No evidence of positive causal effects of maternal and paternal age at first birth on children’s test scores at age 10 years
Main
During the second half of the twentieth century, women’s age at first birth (AFB) increased by about 4–5 years in almost all developed countries1. The consequences of this demographic shift are of great interest to policymakers, the public and academic researchers across a range of scientific disciplines. At the individual level, parents and their offspring may face a trade-off between the negative biological and the positive socioeconomic consequences of postponing parenthood. At the population level, these processes may be of great importance for disease prevalence and social stratification.
Children of older parents are at higher risk of being born with a low birth weight and of developing symptoms of mental-health conditions such as autism and schizophrenia2. However, there are positive associations between older parental age and various measures of children’s educational success3,4,5,6. In the United States, for example, each one-year increase in maternal age is associated with a 0.039 standard deviation (s.d.) increase in children’s mathematics scores4.
The key question is whether the associations between maternal and paternal age and children’s educational outcomes are causal. Only if maternal and paternal age have a causal effect on children’s educational outcomes will a shift in the distribution of paternal or maternal age lead to improved educational outcomes at the population level.
Previous research has mainly relied on family fixed-effects models to identify the causal effects of maternal and paternal age on children’s educational outcomes3,4,5. These studies found similar effect sizes within and across families. The interpretation of these estimates as causal has been questioned because of the possibility of unobserved confounding variables that vary between siblings. In addition, it is unclear whether one should adjust for the offspring’s year of birth. Adjusting for the birth year of the offspring is problematic because it is perfectly collinear with parental age in a family fixed-effects model. However, not adjusting for birth year to avoid the multicollinearity problem results in any parental age effect potentially being driven by the difference in the time period in which different siblings are born7,8.
Here we approach the question of the causal effects of parental age on children’s education using a genetic instrumental variable (IV) design based on Mendelian randomization (MR)9. Specifically, our approach enables us to estimate the causal effects of maternal and paternal AFB on children’s test scores at age 10 years. We use maternal and paternal PGIs for AFB as instruments and condition on the child’s PGI for AFB.
We identify three challenges to the MR approach from recent genetic literature, which we address using the Norwegian Mother, Father and Child Cohort study (MoBa), a Norwegian pregnancy cohort linked to registry data and genotype data on trios. First, the same genetic variants may predict multiple outcomes. For example, AFB-linked genetic variants co-predict the risk of externalizing behaviour and educational attainment10. This phenomenon, known as pleiotropy, can be due to shared biological influences, social mediation or genetic effects on third common causes11. This effect can be more pronounced the further a predicted outcome is from actual biological processes, which makes AFB particularly susceptible to pleiotropy. Pleiotropy leads to a violation of the exclusion restriction assumption that is central to the IV approach, according to which the instrument does not influence the outcome other than through the instrumented variable. We therefore control for PGIs of heritable AFB predictors in the parental generation. Specifically, we control for sexual, contraceptive and smoking behaviour, attention-deficit/hyperactivity disorder (ADHD) and educational attainment10.
Second, because we are interested in the effect of paternal and maternal AFB on children’s test scores, and children inherit 50% of each parent’s DNA, genetic inheritance could bias the results if genes that affect parental AFB directly influence children’s test scores. Therefore, we jointly include parental and child PGIs for AFB in the statistical model. In this way, we minimize the possibility that observed associations between parental and child outcomes are due to shared, instrument-related genotypes.
Third, genetic discoveries consist of a series of simple regression models with few demographic and genetic control variables, and may be confounded by environmental correlates, assortative mating (AM) or population stratification12. These confounding effects could lead to an inflation of causal estimates relative to standard regressions. We control for the PGI of the partner’s AFB to account for parental AM13. To account for grandparental AM leading to potential excess homozygosity, we removed single nucleotide polymorphisms (SNPs) that are out of the Hardy–Weinberg equilibrium based on a P value threshold of 1 × 10−6 (ref. 14). In addition, we control for the respective PGI of inherited SNPs to account for potentially remaining excess homozygosity. To maximize our control for genetic nurture effects and population stratification, we consider the first ten principal components and the education level of the offspring’s grandparents. Finally, use of Norwegian registry data enabled us to avoid several recently highlighted issues of sample selection and resulting ascertainment bias15.
We graphically represent our research design in a directed acyclic graph in Fig. 1.

