Personalized dose selection platform for patients with solid tumors in the PRECISE CURATE.AI feasibility trial

Introduction
Artificial intelligence (AI) is a promising tool for oncology, aiming to support digital pathology, biomarker development, and treatment optimization1,2. One area that may benefit from AI-supported tools is treatment personalization. Oncology doctrine conventionally suggests that higher chemotherapy drug doses lead to higher cancer cell kill and potentially result in increased efficacy. Accordingly, standard-of-care dose selection relies on achieving the maximum tolerated doses (MTD), which are typically established in, and are the primary endpoints of phase I clinical trials. This results in a high initiation dose, and often, dose reductions are toxicity-driven, guided by the severity of the drug-induced side effects. In clinical practice, to avoid toxicity, more than half of the oncologists prescribe initiation doses that are lower than MTD at least 10% of the time3. Alternative strategies include adaptive dose modulations based on evolutionary-based modeling4,5.
CURATE.AI is an indication-agnostic, AI-derived platform that utilizes a patient’s own, prospectively-calibrated small dataset to create an N-of-1 profile that dynamically personalizes only that patient’s dose recommendations6. CURATE.AI is not a conventional AI platform, but rather, it uses the results from an earlier neural network-based study7, and reduces it to a simpler quadratic relation between intervention intensity (e.g., drug dose) and phenotypic response (e.g., tumor marker). This quadratic relation indicates a non-linear relationship, such that when intervention intensity increases, the response either initially increases then decreases, or initially decreases then increases—this could be due to several reasons, which could include intra- and inter-personal differences in pharmacokinetics and pharmacodynamics8, variations in cancer genotype9, tissue–drug interactions, and drug–drug interactions10. It is currently being developed as a clinical decision support system (CDSS) that aids physician decisions to serially identify the optimal dose for the next dosing cycle. Importantly, CURATE.AI is not a black box—a system where its internal workings are not accessible or known. Instead, the quadratic correlation at the foundation CURATE.AI is understandable on a clinician level. The bounds built around the correlation are known and are actively developed and co-created/acquired with the treating physician, ensuring the profile’s relevance to the patient and confidence in the CURATE.AI recommendations. CURATE.AI is being clinically validated through prospective trials in several indications ranging from prostate cancer (NCT02711956)11, cognitive rehabilitative therapy (NCT04848935)12, to hypertension (NCT05376683)13. CURATE.AI’s ability to be applied to various indications is enabled by simplifying the original, neural network-led algorithmic process, incorporating derivatives of clinically relevant factors and its adaptability to various dose-response inputs12,14,15,16,17,18.
Multiple challenges in the sphere of ideation, validation and deployment require addressing before AI and AI-derived solutions realize their potential for clinical oncology1. As such, the objectives of this case series are associated with identification of potential implementation issues and integration of an AI-derived CDSS into clinical workflows. In a formal, pilot feasibility trial design, the primary objective was to assess the applicability, as well as scientific and logistical feasibility of a randomized control trial (RCT), see Supplementary Materials.
Methods
Ethics oversight and reporting standards
The trial underscoring this case series was approved by the relevant review boards (National Healthcare Group Domain Specific Review Board 2020/00334 and National University of Singapore Institutional Review Board 2021-671) and registered at clinicaltrials.gov (NCT04522284). All patients provided informed consent to participate in the study. The case series was performed in accordance with the Declaration of Helsinki and follows CARE reporting standards19, with the exception of including patient perspective.
Additionally, across the main manuscript and the supplementary materials the trial reporting covers the reporting items according to CONSORT-AI20. We additionally drew from the DECIDE-AI reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence21 and from the CONSORT 2010 statement: extension to randomized pilot and feasibility trials22. The updates to the previously published protocol23 are reported according to SPIRIT-AI24 and included as a supplement—see summary of changes with reasons (Supplementary Table 4). Patients were not involved in the development of the research question, the study design, or the conduct of this study.
