother and more complete, and the density of trabecular bone was also increased. However, if the PXB-MS and IL-4-MS are administered at one time (Supplementary Fig. 2), the erosion and damage of the joint surface will be relieved to a certain extent, which also explains the adverse consequences of repeated joint cavity injection itself. At the 12th week after treatment, 3D reconstruction analysis showed that bone-related parameters Tb.Th, Tb.N, BV/TV, BV, and BMD increased significantly compared with those of the control group, but without evident changes in Tb.Sp was observed, indicating the relief of osteoarthrosis and progressive bone repair (Fig. 4c).
Inflammatory cytokines measurements
In order to assess the inflammatory infiltrate in the joint tissue, the levels of inflammatory factors in the joint fluid extracted from the joint cavity were examined by ELISA. Quantitative analysis of the factors revealed that the levels of inflammatory factors PGE2, TNF-α and IL-1β were significantly decreased in the D-MS group and tended to be similar to normal joint fluid with increasing treatment time, suggesting that they greatly reduced the level of inflammation in OA cartilage (Fig. 4e).
Histopathological analysis
The histopathological analysis showed a significantly reduced thickness of the osteoarthritic cartilage in the PBS group compared with the sham group, and typical features of osteoarthritis, such as inflammatory cell infiltration, irregular cartilage surface, and erosion cracks, were most obvious in the PBS group. The treatment inhibited the progression of osteoarthritis by increasing the cartilage thickness and inhibiting chondrocyte apoptosis. Safranin O‐Fast Green (F-O) staining was used to evaluate the loss of proteoglycan in the sensitive cartilage. The results are shown in the Fig. 5a–c. The light blue-stained zones corresponded to the superficial articular cartilage area, and the F-O-stained sections from groups receiving different treatments were observed. The surface and deep areas of the repaired cartilage showed strong GAG red staining and disappeared in calcified and subchondral bone tissue, with only blue staining observed. The results of HE and F-O staining showed that at the 6th week of treatment, the articular cartilage damage in DMM rats was significantly greater, and the loss of proteoglycan was more serious than that in the normal group that was not subjected to modelling. An intra-articular injection of drug-loaded microspheres reduced cartilage damage and increased the proteoglycan content in articular cartilage. Moreover, due to frequent joint cavity injection during joint inflammation, the group receiving free PXB and IL-4 at the same time did not show good therapeutic effects. In contrast, synovial inflammation was reduced in the single-dose PXB-loaded microsphere group compared with the group injected with multiple doses of free PXB, but the cartilage repair effect was poor. The single-dose IL-4-loaded microspheres showed a superior repair ability to the daily dose of free IL-4, and abundant proteoglycans were observed in the cartilage (Supplementary Fig. 3). Notably, less cartilage damage and more proteoglycans were detected in the 12-week treatment group than in the 6-week treatment group. In conclusion, anti-inflammatory factors combined with cartilage repair therapy exerted good anti-inflammatory effects and protected cartilage in the knee joints of rats with osteoarthritis.
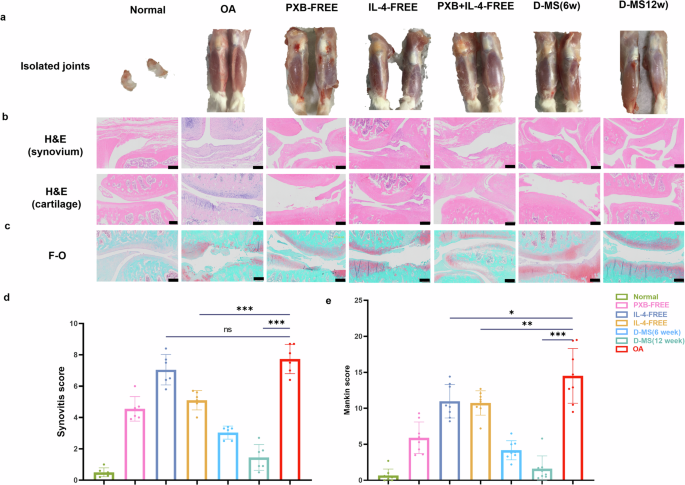
a The meniscus of a normal rat was removed, and the isolated knee joint. b HE staining of knee cartilage and synovium of rats after six weeks of different treatments. Scale bar, 50 microns. c F-0 staining of knee cartilage and synovium of rats. Scale bar, 200 microns. d Synovial scores for synovial inflammation (n = 6) and (e) Mankin’s score (n = 8) based on staining results to evaluate the severity of articular cartilage degradation. Data are presented as means ± SD in (d, e).
