Off-stoichiometry effect on the physical properties of epoxy resins

Introduction
An epoxy resin is a typical thermosetting resin in which a three-dimensional cross-linking structure is formed through the curing reactions of epoxy and amine compounds [1,2,3,4,5,6,7,8]. Epoxy resins are used in a wide variety of applications, such as adhesives [9,10,11,12,13,14,15,16], encapsulants [17], coatings [18], and composites [19], because of their excellent corrosion resistance [20], electronic insulation [21], and mechanical properties [22]. In these applications, epoxy resins come into contact with different materials, such as metals and dissimilar solids, making it important to understand the aggregation states at buried interfaces to ensure their properties and durability. In general, the aggregation states of epoxy resins at the interface differ from those in the bulk [23,24,25,26], and much research has been devoted to develop a better understanding of the cross-linking structure in close proximity to the interface [27,28,29,30,31,32,33,34] as well as the bulk [35,36,37,38,39,40,41,42,43].
The curing reaction of epoxy resin at the interface was examined using attenuated total-reflectance infrared (ATR-IR) spectroscopy, and the reaction was reported to proceed more slowly at the interface than in the bulk region [44]. This was explained in terms of the slowed diffusion of reactants near the solid interface. On the other hand, sum-frequency generation (SFG) spectroscopy [45,46,47,48,49,50,51,52,53,54], which has much better depth resolution than ATR-IR at <1 nm, revealed that the curing reaction of epoxy resin at the outermost quartz interface was faster than that in the bulk [27]. This result was attributed to the densification and orientation of the reactants at the interface, which facilitated their encounter. Molecular dynamics (MD) simulations supported this finding [24,25,26] and provided some additional information that the initial reaction of the epoxy compound was faster because of the segregation of smaller amine compounds at the outermost interface [23, 27]. Interfacial segregation has been widely studied and is well understood [55,56,57]. The difference in the aggregation states at the outermost interface compared with those in the bulk is due not only to the unique reaction kinetics near the interface but also to deviations from stoichiometry that should be considered.
Previously, using angular-dependent X-ray photoelectron spectroscopy (ADXPS) [58,59,60], we examined the segregation phenomenon in a stoichiometric mixture of epoxy and amine compounds at the copper surface [23]. At that time, X-rays were guided into the epoxy resin through the ultrathin copper layer. The results showed that the amine compounds preferentially segregated at the copper interface and extended ~10 nm [23]. MD simulations demonstrated that this amine segregation was driven by the size disparity between the compounds [24,25,26]. Consequently, since the epoxy was smaller than the amine, the epoxy compound was enriched at the interface [25].
As mentioned in the first paragraph, considering the various applications of epoxy resins [4, 61, 62], a better understanding of the cross-linking network structure at adhered interfaces is crucial. However, the effects of off-stoichiometry on the cross-linking structure are not well understood, even in the bulk [63,64,65,66,67,68,69,70,71,72,73]. Therefore, in this study, we investigated changes in the bulk cross-linking structure when the mixing ratio of epoxy and amine deviated toward an excess of amine. The reason for studying the condition with excess amine is that we ultimately aim to gain a better understanding of the issues at the interface, where amine often segregates. Off-stoichiometric cured epoxy resins were evaluated for cross-linking density, mass density, molecular weight of soluble components, glass transition temperature, tensile deformation properties, and aggregation states.
Experimental
Materials and preparation of epoxy resins
Diglycidyl ether of bisphenol A (DGEBA) and 4,4′-diaminodiphenylmethane (DDM) were used as the epoxy and amine compounds, respectively. Figure 1 shows the chemical structures of DGEBA and DDM. Both were purchased from Tokyo Chemical Industry Co., Ltd. and were used without further purification.