Experimental design
PRECISE CURATE.AI was an open-label, single-center, single-arm, prospective feasibility trial in patients diagnosed with advanced solid tumors at/for palliative-intent treatment with single-agent capecitabine, capecitabine in combination with oxaliplatin (XELOX), capecitabine in combination with irinotecan (XELIRI) provided in typically three-week-long cycles. Biologics such as bevacizumab were permitted as clinically indicated. All recruited patients received treatment augmented by CURATE.AI dose recommendations for capecitabine only, while other drug doses were prescribed at physicians’ discretion. The doses of other drugs prescribed were determined independent of the CURATE.AI augmented capecitabine doses (e.g., physicians did not increase oxaliplatin or irinotecan dose if capecitabine dose was decreased for that particular cycle). The dosing range for the recommendations was prespecified as 50–100% of the capecitabine standard-of-care dose, defined as 1000 mg/m2 for capecitabine in combination, and as 1250 mg/m2 single agent capecitabine (unless adjusted by users to account for patient’s comorbidities and organ dysfunction). User-communicated changes to the dosing range for any of the dosing events were incorporated into CURATE.AI operations as data inputs (Supplementary Table 5).
Recruitment sites and recruitment criteria
The recruitment was open in two Singapore hospitals: National University Hospital (NUH) and Ng Teng Fong General Hospital (NTFGH) from 20th August 2020 to 10th Nov 2021, however, all patients were recruited at NUH. The recruitment was concluded after reaching the pre-specified recruitment objective of ten patients. No patient replacement was pursued.
Candidate participants were identified by the physician co-investigators during the routine clinical practice—patients diagnosed with advanced solid tumors at/for treatment with single-agent capecitabine, capecitabine in combination with oxaliplatin (XELOX), or capecitabine in combination with irinotecan (XELIRI). Additionally, the recruited patients fulfilled the following criteria: above 21 years of age, ECOG performance status of 0–2, treatment received not for curative intent, measurable disease defined by raised response marker (e.g., CEA, CA19-9), laboratory criteria within the 21 of starting treatment: a) Absolute neutrophil count (ANC) ≥ 1000/mm3 and platelet ≥50,000/mm3; b) total bilirubin ≤1.5 × the upper limit of the normal range (ULN). Alanine aminotransferase and aspartate aminotransferase (AST) ≤ 3 × ULN or ≤5 ULN if involvement of the liver; c) calculated creatinine clearance ≥30 mL/min or creatinine <1.5 × ULN. Patients who were lactating or pregnant, patients with any contraindication and/or hypersensitivity to the planned treatment, patients with mental disorder expected to affect adherence were excluded from the recruitment. All participants signed informed consent before the trial-related procedures. No blinding was performed.
Clinical decision support system—CURATE.AI
CURATE.AI is a mechanism-agnostic platform that enables dynamic generation of personalized dose recommendations based on a quadratic relationship between intervention intensity (e.g., drug dose) and phenotypic response (e.g., tumor marker). The dynamic nature of the platform results from the continuously changing patient profile as new data are added longitudinally. The input data used to generate the recommendations consist exclusively of the data of the patient whose doses were being optimized, which aligns with algorithmic fairness principles. The information flow for CURATE.AI-aided capecitabine dose selection is demonstrated in Fig. 1.
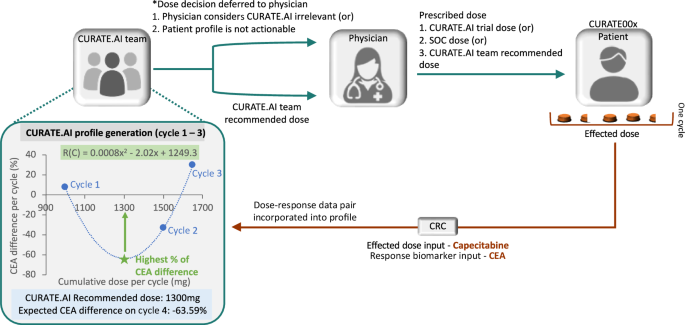
CURATE.AI team recommended dose—dose recommendation by the CURATE.AI team to the physician; Effected dose—drug amount taken by the patient over the duration of the dosing cycle, based on the patient adherence and any dosing interruptions; Dose decision deferred to the physician—dose selected in the process in which CURATE.AI was considered irrelevant; Prescribed dose—dose that was prescribed to the patient by the physician; SOC dose—dose based on projected standard-of-care dose; CURATE.AI trial dose—dose deferred to the physician, where the physician selected a dose that is not standard-of-care dose to aid CURATE.AI calibration, e.g., an initial dose selected by the treating physician that is lower than standard-of-care dose. CRC—clinical research coordinator—was responsible for de-identifying the patient data before transferring it to the CURATE.AI team.