The cartilage was assessed by histologic examination, and disease-related histological changes in OA cartilage were confirmed according to a modified Mankin scale and Synovitis score (Mankin score on toluidine blue and hematoxylin/eosin-stained samples, OA severity: cartilage loss, subchondral bone changes, osteophyte; Synovitis score on synovial membrane inflammation). As expected, the total Mankin and Synovitis score were significantly higher in the OA and IL-4-FREE groups compared with the PXB and D-MS groups in the bone joint and synovial membrane. The assessment demonstrated that the alleviated cartilage damage can be observed in the D-MS groups compared with other control groups (Fig. 5d, e).
Immunohistochemical and immunofluorescence staining
Immunohistochemical staining and the quantitative analysis (Fig. 6a, b) showed that compared with those in the PBS and IL-4 (free) groups, the expression levels of the inflammatory factors TNF-α and PGE-2 in synovial tissue and chondrocytes in the PXB (free) group and the PXB + IL-4 (free) and D-MS groups were decreased. The IL-4 (free) group, the PXB + IL-4 (free) group and D-MS group showed increased expression levels of collagen, aggrecan and IL-10, and the highest levels were detected in the D-MS group. iNOS + /CD68+ macrophages were considered as pro-inflammatory macrophages, and CD206 + /CD68+ macrophages were considered as reparative macrophages in the rat synovial membrane.
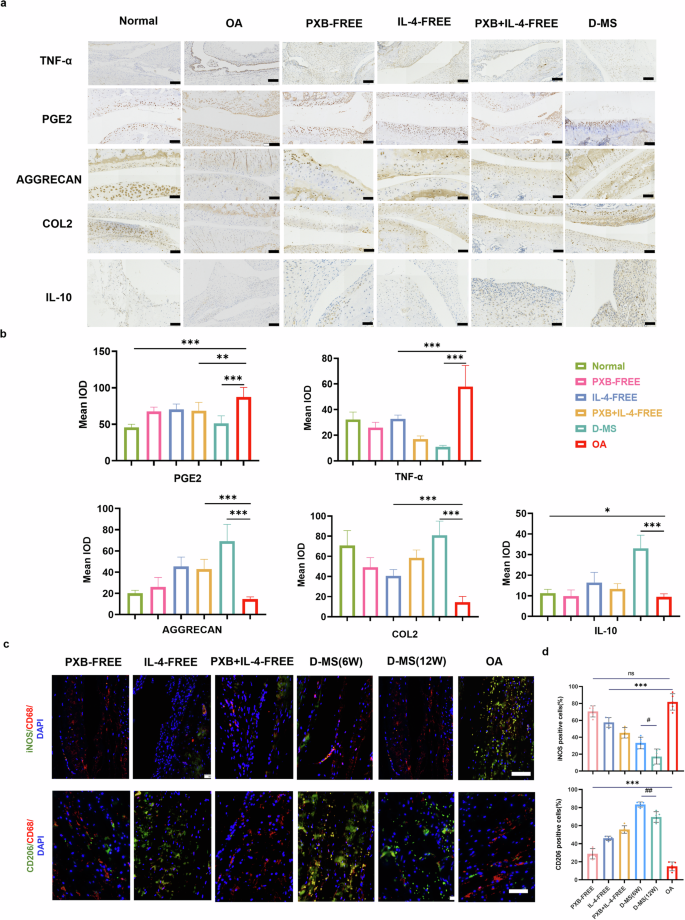
a Immunohistochemical analysis of TNF-α, COL2, AGGRECAN, PGE2, IL-10 expression in cartilage and synovium. Scale bar, 50 microns. b Quantitative immunohistochemistry analysis (n = 3). c Macrophages were detected by co-immunofluorescence staining for CD206 (green, anti-inflammatory)/CD68 (red, pan-macrophage), and iNOS (green, pro-inflammatory)/CD68 (red, pan-macrophage). Scale bars: 50 μm. d Quantification of CD68, CD206 and iNOS-positive cells (n = 5). Data are presented as means ± SD in (b, e).
