Pharmaceutical and clinical implications of proton pump inhibitors with dual antiplatelet therapies: a systematic review

Introduction
Proton pump inhibitors (PPIs) are widely used drugs for various gastrointestinal conditions, including gastroesophageal reflux disease, prevention of aspirin (ASA) and non-steroidal anti-inflammatory drug (NSAID)-induced gastrointestinal toxicity, peptic ulcer disease, Helicobacter pylori eradication, Zollinger-Ellison syndrome, and functional dyspepsia1. Their gastroprotective effects are often concomitantly employed with P2Y12 receptor inhibitors, such as clopidogrel, prasugrel, and ticagrelor2. Combinations of aspirin and one of the P2Y12 receptor inhibitors, referred to as dual antiplatelet therapy (DAPT), are recommended in the management of cardiovascular diseases, such as acute coronary syndrome, cerebrovascular accidents, or peripheral arterial disease, especially when patients require percutaneous interventions with stenting2. DAPT has been shown to increase the risk of gastrointestinal bleeding, especially within 30 days of initiating therapy, prompting the prescription of gastroprotective agents, like PPIs or histamine-2-receptor antagonists (H2RA)2. In patients with chronic coronary disease, the use of PPI can be effective in reducing the risk of gastrointestinal bleeding3.
Much controversy exists regarding the risks versus benefits of concomitant use of PPIs and DAPT due to concerns that PPIs may reduce the efficacy of DAPT due to shared metabolic pathways4. However, the 2023 European Society of Cardiology (ESC) (class I recommendation, level of evidence B) and the 2023 American College of Cardiology (ACC) guidelines strongly recommend the use of PPIs with DAPT to mitigate the risk of gastrointestinal bleeding, citing no clear evidence that PPIs increase the risk of adverse cardiovascular outcomes3,5,6. Furthermore, newer studies have also implicated PPI use as a risk factor for the onset or progression of cardiovascular disease, which may be counterproductive to DAPT7.
PPIs are membrane-permeable, acid-labile weak bases1. The oral formations are absorbed in the proximal small bowel1. They are packaged in various delivery systems, such as enteric-coated tablets, gelatin capsules, coated granules, as a powder for suspension, or combined with bicarbonate to prevent premature activation and degradation by luminal gastric acid1. Intravenous formulations bypass the gut and provide immediate acid suppression1. Oral PPIs are ingested in a prodrug form and activated through acid-catalyzed cleavage in the acid-secretory canaliculi of the parietal cells into active sulfenic acid or sulfonamide1,6. These compounds reduce acid secretion by covalently binding and inhibiting the hydrogen-potassium adenosine triphosphatase enzyme, also known as the proton pump, on gastric parietal cells until new replacement pumps can be synthesized, a process that takes up to 36 h) (Fig. 1)1,8,9.
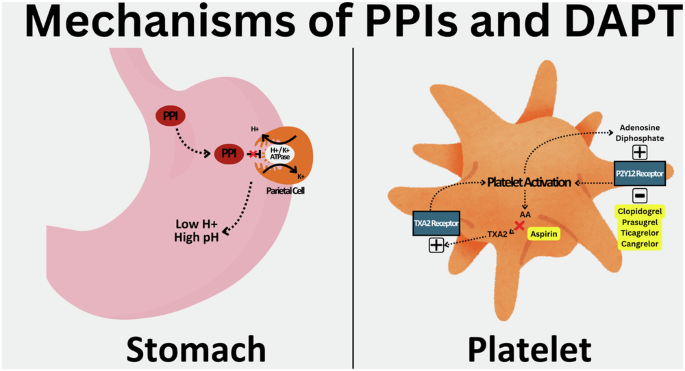
ATPase adenosine triphosphatase, PPI proton pump inhibitor, TXA2 thromboxane A2.