Parental PGI AFB is the instrumental variable for parental AFB. The outcome is the child’s test score. To satisfy the independence and exclusion restriction assumption, we introduced several control variables. The following parental PGIs were included: (1) parental PGI age at first sexual intercourse; (2) parental PGI for smoking (3) parental PGI for contraception use; (4) parental PGI for ADHD; and (5) parental PGI for education.
Results
The results of the IV models are reported in Table 1. We report estimates, obtained in separate models, for both maternal and paternal AFB. We also report results for the corresponding cross-sectional ordinary least squares (OLS) regression models.
The OLS models showed positive associations between both maternal and paternal AFB and children’s education, a result consistent with previous research3,4,5,6. Without control variables, a 1-year delay in the mother’s AFB was associated with 3.7% of a s.d. (confidence interval (CI): 3.4%, 4.0%) increase in test scores in fifth grade (around age 10) (model 1 OLS). This association was slightly smaller for the father’s AFB, with 2.3% of a s.d. (CI: 2.0%, 2.5%) increase in achievement (model 1 OLS). Adding the control variables and parental PGI for education reduced the estimates to 1.4% (CI: 0.9%, 1.8%) for mothers and 0.9% (CI: 0.6%, 1.2%) for fathers (models 13 OLS). These associations cannot be interpreted causally because of the potential remaining unobserved confounding.
The first-stage F statistics of the IV models were substantial, and our analyses did not suffer from weak instrument bias. The IV models without our control variables showed a strong inflation of the estimates compared with the OLS models for both maternal (12.6%; CI: 10.5%, 14.7%) and paternal (19.8%; CI: 15.6%, 23.9%) AFB (model 1 IV). However, once we included the control variables in the IV models, the effects became nonsignificantly different from 0 (model 12 IV). Once we added a control for the parental educational attainment PGI, the estimates turned around, and we even found evidence for a negative causal effect of AFB on test scores at age 10 in the full model. In detail, the estimates for both maternal and paternal AFB become significantly negative (mothers: −8.8% (CI: −15.5%, −2.1%); fathers: −19.3% (CI: −35.1%, −3.6%); model 13 IV). Although the inclusion of the PGI of education may have reduced bias by controlling for a confounder, it could also have introduced overcontrol bias if the PGI for education picked up part of the effect that actually belongs to the PGI for AFB. The correlation between the PGI for AFB and the PGI for education is shown in Supplementary Table 3. We therefore conservatively conclude that our results do not provide support for the idea that parental age positively affects children’s educational performance.
To unpack these results, we analysed our model specifications in detail, performing analyses with covariates added stepwise (Fig. 2). We observed two major shifts in the IV estimates across specifications. First, the full PGI of AFB for both parents were positively predictive of children’s test scores when both indirect genetic effects and inherited alleles were combined. Once the model was controlled for the child’s PGI of AFB and identified nurture and ancestry effects for the instrument, respectively, the estimate approached zero. Second, inclusion of the parental PGI for educational attainment turned the estimates negative. In summary, our conservative conclusion is that there is no robust evidence of positive effects of paternal and maternal AFB on children’s test scores at age 10 in Norway.
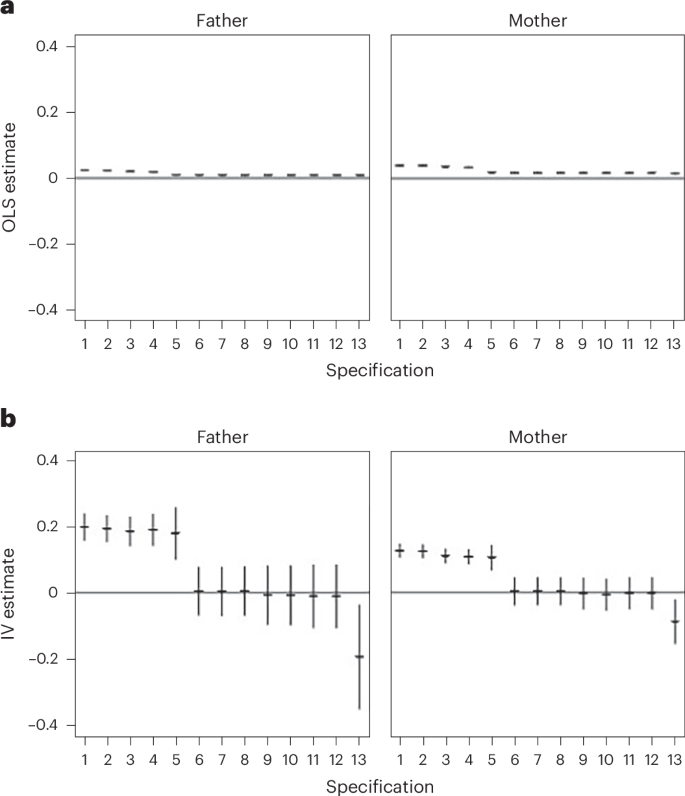
a,b, Estimates obtained for OLS (a) and IV (b) models for both maternal and paternal AFB. The following control variables were included stepwise: (1) none; (2) first ten genetic principal components; (3) grandparental education; (4) partner’s PGI for AFB; (5) parental education; (6) child PGI for AFB; (7) child’s birth cohort (continuous); (8) child’s sex; (9) parental PGI age at first sexual intercourse; (10) parental PGI for smoking; (11) parental PGI for contraception use; (12) parental PGI for ADHD; and (13) parental PGI for education. Plots show point estimates and CIs; the CIs are very small for the OLS estimates. n = 15,670 (maternal) and 15,593 (paternal) for both a and b.