In vitro experiment has confirmed that IL-4 can regulate macrophage polarization in OA synovial membrane (Supplementary Fig. 4). The surface marker is an indicator of a macrophage phenotype. To assess the polarization of RAW264.7 macrophages toward the M2 phenotype was assessed using staining for CD206 (M2-specific), CD86 (M1-specific), and CD11b (macrophage) surface markers by flow cytometry. Gating strategies of CD86 + M1-macrophages and CD206 + M2-macrophages in Supplementary Fig. 5. The results shown that LPS and IFN-γ stimulation significantly increased the proportion of M1-type cells compared with the blank group. Compared with the control group, increased expression of CD206 was observed in RAW264.7 treated with IL-4 in a dose-dependent manner. In contrast, the proportion of M1 type macrophages was subsequently decreased. Based on this result, a simultaneous increase in expression of M2 markers and decrease in M1 markers confirmed polarization of macrophages to the M2 phenotype after stimulating with IL-4 cytokine.
We further detected the phenotype of macrophages through immunofluorescence staining, which showed that while in the OA group, more iNOS positive macrophages presented M1 type macrophages. The higher CD206 positive cells ratio and the lower iNOS positive cells ratio in D-MS groups than other treatment groups (Fig. 6c, d, Supplementary Fig. 6). In particular, the polarization of M1 macrophages into the M2 phenotype, which contributes to tissue remodeling and repair through the release of anti-inflammatory cytokines, would be an effective treatment for OA.
Discussion
The clinical treatment strategy for osteoarthritis proposed in this protocol is to combine the NSAIDs PXB with the macrophage polarizing factor interleukin-4 (IL-4) and prepare co-loaded double-layer microspheres of these two drugs. Since chondrocytes are the only cell type in articular cartilage, when they exhibit metabolic dysfunction, such as in the progression of osteoarthritis, researchers have explored cartilage damage extensively37. In the present study, the drug PXB loaded in the outer layer of inflammatory OA chondrocytes have shown the capacity to inhibit the inflammatory response. Meanwhile, macrophage regulatory factors can repair cartilage, which can be combined with treatment strategies to improve the long-term prognosis. This work also built a platform for the step-by-step delivery of two drugs with different properties, showing great potential for clinical translation to clinical conditions other than OA.
As shown in this study, intra-articular injections of PXB and IL-4 provided some relief of joint lesions and pain-related behavior in a DMM-induced mouse model of OA. However, even when PXB was co-administered with the rapidly metabolized cytokine IL-4, it showed substantial joint damage regardless of in vitro inflammatory exudation and repeated swelling of the joint surface as it still required repeated injections (once daily). The combination of drugs does not seem to exert a satisfied therapeutic effect. However, no significant cartilage recovery was observed after microspheres loaded with PXB or IL-4, respectively, were administered. Our analysis of the causes suggests that it may be due to persistent joint friction caused by meniscectomy, or the persistence of inflammatory invasion due to joint inflammation, despite the absence of manual injury or inflammatory irritation caused by repeated injections of invasive therapies. In previous studies, biomaterials that encapsulate NSAIDs used for OA treatment have been designed to maintain their local release, providing long-term relief of OA-related pain and alleviating systemic toxicity and side effects38,39. It is a promising method to solve the problems of repeated injection-related infection, such as cardiotoxicity and gastrointestinal reactions caused by oral NSAIDs, and poor patient compliance caused by the chronic nature of osteoarthritis. The superiority of the combination strategy double-layer microspheres administration containing two therapeutic agents also reveals the need for long-term treatment of osteoarthritis, a degenerative disease in which the combination of the two agents is prepared as a single-dose preparation40.