Chemical structures of a DGEBA and b DDM
A primary amino group reacts with an epoxy group to form a secondary amino group, which can further react with another epoxy group to form a tertiary amino group [2, 3]. In these reactions, the stoichiometric molar ratio of epoxy to amine is 2:1. Table 1 listed various values of ϕamine, representing the excess amount of amine, along with the corresponding molar ratios of DGEBA to DDM used in the experiments [66, 73]. The mixture of DGEBA and DDM, which was heated to 363 K, was placed on an aluminum plate with a silicone rubber mold, and then pre-cured at 363 K for 3 h. All samples were further post-cured at 453 K for 12 h.
Characterization of the cross-linking structure
To track the progress of the curing reactions, the consumption of epoxy and amino groups was examined via Fourier transform infrared (FT-IR) spectrometry. FT-IR spectra for the post-cured epoxy resins were collected using an FT-IR-620 spectrometer (JASCO Co.) at 298 K. All spectra were acquired with a resolution of 4 cm−1, with a total of 64 scans accumulated for each measurement.
The cross-linking density (ν) for the cured epoxy resins was estimated based on the swelling ratio in a good solvent using the Flory–Rehner equation [74,75,76]. The cured epoxy resin with a given weight was immersed in tetrahydrofuran (THF) at 298 K to reach equilibrium swelling. The equilibrium weight was recorded after gently removing THF from the sample surface with blotting paper. The mass density (ρ) of the cured epoxy resin was examined by a gas pycnometer (ULTRAPYC 1200e, Quantachrome Instruments Inc.) with helium gas as a probe at 296 K. The target pressure of the helium gas was set at 117 kPa. The measurement was repeated 100 times to obtain an average value.
To extract soluble components not attached to the main chain of the network, the epoxy resins were immersed in THF at room temperature for a few weeks. During the immersion, the supernatant was replaced with fresh THF several times. The evaporation of THF from the collected supernatant yielded a viscous orange-colored liquid. The resultant liquid was dried at room temperature for 12 h, and its weight was then measured to calculate the weight fraction of the soluble component.
The average molecular weight (Mw) of the soluble components was measured via gel permeation chromatography (GPC, JASCO Co.). Two connected columns (Shodex KF-803L, Resonac Co.), with a target Mw range of 1 × 102–5 × 104 and an exclusion limit Mw of 4 × 104, were used, along with a PU-4180 pump, a CO-4060 column oven, and an RI-4030 refractive index detector (JASCO Co.). THF containing 0.04 wt% triethylamine was used as the mobile phase. The column temperature and flow rate were set to 313 K and 0.5 mL min−1, respectively. Monodisperse poly(methyl methacrylate) (PMMA) was used as a standard.
The cured epoxy resins were subjected to wide-angle X-ray scattering (WAXS) measurements at the BL05XU beamline of SPring-8 (Japan). The wavelength of the incident X-ray and the sample-to-detector distance were 0.10 nm and 273 mm, respectively. By circularly averaging the two-dimensional pattern captured on a PILATUS 2 M (DECTRIS Ltd.), a one-dimensional scattering profile of the sample was obtained.
MD simulations were conducted for the epoxy resins with various ϕamines. A stoichiometric mixture consisting of 200 DGEBA and 100 DDM was placed in a cubic unit cell with a side length of 5 nm for ϕamine = 1.0. For ϕamine = 1.2, 1.4, 1.6, and 1.8, 200 DGEBA were mixed with 120, 140, 160, and 180 DDM, respectively. The curing reactions of epoxy and amine compounds were simulated using a previously reported technique to construct cured epoxy resins [77,78,79,80].
Characterization of the physical properties
The glass transition temperature (Tg) of the post-cured samples was determined via differential scanning calorimetry (DSC6220, Hitachi High-Tech Science Co.). The samples were heated to 473 K at a rate of 10 K min−1 under a dry nitrogen purge and then cooled to 303 K. The third heating scan was used to characterize Tg.
The mechanical properties of the epoxy resins were characterized via tensile tests using a TENSILON RTF-1310 (A&D Co., Ltd.). The specimens for the tensile tests were prepared in a silicone rubber mold on an aluminum plate, as mentioned above. The shape of the specimens conformed to the Japanese Industrial Standards (JIS), K6251-7, which specifies a dumbbell shape with a gauge length of 12 mm and a width of 2 mm. The tensile test was conducted at room temperature with a crosshead speed of 10 mm min−1.