Discussion
Numerous studies have reported positive associations between maternal and paternal age and children’s educational outcomes, even within families3,4,5,6. However, it has been unclear whether these positive associations are due to underlying positive causal effects of higher maternal and paternal age. Using an MR approach, we did not find robust evidence for positive causal effects of maternal and paternal AFB on children’s test scores at age 10.
Specification analyses revealed that the positive association was due to a null effect once the children’s PGI and correlations between different PGIs were taken into account. In an IV model without further controls, we observed a large positive effect of parental age, which we consider implausible and unreliable. This effect is probably due to unobserved confounding. Controlling for children’s PGI for AFB reduced the IV estimate. Finally, we observed a strong change in the IV estimate when we included the PGI for parental educational attainment. This is unsurprising as the genetic association between the PGI for education and the PGI for AFB is well established, and the education PGI is known to be one of the strongest PGIs in behavioural science. However, at the same time, the PGI for education is a proxy measure, which captures all factors related to education, including diseases, cognitive skills and personality traits. For this reason, conditioning on the PGI for education could also introduce overcontrol bias.
Although IV approaches in general, and MR approaches in particular, have been challenged for potential violations of assumptions such as the independence assumption, exclusion restriction or population stratification12, we think it is unlikely that the effects of such violations perfectly balance our results towards zero or are responsible for making them negative in the full model. However, we cannot rule out potential biases, particularly due to pleiotropy. Recruitment bias may have been introduced in our study because the data from the genome-wide association study (GWAS) used in our study were largely from the UK Biobank, which had a low participation rate and is known to be an imperfect reflection of the general population. However, it is unclear whether this biases our estimates with respect to the specific research question we analysed.
Our results do not support sociological expectations of positive effects of higher maternal and paternal age on children’s education. The positive associations between parental ages and children’s academic performance have been attributed by social scientists to higher socioeconomic status and the greater resources that parents accumulate as they age6. In addition, it has been argued that higher parental age leads to more stable relationships and better parenting5, which is beneficial for children’s academic performance. Our results do not provide evidence to support these expectations, although uncertainty remains owing to the large CI values.
Our findings also do not support biological theories that predict even negative effects of paternal and maternal age. Biologists have observed potentially deleterious mental-health consequences of increasing parental ages, particularly related to the paternal accumulation and inheritance of de novo mutations across the life course and increased psychiatric problems in children. In addition, age-related methylation changes16, which may modify parenting behaviour, may influence children’s school performance, including episodes of psychosis17,18,19. Although our results do not directly address these potential mechanisms influencing children’s test scores, and mathematical models have shown that de novo mutations are unlikely to have large effects in general20, they do not provide evidence for robust negative effects of parental age. The possibility remains that positive social consequences of parental age effects and negative biological consequences cancel each other out.
The MR approach has been promoted21 and challenged22 in the social sciences. Our study showed that its naive use can lead to misleading, inflated and implausible results. The quantitative solutions to challenges in genetic discovery and the genetic architecture of human behaviour—in particular, polygenicity and pleiotropy—suggest that applications can be informative as complements to the literature.
Our results were obtained with respect to a specific society. Norway has a high per capita income compared with other developed countries, and has a low level of income inequality. In addition, the Norwegian government provides generous child benefits. For this reason, the positive causal effect of parental age on child education may be smaller in Norway than in other societies, such as the United States. Our results are also specific to the analysis of the AFB, and other research designs are needed to study the effects of parental age at higher order births.