Low-dose high-potency drugs, such as cytokines (such as IL-4) and selective high-potency nonsteroidal anti-inflammatory drugs, should be prioritized when selecting drugs loaded in double-layer microspheres with long-term effects. PXB is also a highly selective anti-COX-2 agent that is safe at low therapeutic doses in the short term and does not interfere with later secondary bone healing41. The systemic side effects following the controlled release of NSAIDs after IA injection have been far less studied. During this study, the application of D-MS in an OA rat model showed good safety in organs responsible for drug metabolism (heart, liver, spleen, lung, and kidney). Especially cardiotoxicity, a common adverse reaction of systemic application of NSAIDS, was not found. However, repeated administration of free PXB occasionally resulted in hemosiderin deposition in cardiac tissue sections, suggesting the possibility of accidental cardiotoxicity and side effects of NSAIDs. Moreover, the histopathological imaging analysis showed that repeated free IL-4 injections or the combination of free PXB and IL-4 caused mild renal edema but no significant protein tubular exudation or necrotic foci. No apparent pathological changes were observed in other organs in treatment groups, which preliminarily determined local injection was safe (Supplementary Fig. 7). During the treatment process, the weight of the rats changed and kept rising, indicating that the administration had little effect on their normal growth (Supplementary Fig. 8).
Here, a biological material platform characterized by its degradation products in cells after examining its safety and biodegradability and its degradation rate by adjusting the ratio of monomers, the base type (acid and ester side), molecular weight, size and other parameters to control the retention, suggesting that it can be applied to internal joints for a relatively long time of local delivery to achieve controlled drug release in individuals with OA42,43,44. In biomedical applications, the LA:GA ratios of PLGA are 85:15, 75:25, and 50:50. Studies have shown that the degradation rate of PLGA decreases with an increasing LA:GA ratio. An exception was the 50:50 PLGA, which showed a faster degradation rate45. In the present study, the ratios of LA:GA were 75:25 and 50:50. The two materials had different degradation characteristics, and 75:25 also contained an ester group, which had a slower release rate and higher encapsulation efficiency for water-soluble drugs. We compared the release rates of the two drugs from double-layer microspheres in different pH environments. In the in vitro release system at pH 6.8, both drugs were released from the microspheres at an accelerated rate, with the lipid-soluble PXB distributed to the outside of the microspheres for slower release and IL-4 encapsulated in the innermost part of the microspheres, where lower pH had a larger impact on the release rate. In the inflammatory stage of osteoarthritis, the pH in the joint cavity was acidic. The biological activity of active proteins and polypeptides is lost rapidly in the acidic microenvironment, suggesting that the inflammatory response in the inflammatory stage of arthritis must first be relieved to prevent the rapid release of IL-4, which is conducive to the long-term slow release of IL-4 to promote the gradual repair of cartilage.
Regarding the particle size of microspheres determination, some researchers have demonstrated that each particle with different diameters will interact with different types of macrophages in different flow environments. For example, some drug delivery methods need to enhance macrophage phagocytosis of cells, then microspheres between 1 and 4 μm in diameter are usually used for vaccination46. However, some applications (subcutaneous, inhalation, intra-articular depot injection, etc) require reduced phagocytosis to achieve sustained release47. Particles smaller than 5 μm in diameter are eliminated by phagocytosis by synovial resident and recruited macrophages, which may lead to an inflammatory response that may exacerbate inflammation in patients with osteoarthritis when injected intra-articularly38. In addition, the synovial structure has a limited permeability, and the intercellular space is 0.1–5.5 μm48,49. Therefore, to ensure the long-term stable release performance of the osteoarthritis treatment microspheres and increase the retention time of the microspheres in the joint cavity, the prepared intra-articular injection microspheres are mostly range from 5.5 to 10 micrometers. In this study, by examining various factors that affect the particle size of microspheres (PLGA concentration, water phase/oil phase volume ratio, outer water phase concentration, etc.), the microspheres suitable for joint cavity injection are finally screened out. Notably, advanced microfluidic technology has been used to prepare microspheres with a controllable, highly dispersed and uniform size to control drug release performance, providing an efficient route for preparation of safe and high-performance slow and controlled release microspheres50,51.