Results and discussion
The mixture of DGEBA and DDM with various ϕamines was pre-cured at 363 K for 3 h and then post-cured at 453 K for 12 h. Figure 2 shows the FT-IR spectra for the post-cured epoxy resins with ϕamines of 1.0, 1.2, 1.4, 1.6, and 1.8. The spectra for DGEBA and DDM are also shown as references. For clarity, each spectrum is vertically shifted. The spectrum of DGEBA shows an absorption peak at 4528 cm−1, which is attributed to the combination of the stretching and bending vibrations of epoxy groups [76, 81]. DDM exhibits two absorption peaks at 5034 and 6630 cm−1. The former is attributed to the combination of the stretching and bending vibrations of primary amino groups, and the latter is attributed to the combination of the symmetric and asymmetric stretching vibrations [76, 81]. The peak at 6630 cm−1 also corresponds to the symmetric stretching vibrations of secondary amino groups, if present [82]. For all post-cured epoxy resins, the peak due to epoxy groups disappeared, indicating that the consumption of epoxy groups was apparently complete. The intensity of the peak observed at 6630 cm−1 after post-curing increased with increasing ϕamine, making it clear that an excess amount of primary and/or secondary amino groups remained even after post-curing.
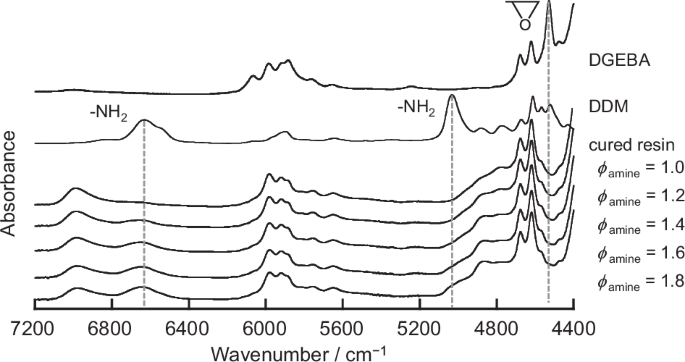
FT-IR spectra of DGEBA, DDM, and cured epoxy resins with ϕamines of 1.0, 1.2, 1.4, 1.6, and 1.8
The ν values for the post-cured epoxy resins with various ϕamines were estimated by the swelling test with the Flory–Rehner equation. Figure 3a shows the relationship between the ϕamine and ν values. The ν decreased with increasing ϕamine. Specifically, the cross-linking density decreased as the excess amount of the amine increased. Thus, unreacted amines and dangling chains, where one end attached to the network and the other was free, most likely appeared, and topological defects formed in the network [83]. Figure 3b shows the correlation between the ϕamine and ρ values. As ϕamine increased, ν decreased, and the ρ value also decreased. Since the lower cross-linking density induces a fractional amount of free volume, this decrease in the mass density could be reasonably understood [84].