Depending on an indication, the data used to construct the low-order relationship in CURATE.AI can be differentially transformed. In the version of CURATE.AI used in this study, the data inputs were as follows: total capecitabine dose taken over the course of the cycle (effected dose—the prescribed dose adjusted by the pharmacovigilance information), and the resulting response biomarker level change expressed as a percentage of the pre-cycle level. Selected additional data pertaining to the patient medical history were also included in the pre-processing of the data and interpretation of the CURATE.AI algorithmic operations. The input data, their source, and pre-processing are specified in Supplementary Table 5. As the data were collected prospectively, missing data or quality issues were expected to be rare and resolvable. A second-order polynomial regression model was used to fit the available dose-response data pairs and generate a personalized profile. After the calibration stage was completed and the profile was generated, the CURATE.AI dose recommendation intent changed from “calibration intent” to ‘efficacy-driven’ and the CURATE.AI recommendation based on the personalized profile generated, as shown in Fig. 1, aimed to achieve a biomarker response in line with the clinical objective of the treatment. The dose-response data pairs for the subsequent cycles were incorporated into the profile in the same manner, such that the profile and the CURATE.AI dose recommendations dynamically evolved along the patient’s journey. In the case of systemic changes such as regimen change (e.g., change from XELOX to XELIRI), CURATE.AI recommended “recalibration” dose(s), aiming to collect a new set of dose-response data pairs to generate an updated or new profile.
Treatments and procedures
After enrollment, standard-of-care longitudinal blood draws were performed to collect the patient’s biomarker levels before and after each cycle. Research-specific intracycle blood draws were optional and did not exceed more than two per cycle. Patients underwent monitoring and other planned or emergency treatments as per standard-of-care. The patient engagement was planned as no longer than 12 months with survival follow up for three years every six months.
Users of CURATE.AI recommendations
The users of CURATE.AI recommendations were the physicians engaged as a principal investigator and two co-investigators. The principal investigator took part in the conceptualization of the study, while the co-investigators were introduced to the principles of CURATE.AI during a site initiation visit (SIV), prior to the first patient enrollment. Although no formal training was provided, all users had additional opportunities to familiarize themselves with CURATE.AI through grand rounds and seminars as well as published literature. The CURATE.AI output was presented to the users at each dosing event via email as a recommendation sheet that included information on the dose recommendation and the recommendation’s intent. The users were free to ask additional questions to the CURATE.AI team at any time.
Human oversight of CURATE.AI
All steps of the CURATE.AI dose recommendation generation involved human oversight. Prespecified patient data (Supplementary Table 5) were anonymized and provided by the CRC to the CURATE.AI team via email together with the recommendation delivery deadline with a median of two working days (range of one-six days). At least two, trained individuals from the CURATE.AI team were involved in each dosing event to pre-process the input data, interpret the algorithmic operations result, and validate the recommendation in the context of the patient journey (e.g., expected patient medication adherence) before providing it to the users. The users were free to accept or reject the CURATE.AI recommendation or to deem it irrelevant to the particular dosing event, e.g., due to the tight drug administration schedule.
The treatment response was monitored with the Response Evaluation Criteria in Solid Tumors (RECIST). RECIST classifies treatment responses of target lesions as complete response, partial response, progressive disease, and stable disease. If the patient experienced clinically relevant grade three or four non-hematological toxicity (according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0) at a particular dose, the next dose recommendation by CURATE.AI was restricted to a lower dose.