PPIs are highly protein-bound and metabolized by hepatic P450 enzymes1. Omeprazole and esomeprazole are two PPIs that are metabolized by CYP2C19, while rabeprazole, lansoprazole, and dexlansoprazole are metabolized by CYP2C19 and CYP3A4, to a lesser extent1. Pantoprazole is primarily degraded by CYPC19 O-methylation and sulfate conjugation, which results in the lowest potential for cytochrome induction or inhibition1. Clinical responses to different PPIs can be attributed to genetic variations in CYP219 enzymes1,6. Elderly populations and those of Asian descent typically have increased bioavailability of PPIs due to alterations in CYP2C19 metabolism and, therefore, require close attention to dosing10. Most PPIs are renally excreted following metabolism, though lansoprazole and dexlansoprazole are also excreted via the biliary tree1. The serum half-life of single-release PPIs is only 1 to 2 h, although extended-release PPIs may be on the horizon1.
Dual antiplatelet is comprised of aspirin and one drug from the PY212-receptor inhibitor class11. Low-dose aspirin inhibits the thromboxane A2 pathway through irreversible acetylation of the cyclooxygenase 1 (COX1) enzyme, which blocks arterial vasoconstriction and platelet activation, preventing thrombosis (Fig. 1)11. It also irreversibly inhibits the cyclooxygenase 2 (COX2) enzyme, which has an anti-inflammatory effect, as well as inhibition of prostaglandin formation, which has a pro-inflammatory effect, but to a lesser degree. The optimal dosing of aspirin is between 75 and 100 mg for maximal antithrombotic effect and a low risk of bleeding, with the effects lasting the lifespan of a platelet (8-10 days)11.
P2Y12-receptor antagonism is the complement to aspirin in DAPT. P2Y12 is the predominant receptor involved in adenosine diphosphate-stimulated platelet activation of the glycoprotein IIb/IIIa receptor, leading to platelet degranulation, thromboxane production, and platelet aggregation (Fig. 1)12. Three oral agents (clopidogrel, prasugrel, ticagrelor) and one IV agent (cangrelor) are available in this class11. Clopidogrel and prasugrel are prodrugs that require metabolism by the cytochrome P450 enzymes to become active11. Prasugrel has a quicker onset of action and is more potent than clopidogrel11. It is contraindicated in patients with a history of previous cerebrovascular accidents or transient ischemic attacks11. Ticagrelor is ingested in its active form and is an irreversible P2Y12 inhibitor with potency and onset of action akin to prasugrel11. Cangrelor reversibly inhibits the P2Y12 receptor with potent platelet inhibition within minutes of intravenous administration11. It also has a rapid offset within 1 h for platelet aggregation to improve to baseline levels11. As seen with PPIs, genetic variations in the CYP2C19 and CYP3A4 genes convey variable efficacy for these drugs, with clopidogrel primarily affected and ticagrelor affected to a lesser extent11,13. Prasugrel is unaffected by genetic pleomorphisms11,13.
This comprehensive systematic review aims to provide an in-depth analysis of the interactions between PPIs and dual antiplatelet agents, shedding light on their mechanisms and clinical implications and suggesting areas for treatment optimization. It will also address recent advancements in personalized medicine and the potential role of genetic factors in influencing individual responses to PPIs and antiplatelet drugs to optimize treatment outcomes and minimize adverse effects.
Results
Four hundred and twenty-one papers were reviewed, and 62 met the inclusion criteria (Fig. 2). Eighteen of the 62 papers evaluated potential pharmaceutical interactions between PPIs and DAPT, and 44 assessed the clinical implications of concomitant therapy. A brief overview of the included studies can be found in Tables 1 and 2.
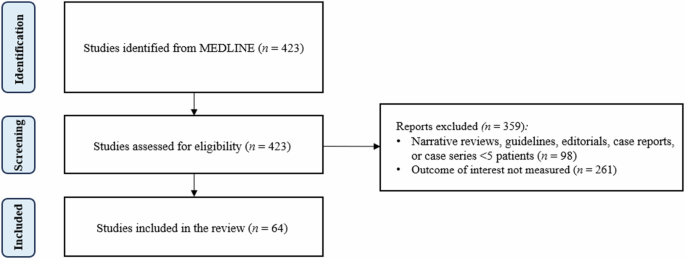
A PRISMA flow diagram of study selection.