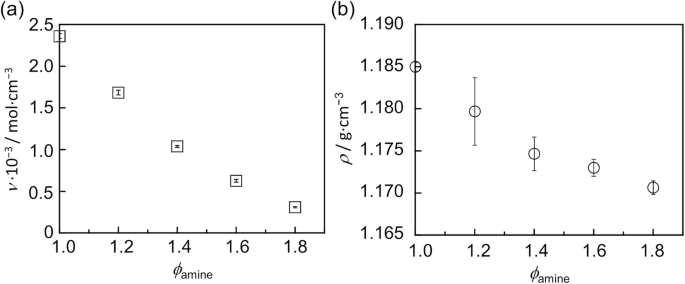
Dependence of the a cross-linking density and b mass density on ϕamine
Direct characterization of the cross-linking architecture in cured epoxy resins is extremely difficult because of their infusibility at relatively high temperatures and insolubility in organic solvents. However, unreacted monomers and isolated chains with low-molecular weights, which are not covalently attached to the network expanded through the system, exist in the cured epoxy resin. These components can be extracted using a good solvent, such as THF. Figure 4a shows the weight fraction of the soluble components as a function of ϕamine. The weight fraction is defined as the weight of the extract divided by the weight of the initial specimen. A straight line corresponds to the predicted weight fraction of the excess amine based on stoichiometry, assuming that all amines remain in their monomeric form. Although the weight fraction of the soluble components increased with increasing ϕamine, the experimental values were consistently much smaller than the predicted line. Thus, it seems reasonable to consider that unreacted components, distinct from the soluble components, are present in the residue. These are likely to be primarily dangling chains within the network, but it is also possible that isolated chains with high molecular weights are physically trapped in the network and thus unable to dissolve. The soluble components obtained were then subjected to molecular weight measurements using GPC. Figure 4b shows the chromatograms of the soluble components extracted from the epoxy resins with ϕamines of 1.2, 1.4, 1.6, and 1.8. While the x-axis represents the retention time after the injection of a solution into the instrument, the secondary x-axis at the top of the figure indicates the relative molecular weight based on PMMA standards. Various peaks were observed for all samples. Notably, a broad peak that appeared in a shorter retention time region shifted to a longer time as the ϕamine increased, meaning that the molecular weight of the soluble components increased with increasing ϕamine. This will be further discussed in the following MD simulation.
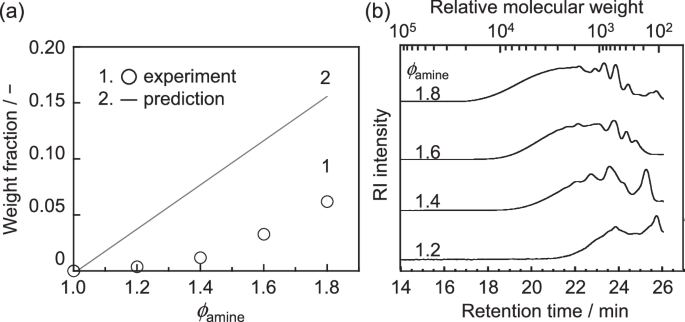
a Dependence of the soluble component weight fraction on ϕamine. b GPC chromatograms of the soluble components extracted from the epoxy resins with various ϕamines
Figure 5 shows snapshots of the network structure evolved in the cured epoxy resins with ϕamines of (a) 1.0 and (b) 1.8, where the reaction conversion was set to 90%. A higher reaction conversion would result in cramped cross-linking, causing excessive and localized residual strain in the system. Hence, the structure at 90% conversion was used for the following discussion. Green represents isolated chains, which are considered the soluble components in the experiment. Red represents dangling chains composed of DGEBA and/or DDM. Blue denotes the percolated network spanning the entire system, excluding isolated and dangling chains. Figure 5 clearly shows that the system with a ϕamine of 1.8 contains more isolated and dangling chains than the system with a ϕamine of 1.0.