Overall, this study highlights that the treatment strategy combining the two drugs not only modulated the early inflammatory environment but also repaired cartilage and improved the prognosis. In subsequent studies, the results of this study should be further confirmed in models with more similar cartilage repair and regeneration abilities to humans by increasing the study sample size and using larger mammalian models.
Methods
Preparation of double-layer microspheres
Microspheres containing two drugs with different physical and chemical properties were prepared using two double emulsion solvent volatilization methods. For detailed preparation methods, please refer to the supplementary information.
Characterization of double-layer microspheres
According to the manufacturer’s instructions, the microspheres were suspended in solution and observed, and the particle size distribution was measured using a super depth of field three-position microscopic system (Keyence VHX-5000, Japan). The freeze-dried powdered double-layer microspheres were attached to the conductive adhesive, and the morphology of microspheres was observed using Focused ion beam-scanning electron microscopy (FIB-SEM) (Zeiss Pioneer Two, Germany). In some sense, changes in the sizes of monolayer and bilayer microspheres determined using scanning electron microscopy indicated the successful formation of double-layer microspheres.
The amount of PXB released from D-MS was quantified by measuring the absorbance at 265 nm until the in vitro release experiment was complete. The released BSA samples were stored in a refrigerator until quantification with ELISA kits. The released PXB was filtered through a 0.22 μm organic filter, transferred to HPLC vials and analyzed using HPLC at an ultraviolet absorption wavelength of 265 nm with a HPLC system (Agilent 1200, USA). The chromatographic conditions for PXB were as follows: λ max = 265 nm and mobile phase of acetonitrile: ammonium acetate buffer (10 mM, pH 5.0) = 55:45 (v/w). Measurements and analyses were performed in triplicate. The drug encapsulation efficiency and loading efficiency were then calculated using the following equations:
X-ray diffraction (XRD) was used to characterise the phase purity and crystal microstructure of the composite microspheres. Fourier transform infrared spectroscopy (FT-IR) was used to qualitatively analyse the chemical groups in the composite microspheres to determine their compositions. Thermogravimetric analyser (TGA) was used to study the thermal stability of the composite microspheres. Detailed information on the above-mentioned methods is provided in the supplementary information.
In vitro release behavior
According to the previous research basis of the authors, due to the relatively trace amounts of cytokines, the detectability of cytokine content in the release system was challenged in the long-term release experiment in vitro. Bovine serum albumin (BSA) was used as model protein since it is one of the most stable and widely-used proteins for evaluating novel sustained release drug delivery system52,53. The double-layer microspheres (50 mg) loaded with the model protein and PXB were placed in a dialysis bag (molecular weight cut off of 7000 Da) with 2 ml of release medium and suspended in a 30 mL test tube. The release medium was phosphate-buffered saline (PBS, pH 7.4 and 6.8, 37 °C) containing Tween 80 (0.5 wt%). Tubes were oscillated at 100 r/min in a steam bath thermostatic vibrator (MiuLab ES-60, China). At the desired time intervals, 2 mL of the release medium were extracted, and then the same volume of fresh medium was added.
In addition, some of the microspheres were removed every other week, the morphology of the microspheres was characterized using SEM after freeze-drying. Morphological changes in the microspheres were observed during the release process.
Osteoarthritis induction in vivo
Rat models of osteoarthritis was constructed to evaluate the anti-osteoarthritis efficacy and safety of double-layer microspheres in vivo. All in vivo experiments using rats in this study were conducted in compliance with animal welfare ethical regulations and approved by the Animal Use and Care Committee of Shandong University (Approval number: SYXK20190005). Animal models of osteoarthritis established using medial meniscectomy (MM) have the advantage of slower disease progression than other surgically induced models, such as anterior cruciate ligament transection. Fifty healthy male Wistar rats aged 6 weeks and weighing 200–250 g were anesthetized with 3% sodium pentobarbital (40 mg/kg) through an abdominal injection. An incision was made at the side of the patella of the medial knee of the right leg, and the medial paraligaments were cut and resected layer by layer. After opening the joint capsule, the patella was evaginated, and the medial meniscus was resected at the knee bend. The surgical incision was closed and the articular cartilage surface of the tibial plateau was protected. Only the knee joint capsule was cut and exposed on the left leg, and the medial collateral ligament and meniscus were not excised. After the operation, anti-infective drugs were continuously administered and the vital signs of rats in each group were monitored.