Management of patient safety
To ensure patient safety, CURATE.AI recommendations followed guidelines as per protocol23: (1) there was a predetermined safety range for dosing (50–100% of dose used in standard-of-care treatment), (2) all recommendations were to be within a patient-specific safe dosing range set by the clinical investigator, which accounted for personal medical history and clinical context, (3) should CURATE.AI not have been able to recommend a dose that fulfilled the above requirements, a recommendation was not generated, and the clinical investigator decided the dose based on standard-of-care.
While adverse events were not directly included within the regression model, patient adverse events were looked out for by clinicians within the workflow, and relevant actions—which include, but are not limited to the pausing of treatment, or narrowing the patient-specific safe dosing range—would be taken by the clinicians to ensure patient safety.
Outcomes—applicability and implementation factors
The primary outcome measure was the percentage of participants in whom we successfully applied CURATE.AI profile, as concluded by expert judgment based on the numerical assessment of the primary outcome measure (Supplementary Table 3). This was the main outcome used to judge the feasibility of the RCT according to ‘the traffic light system’ defining progression criteria22. The applicability of CURATE.AI was further defined into five sub-measures, assessing: (1) if the marker error is sufficiently small to allow for accurate predictions, (2) if the profile can be generated sufficiently early, (3) if a dose-dependent relationship is observed, (4) if the profile is actionable, and (5) if systematic changes in the participant are readily assimilated into the CURATE.AI algorithm. The expert panel consisted of three medical oncologists, part of the phase I clinical trial unit of the National University Cancer Institute, Singapore. The panelists were provided with the primary and secondary outcome measures and the individual patient journey graphs. The secondary outcome measures and the exploratory outcome measures focused on scientific and logistical feasibility as part of identifying relevant implementation factors, as well as efficacy and toxicity, respectively (Supplementary Table 3).
Statistical analysis
Kolmogorov-Smirnov test was used to assess data normality for datasets with more than 30 datapoints. Normally distributed data are presented as mean ± standard deviation (SD). Non-normally distributed and small data sets are presented as median and interquartile range (IQR).
Role of funding sources
The study was performed as an independent, investigator-sponsored study. Funders had no role in study design, data collection, data analysis, data interpretation, or manuscript writing. The corresponding authors had full access to the data and carried the final responsibility for the submission of the manuscript.
Results
Ten patients were recruited who were intended for palliative-intent treatment with: single-agent capecitabine (one patient), XELOX (six patients), and XELIRI (three patients). De-identified, patient-specific information is presented in Table 1.
Carcinoembryonic antigen (CEA) and CA125 were used to monitor the disease and serve as inputs for CURATE.AI in the treatment of nine and one patient, respectively. Three patients reached efficacy-driven dosing stage, with the longest engagement in the trial of 12 cycles (Supplementary Table 1). Their medical journeys were highly varied, with CURATE.AI being adapted to the encountered circumstances (Fig. 2; Supplementary Materials). Of note, only three patients—CURATE002, 007, and 011—were responders to CURATE.AI-guided treatment. They had different CURATE.AI profiles and their treating physicians received different, dynamically adjusted CURATE.AI dose recommendations. Accordingly, each patient received bespoke, dynamically modulated doses of capecitabine. Seven patients did not reach the efficacy-driven dosing stage (Supplementary Results; Supplementary Fig. 1).