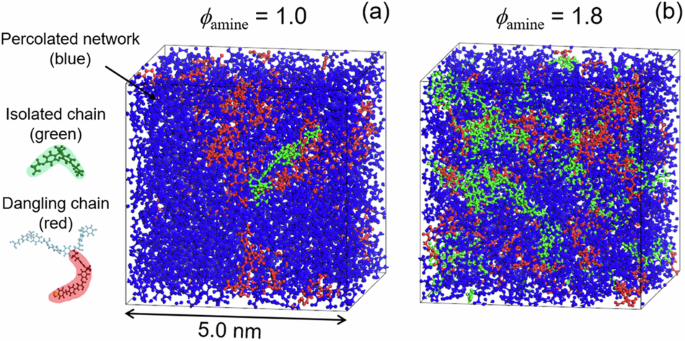
Snapshot of the simulation cell for the cured epoxy resins with ϕamines of a 1.0 and b 1.8 at 90% conversion
Figure 6a shows the volume fraction of the three components as a function of ϕamine. At a ϕamine of 1, the fraction of isolated chains was near zero, whereas that of dangling chains was 0.14. As the ϕamine increased, the fraction of both the isolated and dangling chains increased. Concurrently, the volume fraction of the percolated network decreased. This decrease was likely associated with a decrease in the cross-linking density and mass density, as shown in Fig. 3. Figure 6b shows the number-average molecular weight (Mn) of isolated chains and dangling chains plotted against ϕamine. The Mn value of isolated chains increased with increasing ϕamine. This result was consistent with the GPC results for the soluble components, as shown in Fig. 4b. The quantitative discrepancy with the GPC results was likely caused by the small size of the simulation system. An increase in Mn associated with increasing ϕamine was also observed for dangling chains. When ϕamine is equal to unity, a dangling chain is composed solely of either a DGEBA or DDM monomer. Their molecular weights are 340.4 and 198.3, respectively. As the ϕamine increased, the number of DDM-terminated dangling chains increased. Thus, it is most likely that the excess amount of amine induces an increase in not only the amount of isolated and dangling chains but also their molecular weight.
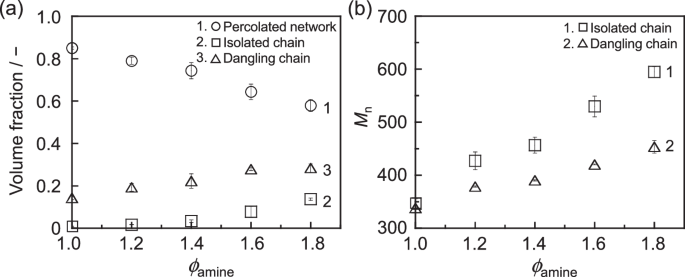
a Dependence of the volume fraction of the percolated network, isolated chains, and dangling chains on ϕamine. b Relationships between the ϕamine and the number-average molecular weight of isolated and dangling chains
Figure 7 shows the ϕamine dependence of the Tg value, which was defined as the midpoint of the baseline shift in the DSC thermogram. As the ϕamine increased, the Tg decreased from 442 to 380 K. The ϕamine dependence of Tg was consistent with the behavior of cross-linking density and mass density [83]. This trend could be explained by the plasticization of topological defects formed under off-stoichiometric conditions [84,85,86,87,88]. That is, the decrease in Tg was likely caused by the presence of the excess amine remaining as unreacted substances, which enhanced the chain mobility in the cured epoxy resin. Additionally, the presence of dangling chains increased with increasing ϕamine, leading to a reduction in the cross-linking density. Based on the results for the cross-linking density and mass density together with the Tg results, it is evident that all cured epoxy resins were clearly in a glassy state at room temperature and became less dense as the ϕamine increased.
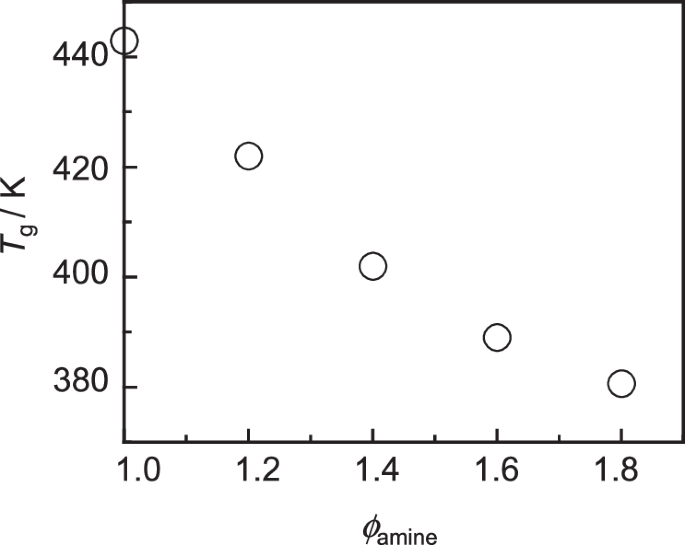
Tg as a function of ϕamine
Figure 8a shows the stress–strain (S–S) curves for the cured epoxy resins with ϕamines of 1.0, 1.2, 1.4, 1.6, and 1.8 obtained from tensile tests. The cured epoxy resins with ϕamines of 1.0 and 1.2 exhibited yielding behavior before reaching rupture, as is commonly observed for glassy polymers. In contrast, at ϕamines of 1.4, 1.6, and 1.8, necking behavior was observed after yielding. Figure 8b shows the fracture energy (Gf) and Young’s modulus (E) as a function of ϕamine. The Gf value decreased with increasing ϕamine. This result can be understood if the stress concentration is assumed to more likely to occur as the ϕamine increases because of the increased presence of the topological defects, such as isolated chains and dangling chains. In contrast to the trend for Gf, the E value increased with increasing ϕamine. Given that both cross-linking density and mass density decrease with increasing ϕamine, it is intuitively difficult to understand why Young’s modulus increased with ϕamine.