It is important to highlight both the advances our data provide for our modelling approach compared with standard MR approaches, as well as the remaining limitations. In particular, the MoBa data enabled us to account for AM. However, the correction for AM, which controls for partner PGI, remains incomplete owing to missing heritability, which may potentially inflate causal estimates. We also controlled for population stratification, including genetic principal components and grandparental education. However, these controls also remain incomplete, and future studies should aim to use results from within-family GWAS or perform within-family prediction23 when such data are available.
Finally, given the uncertainty in our estimates and the fact that we provided only one specific modelling approach to causality, we do not provide a definitive answer but add new evidence to the literature that contradicts the idea that parental age leads to better education for children, at least independent of parental education and other correlated factors that should be taken into account. Our results also implied that the effects of postponing fertility at the population level largely depend on age-related conditions that seem to compensate for potential negative effects of ageing, all of which can be replicated in even larger studies using different research designs. It is still possible that social and biological processes act in opposite directions, as predicted by the theories, but cancel each other out. At the macro level, this interpretation implies that increasing AFB will not lead to improved educational outcomes at the population level.
Methods
Data
We used data from MoBa14,24, which is a population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health. Participants were recruited from all over Norway from 1999 to 2008. The women consented to participate in 41% of cases. No compensation was paid. The cohort includes approximately 114,500 children, 95,200 mothers and 75,200 fathers. The current study is based on version 12 of the quality-assured data files, which was released for research in 2019. The establishment of MoBa and the initial data collection were based on a licence from the Norwegian Data Protection Agency and approval from the Regional Committees for Medical and Health Research Ethics. Data from the MoBa cohort are regulated by the Norwegian Health Registry Act. Our sample was restricted to first-born children born in Norway between 2001 and 2008 to focus on the causal effects of maternal and paternal ages AFB. The sample size is 15,670 for the models using maternal AFB and 15,593 for the models using paternal AFB. Given these sample sizes and an alpha set to the standard value of 0.05, the power of our analysis to identify an effect size of 0.034 s.d. (OLS estimate in model 1) is 0.99 for the IV analysis. Overall, 50.7% of the children in the sample are female.
We report the first-stage results in Supplementary Table 1 and the reduced-form estimates in Supplementary Table 2. Supplementary Table 3 reports a correlation matrix among the variables included in our analysis.
Phenotypic measures
We measured children’s academic performance using national standardized tests in mathematics, reading and English in fifth grade (around age 10). We analysed a measure that averages across subjects. If there were missing values in one subject, we averaged using the other test scores. We standardized test scores within each individual and birth year to have a mean of 0 and a s.d. of 1. For the AFB, we included only children for whom both parents registered as first-time parents.
Genetic measures
Genotyping of MoBa has been performed in different projects with various selection criteria, genotyping arrays and genotyping centres13. Phasing and imputation have been performed using the publicly available Haplotype Reference Consortium release 1.1 panel as a reference. The MoBaPsychGen pipeline, comprising pre-imputation quality control (QC) based on SNPs and individuals, phasing, imputation and post-imputation QC based on current best-practice protocols, was implemented to account for the complex (relatedness) structure of the data. In total, 6,981,748 SNPs passed through the pipeline. Our PGI constructions were based on all these SNPs. Each PGI construction was based on summary statistics provided by the trait-specific GWAS.
Analytical strategy
We used a MR approach to estimate the causal effects of parental age on a child’s education9. We instrumented maternal and paternal AFB using genes associated with AFB in a previous genetic discovery study10 and summed the results to create a PGI to ensure sufficient statistical power. We use the parental PGI for AFB as the instrument while conditioning on the child’s PGI for AFB. We also considered population stratification controlling for grandparental education and the first ten genetic principal components, AM controlling for partner’s PGI for AFB, demographic characteristics (birth year and child’s sex), and controlling for the PGIs for age at first sexual intercourse, age at smoking initiation, age at first use of oral contraceptives, ADHD, and educational attainment, which have previously been linked to the PGI for AFB10 and may influence parental nurturing behaviour relevant to child test scores. All statistical tests in our regression models are two-sided. For comparison reasons, we also report OLS regression models that estimate the associations between parental age and children’s test scores, without controlling for the endogeneity of AFB.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Responses