Retention of microspheres in vivo
One week after OA induction in vivo, the rats were divided into four groups (n = 3). The joints of rats were injected with water-soluble fluorescent dyes Rhodamine b (Rb-free), fat-soluble iodide IR780 (IR780-free), Rhodamine b loaded double-layer microspheres in the inner layer (Rb-D-MS) or IR780 loaded double-layer microspheres in outer layer (IR780-D-MS), respectively. 100 μL of free fluorescent dyes or microspheres loaded with the same amount of fluorescent dyes were injected intra-articularly on day 0 with a needle. At regular intervals, rats were anesthetized for in vivo fluorescence intensity measurement of these two kinds of dyes with an IVIS small animal living imaging device (Perkin Elmer IVIS Spectrum, USA). The Rhodamine b group was scanned with an excitation wavelength of 555 nm and emission wavelength of 588 nm, while the IR780 iodide group was scanned with an excitation wavelength of 675 nm and emission wavelength of 780 nm. Living Image 4.3 software (Perkin Elmer IVIS Spectrum, USA) was used to analyze the data. Four weeks later, the rats were euthanized by inhaling excess carbon dioxide, the leg tissues were collected, after the skin was removed and samples were scanned again.
Injecting drugs and preparations into the joint cavity
The treatment protocol to evaluate the therapeutic effect in vivo is shown in Fig. 4a. Four weeks after modeling, the operation group was randomly divided into 5 groups: OA group, PXB (free) group, IL-4 (free) group, PXB + IL-4 (free) group, and D-MS group. PXB (free) was administered at a dose of 100 mg/0.1 mL once a week, PBS and IL-4 (free) were administered at a dose of 20 ng/0.1 mL once a day for 4 weeks, and double-layer D-MS was injected into the articular cavity all at once. Vernier calipers were used to monitor the circumference of both knees. Results were presented as the difference in knee diameter (OA modeling side – healthy side).
In vivo therapeutic effect assessment on osteoarthritis
In week 6 after treatment, as shown in Fig. 4d, groups with less frequent dosing, such as the PXB (free) group and the D-MS group, showed a lower degree of inhibition of swelling of the joints. Especially in the D-MS group with the extension of treatment time, and the joint circumference of the two feet was similar at week 12. Extending the retention of the drug in the joint, resulting in its continued action throughout the study period, significantly reduced joint swelling.
Detection of inflammatory cytokines in joint fluid
At the 6th week of treatment, 1 mL of normal saline was injected into the joint cavity, and the joint fluid was extracted from the treatment side and the control side and stored at −80 °C. The contents of PGE2, IL-1β and TNF-α in the joint fluid were determined according to the instructions of the ELISA kit (Absin, China). First, the collected liquid was centrifuged at 1000 × g for 20 minutes to remove particles and polymers. Then, the OD value was read at 450 nm using an ELISA reader within 30 min according to the test procedure.
Micro-CT scanning
After extracting the joint fluid from the treatment side and the control side, all rats in the OA control group and half of the rats in the preparation group were euthanized, and microcomputed tomography (CT) scans and histological analyses were performed. The tibial plateau and the articular surface of the femoral condyle of the right knee joint were removed from rats in each group, the synovial tissue was not damaged, and the bone surface, especially the cartilage surface, remained smooth after the injection and was fixed with 4% paraformaldehyde. A micro-CT imaging system (PerkinElmer Quantum GX2, Japan) for small animals was used to scan each sample was scanned layer by layer (the scanning parameters: scanning resolution, 18 μm; voltage,90 kV; current, 88 μA) to obtain the scanned image.