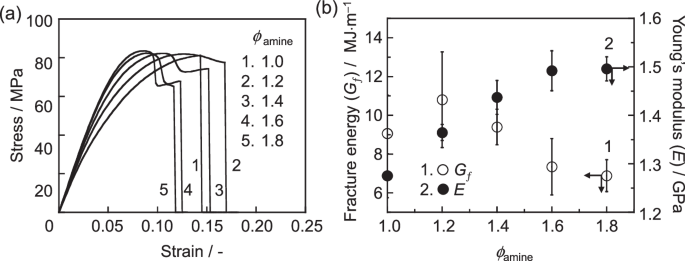
a Stress–strain curves and b fracture energy and Young’s modulus derived from (a) as a function of ϕamine for the cured epoxy resins
Notably, the origin of elasticity in polymer materials in a glassy state is related to the energy associated with the torsion and/or rotation of C–C bonds. Given this, the rotational motion of chains, and consequently the local steric hindrance between repeating units, could be a contributing factor. This phenomenon is recognized as the antiplasticization effect [86]. Based on the molecular structure shown in Fig. 1, phenyl groups are associated with resistance to deformation. Thus, the WAXS measurements were conducted to examine the changes in the distance between phenyl groups as a function of ϕamine.
Figure 9a shows the WAXS profiles for the cured epoxy resins with ϕamines of 1.0, 1.2, 1.4, 1.6, and 1.8. For all samples, a broad peak was observed at a scattering vector q (=(4π/λ) × sin θ, where λ is the X-ray wavelength and 2θ is the scattering angle) of ~13 nm−1. This peak shifted to a higher q value with increasing ϕamine. Based on the q value at the peak maximum, the correlation length (l), corresponding to the distance between phenyl groups [80], was calculated. Figure 9b shows the relationship between the ϕamine and l. The l value, initially 0.5 nm at ϕamine of 1.0, slightly decreased with increasing ϕamine, reaching 0.48 nm at a ϕamine of 1.8.