Drug-drug interactions of PPIs and DAPT
As there are no known shared metabolic pathways between PPIs and aspirin, only one study has been performed over the past ten years to investigate potential drug-drug interactions. This prospective case-control study by Ozel et al. in 2023 found no statistically significant change in the antiaggregant activity of aspirin between 75 patients who were administered lansoprazole plus aspirin and 67 patients who only received aspirin14. This finding suggests that PPIs likely do not suppress the effects of the aspirin component of DAPT therapy.
On the other hand, the shared metabolic pathways between PPIs and P2Y12 receptor inhibitors have generated many studies to examine the potential drug-drug interactions (Fig. 3). Many of these studies assessed the antiplatelet aggregation activity of individual P2Y12 receptor inhibitors in the presence of PPI therapy with mixed results. Others have investigated the effect of CYP2C19 polymorphisms on P2Y12 receptor inhibitors and PPI metabolism.
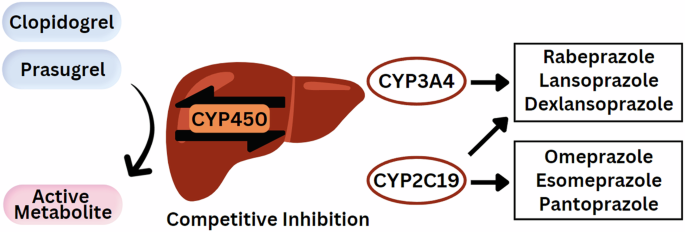
Degradation of proton pump inhibitors by the CYP450 hepatic enzyme system competitively inhibits the activation of the clopidogrel and prasugrel prodrugs.
In vitro studies by Nagata et al. and Tsantes et al. in 2013 measured the adenosine diphosphate-induced platelet aggregation index (ADP-PATI) via ADP-light transmittance aggregometry, INNOVANCE PFA-100 P2Y, flow-cytometric vasodilator-stimulated phosphoprotein (VASP)-phosphorylation assay, and multiple electrode aggregometry in patients with coronary artery disease on chronic clopidogrel and aspirin therapy with and without concomitant PPI15,16. PPI co-administration did not alter the antiplatelet effect of clopidogrel on all laboratory testing modalities15,16. Conversely, Arbel et al. in 2013 found patients prescribed clopidogrel and aspirin therapy plus either omeprazole or pantoprazole to be associated with a higher on-treatment high platelet reactivity (HPR), an independent risk factor for stent thrombosis and acute coronary syndrome due to an increased platelet activation and aggregation at sites of plaque rupture17. Furthermore, CYP2C19 polymorphisms do not alter residual platelet reactivity levels; however, one study suggests the CYP2C19*2 genotype may contribute to higher reactivity16,18.
Prospective studies like Brendel et al. in 2013, Martinez-Quintana et al. in 2014, and Liu et al. in 2016 have also corroborated the findings that PPIs likely do not interfere with the platelet inhibitory effects of DAPT and do not translate to increased risk of new acute coronary syndrome or disease relapse19,20,21. Interestingly, a randomized, crossover study by Andersson et al. in 2014 found the active metabolite of clopidogrel and the ADP-PATI to be reduced by up to 50 and 17%, respectively, and when combined with esomeprazole, omeprazole, or lansoprazole; however, these reductions were insignificant in the presence of aspirin, suggesting that the antiplatelet effect of PPI on P2Y12 receptor inhibitors may be nullified by concomitant aspirin therapy22. Along these lines, Collet et al. in 2014 demonstrated that higher doses of clopidogrel do not overcome the antiplatelet inhibition of PPIs, but transitioning to alternative P2Y12 receptor antagonists like prasugrel does mitigate this effect23. Nicolau et al. in 2015 found that patients who underwent percutaneous coronary revascularization on prasugrel and aspirin exhibited lower median on-treatment HPR and subsequently lower rates of acute coronary syndrome compared to clopidogrel24. These findings suggest that alternative P2Y12 receptor antagonists like prasugrel may be more pharmacodynamically beneficial in patients who require concomitant PPI therapy compared to clopidogrel.