The trabecular bone thickness (Tb.Th), trabecular bone number (Tb.N), trabecular bone separation (Tb.Sp), bone volume fraction (BV/TV), bone volume (BV), and bone mineral density (BMD) were quantitatively analyzed using a micro-CT data processing workstation (PerkinElmer Quantum GX2, Japan). BMD was calculated from 5 standard hydroxyapatite (HA) models (0, 50, 200, 800 and 1200 mg HA/cm3) under the same conditions by selecting the cross-section of the same size of the volume model in the 3D image and selecting the volume model region of the same size in the cross-section to calculate the average density of each region. A standard curve was drawn to determine the density of the sample. The remaining mice were euthanized at 12 weeks after treatment and underwent a micro-CT scan and histological analysis.
Histological examination
After micro-CT scanning, tissues were again fixed with 4% paraformaldehyde, and gradient decalcification was performed with an EDTA solution to determine the different therapeutic effects of double-layer microspheres on osteoarthritis. After decalcification, the tissues were stained with hematoxylin and eosin, ferro-solid green and antibodies against tumor necrosis factor-α (TNF-α) (1:400; GB11188; Servicebio), type II collagen (COL2) (1:400; GB11021; Servicebio), aggrecan (1:500; GB11373; Servicebio), prostaglandin E2 (PGE2) (1:100; DF7107; Affinity Biosciences) and interleukin-10 (IL-10) (1:500; GB11108; Servicebio) using immunohistochemistry. The degenerative changes of the cartilage were graded histologically using the Mankin score and Synovial score for assessment of cartilage structure, cartilage cells, and tidemark integrity.
In addition, the safety of the double-layer microspheres was investigated in rats by assessing potential toxicity in the heart, liver, spleen, lungs and kidneys. These organs were embedded in paraffin, sectioned and stained with hematoxylin and eosin, according to the manufacturer’s instructions. The stained sections were observed and photographed using a VS120 virtual slide microscope (Olympus Corp., Tokyo, Japan).
Immunofluorescence staining
The paraffin-embedded sections of articular synovial tissues and cartilage tissues of rat knee joints were collected to perform immunofluorescence staining for identifying M1 or M2 phenotypes of macrophages. Tissue sections were stained with anti-CD68 (1:200; ab955, Abcam) and anti-inducible nitric oxide synthase (iNOS) (1:200; GB11119, Servicebio) for M1 macrophages, or anti-CD68 and anti-CD206 (1:400; GB113497, Servicebio) for M2 macrophages.
Safety evaluation of microspheres
Intra-articular injections of double-layer microspheres were well tolerated, and no abnormal behavior or infection was observed in rats after a single injection. The body weight of rats increased for all rats during the experimental period (Supplementary Fig. 8), and no deaths were observed. The histopathological results obtained in the 6th and 12th weeks of treatment also revealed no inflammatory cell infiltration in the synovium of the rat joints in the double-layer microsphere administration group, suggesting good safety of the preparation.
In addition, the safety of the double-layer microspheres was investigated in rats by assessing potential toxicity in the heart, liver, spleen, lungs and kidneys. These organs were embedded in paraffin, sectioned and stained with hematoxylin and eosin, according to the manufacturer’s instructions. The stained sections were observed and photographed using a VS120 virtual slide microscope (Olympus Corp., Tokyo, Japan).
Statistical analysis
GraphPad Prism version 8.0.2 (GraphPad Software Inc.) and Origin Lab software (Origin Lab Software Inc.) were used for data analysis and drawing all figures. All data were presented as mean ± standard deviation. Analysis of variance was used to determine differences within groups, and the two-tailed unpaired Student’s t test was used to analyze differences between two groups. One-way ANOVA was used when comparing multiple groups, followed by a Dunnett test as appropriate for subsequent pairwise (group) comparisons. P < 0.05 was considered statistically significant with */# representing 0.01 < P /# < 0.05, **/## representing 0.001 < P < 0.01, and ***/### representing P < 0.001.

Responses