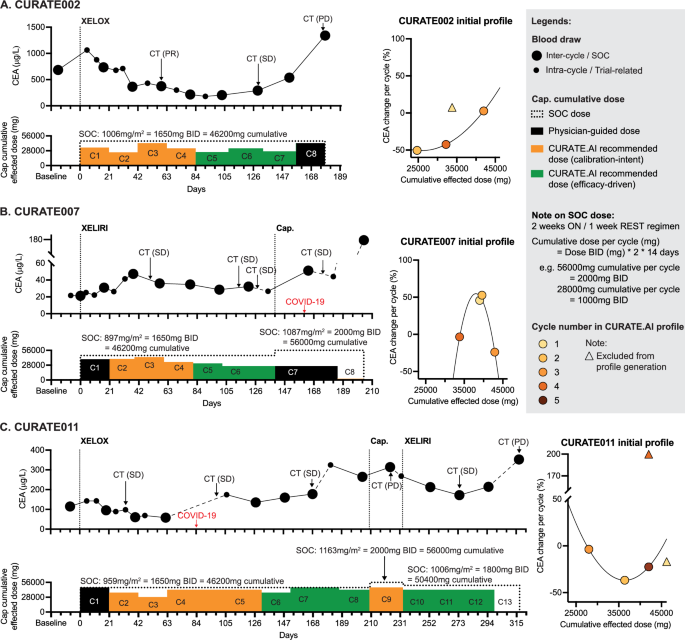
Effected dose of capecitabine in each cycle vs patient response as measured with longitudinal CEA changes. Patients’ CURATE.AI profiles were generated based on the initial effected dose-CEA response data pairs. Each patient had their own, personalized CURATE.AI profile generated based on their own data only. Standard-of-care (SOC) doses for each patient and each regimen are listed under the regimen’s name. A Patient CURATE002 was diagnosed with sigmoid adenocarcinoma with metastasis to liver and lung and was treated with XELOX. B Patient CURATE007 was diagnosed with colorectal cancer with metastasis to liver and was treated with XELIRI and bevacizumab. C Patient CURATE011 was diagnosed with rectosigmoid cancer and was treated with XELOX. Details of the three patients’ treatment are described in the Supplementary Results. SOC stands for standard-of-care; CT (*) stands for computer tomography; BID means twice a day; PR, SD, and PD stand for partial response, stable disease, and progressive disease, respectively, according to the RECIST criteria; CEA stands for carcinoembryonic antigen. Cap. indicates single-agent capecitabine; XELOX is capecitabine in combination with oxaliplatin; XELIRI is capecitabine in combination with irinotecan.
Difficulties encountered during the implementation of CURATE.AI in the treatment of CURATE002 stemmed from a surge-like behavior of CEA following the initial dose, presenting a scientific challenge to CURATE.AI, specifically, the lack of sustained dose-response relationship. An additional challenge that CURATE.AI needed to incorporate was dose interruptions, which are common, due to logistics or toxicities. In the case of CURATE007, the relevance of CURATE.AI was initially challenged in the first cycle due to practical considerations in the clinical workflow, compounded by consistent challenges in patient adherence. For CURATE011, complications such as COVID-19 infection and associated side effects necessitated regimen changes, prompting the recalibration of the CURATE.AI profile. The obstacles stemming from systemic changes in the patients’ states and adaptation strategies employed by CURATE.AI are further elaborated on in the Supplementary Results.
We assessed feasibility features based on the treatment of all ten patients (see also Supplementary Results). 73.5% (36/49) of the dosing events considered CURATE.AI—it had a limited relevance for the initial dosing decision and complex cases. All (36/36) CURATE.AI dose recommendations were provided on time. Users chose to follow CURATE.AI recommendation for 97.2% (35/36) dosing decisions. Finally, 100% (10/10) patients were prescribed doses with total reduction exceeding 10% compared to the projected standard-of-care, and 80% (8/10) patients adhered to the prescribed doses.
Adverse and unanticipated events
Five patients [CURATE002, 003, 004, 007, 010] experienced grade three toxicities, while no patients experienced grade 4 toxicities. Among the five patients, three patients [CURATE002, 007, 010] experienced grade three toxicities that were assessed as possibly related to capecitabine, such as esophagitis and non-neutropenic fever. Treatment was paused or delayed in these cases, with subsequent resumption.
Discussion
CDSS have shown the potential to enhance the quality of patient care and alleviate the burden of clinical decision-making at the individual patient level, across a spectrum of domains ranging from diagnostics to clinical management25. The onset of AI and big data analytics opened the door to the use of probabilistic modeling and digital twins for personalized treatment decisions, including in oncology26. Of note, CURATE.AI aims to create clinically actionable patient profiles using small data and dynamically generate dose recommendations. Four to five dose-response data pairs were sufficient to generate initial profiles and, in the treatment across three responsive patients, the profiles enabled eleven CURATE.AI dose recommendations, which were all accepted for prescription and provided clinically significant dose changes. The number of cycles needed to generate the profile, however, may also be a limitation of the technology for use in oncology, where several treatment regimens have short progression free-survival durations, precluding sufficient data points to apply CURATE.AI over a long time without the need for recalibration. The small data foundation of CURATE.AI may have played an important role toward enabling CURATE.AI to dynamically adapt to the expected changes in patient response, to allow for the adaptation of treatment in a range of indications with heterogeneous characteristics, and to assimilate unexpected medical events in each patient’s journey. This is particularly relevant to the palliative-intent care population. In our study alone, the ten enrolled patients experienced 29 systemic changes, out of which 19 were assimilated into the CURATE.AI operations.