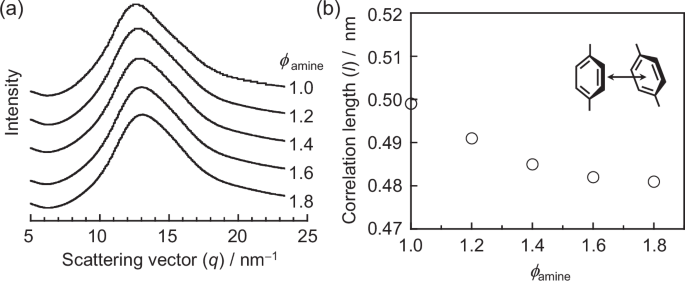
a WAXS profiles for the cured epoxy resins with various ϕamines and b correlation between the ϕamine and l values, which reflect the distance between phenyl groups, as shown in the inset
To verify the plausibility of the molecular picture obtained from the WAXS data, power spectra were calculated based on the distances between phenyl groups obtained from the MD simulations. The result is shown in Fig. 10a, where a peak appears at q = 12–13 nm−1, which is almost identical to that observed in the experiment. Figure 10b shows the l value in real space as a function of ϕamine for the cured and uncured epoxy resins. Although each value for the cured epoxy resins was larger than the corresponding experimental value by ~0.01 nm, it is consistent with the trend that the correlation length decreased with increasing excess amount of amine. For the uncured system, that is, before curing, monomeric DGEBA and DDM were positioned at a stable distance from each other. However, upon being incorporated into the cross-linking structure by the reaction, they became constrained, leading to an increase in the distance between phenyl groups. Since isolated chains and dangling chains are more mobile than chains within the cross-linking structure, this likely results in more stable phenyl group spacing, even when constrained by the cross-linking structure. If this hypothesis is correct, the l value at a given ϕamine should be smaller before curing than after curing and be independent of the ϕamine. This is exactly what is seen in Fig. 10b. Thus, an excess of amine suppressed the widening of the distance between phenyl groups due to the formation of the network structure, resulting in higher stresses during tensile deformation. This interpretation is consistent with the above explanation based on the local steric hindrance between repeating units.
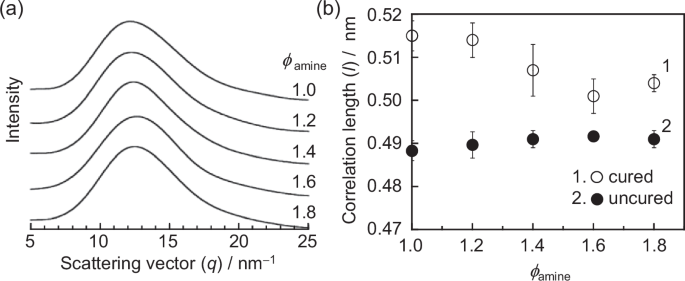
a Power spectra between phenyl groups in the cured epoxy resins at 90% conversion with various ϕamines obtained from MD simulations. b Correlation between the ϕamine and l values of phenyl groups for the cured and uncured epoxy resins
Conclusions
The cross-linking structure and physical properties of the cured epoxy resins, where the amount of amine exceeded the stoichiometric ratio, were studied. FT-IR analysis revealed that peaks derived from epoxy groups disappeared after post-curing, indicating that the reaction of epoxy groups was complete. In contrast, peaks associated with amino groups remained after post-curing, and their intensity increased as the excess amine increased, indicating that amino groups persisted even after post-curing. In addition, as the excess amine increased, unreacted monomers and lower-molecular-weight isolated chains formed alongside the major cross-linking structure. Some of these components were extractable by THF and subjected to GPC analysis. This led to a finding that as the excess amine content increased, the molecular weights of the soluble components also increased. Furthermore, under conditions of excess amine, dangling chains in the cross-linking structure were mostly terminated with amines, as confirmed by MD simulations. The unreacted monomers, low-molecular-weight isolated chains, and dangling chains increased the number density of the topological defects within the network, thereby reducing the cross-linking density and mass density of the cured epoxy resin, which subsequently lowered the glass transition temperature. Tensile tests on the cured epoxy resins revealed that Young’s modulus increased with excess amine. Considering that the origin of elasticity in glassy polymers is related to the rotation and torsion of C–C bonds, the results suggest that the distance between phenyl groups depended on the mixing ratio of epoxy and amine. WAXS measurements and MD simulations revealed that as the curing reaction progressed and the heterogeneity of the network structure increased, the distance between phenyl groups became larger. Therefore, it was claimed that increasing excess amine suppressed the expansion of the phenyl group distances, resulting in an increase in Young’s modulus. Since off-stoichiometric conditions are often observed at adhesive interfaces and at interfaces in filler-filled epoxy resins, the results obtained in this study provide valuable insights for developing more realistic and effective material designs. These findings can be applied to predict and potentially optimize the physical properties of the epoxy interfaces in such systems.



Responses