CURATE.AI is structured to ensure a central role of the user (physician) in each dose selection and our study demonstrated physician adherence to 97.2% (35/36) of CURATE.AI’s recommendations (Supplementary Materials). Physician feedback on the appropriate dosing range for each dosing event was incorporated into CURATE.AI operations. Additionally, the dataset at the foundation of each CURATE.AI dose recommendation was co-created with the physician to ensure the relevance of the data to the specific circumstances of the patient and build trust in the CURATE.AI dose recommendations. Accordingly, collaborative functioning together with awareness, evidence, patient safety, and data were key aspects that defined CURATE.AI adoption by doctors as gauged in a formal, behavioral study27. User-centered design may contribute to realizing the potential of CURATE.AI and other CDSS and their adoption at-scale. A systematic review of higher-level CDSS in oncology concluded that CDSS failed to improve clinical outcome28. Another study identified only sparse evidence that machine learning (ML)-based diagnostic CDSS improved clinical diagnostic performance, as physicians overrode the recommendation in the vast majority of the studies that reported on user feedback29. Human–CDSS interface can be a pivotal element of CDSS design toward implementation, effectiveness, and scale-up.
The toxicity rates in this study (30–50%), within the limitations of the small sample size, were broadly comparable to known toxicity rates for the relevant chemotherapy regimens (~40%)30. The rates of progressive disease at the first standard-of-care computer tomography scan (30%) were comparable to the reported rates (~20–40%) of progressive disease within the first three months of chemotherapy in prior studies31,32. Nonetheless, this study was not designed nor statistically powered to distinguish differences in efficacy and toxicity.
CURATE.AI and this case series are not without limitations. Firstly, most patients that were recruited demonstrated a lack of response to the selected regimens. In future studies, CURATE.AI might be deployed at the later stage, after the patient response to the regimen is confirmed/predicted, e.g., through precision oncology or ex-vivo testing33. Additionally, users’ acceptability of the CURATE.AI recommendations will need to be reconfirmed in a regular clinical setting, with lower human involvement in conveying the data processing results.
Additionally, the use of CEA has its limitations, which include being modulated by other non-relevant factors such as smoking, inflammation, or liver disease, and thus, may introduce error and unreliability within the process34,35. However, CEA’s use is still clinically relevant within a treatment context. The additional logistical benefits include being common, cheap, and accessible. While the use of novel biomarkers, such as circulating tumor DNA, may be interesting, CEA is still a suitable biomarker for use within regular clinical workflow—especially with longitudinal tracking of CEA at a weekly frequency. Feasibility trial-related limitations are further elaborated on in the supplementary discussion.
In conclusion, the patients in this case series received capecitabine at the doses dynamically identified with the assistance of CURATE.AI. CURATE.AI demonstrated high adaptability to clinically-relevant situations encountered by patients, often treated with palliative intent of care. That was possible largely through its small data, longitudinal architecture, and a simplification of the original neural network-based model. The case series further demonstrated high rates of user adherence under a pilot study context, which may be in part due to the high engagement of the physicians in selecting data and boundaries for CURATE.AI operations.
Drawing from the learnings of this feasibility trial, we have assessed that an extension into a future randomized controlled trial can only be performed if key adjustments are made—such modifications include being more specific with patient recruitment criteria, adjusting the CURATE.AI engagement strategy, and logistical measures to reduce hospital visits. New biomarkers will be explored. With a future RCT, we aim to evaluate the effectiveness of a CURATE.AI-assisted treatment strategy compared to the standard-of-care treatment, through assessing patient outcome measures.


Responses