Few studies have investigated how the choice, timing, and dosing of PPIs affect the antiplatelet effects of DAPT. Nii et al., in 2019, found lansoprazole to produce more antiplatelet inhibition compared to esomeprazole, contradictory to what is suspected, as lansoprazole should provide less inhibition of platelet aggregation compared to esomeprazole due to its partial metabolism by CYP3A425. In 2016, Karlsson et al. found pantoprazole to have a dose-dependent antiplatelet inhibition compared to controls26. Furuta et al. in 2017 compared the effects of dose timing of omeprazole, esomeprazole, lansoprazole, and rabeprazole with respect to clopidogrel and aspirin consumption on platelet aggregation27. Mean platelet aggregation inhibition was significantly decreased with all four PPIs compared to clopidogrel alone, regardless of morning or evening PPI administration27. Interestingly, however, administration of rabeprazole 4 h following clopidogrel produced results similar to clopidogrel alone in the morning, suggesting that the timing of rabeprazole administration might minimize potential drug-drug interactions27.
The use of histamine-2-receptor antagonists (H2RA) instead of PPIs may help avoid any potential drug-drug interactions between PPIs and DAPT. Several studies by Furtado et al. in 2016, Harvey et al. in 2016, Choi et al. in 2017, and Wu et al. in 2018 compared the effect of H2RAs, specifically ranitidine, against PPIs on the antiplatelet effects of DAPT28,29. As suspected, ranitidine did not alter platelet aggregation, on-treatment HPR, or CYP2C19 enzyme activity, compared to significant changes by omeprazole28,29. Interestingly, pantoprazole produced equivocal findings to ranitidine, suggesting pantoprazole should be preferred over omeprazole if PPIs are necessary30,31.
Clinical implications of combined PPI and DAPT
Despite demonstrating potential pharmacokinetic and pharmacodynamic effects on DAPT’s antiplatelet functions, many studies have suggested that these effects may not translate to adverse clinical outcomes32,33. Overall, co-prescription of DAPT and PPIs have been associated with reduced rates of major adverse cardiovascular and cerebrovascular events, including acute coronary syndrome, stent restenosis or thrombus, hemorrhagic or thromboembolic events, cardiovascular disease-related mortality (Fig. 4)32,33. All-cause death was also unchanged with or without concurrent PPI therapy34,35,36. A minimal number of studies suggest the alternative37,38. Unsurprisingly, the concomitant use of PPIs with DAPT consistently demonstrates significant reductions in the rate of gastrointestinal bleeding due to DAPT, even in the presence of high-dose aspirin36,38,39,40,41,42.
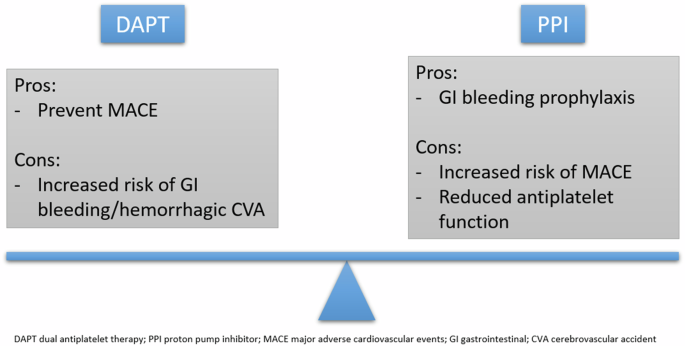
Balancing the pros and cons of DAPT with concomitant PPI therapy remains a significant challenge to clinicians to minimize adverse cardiovascular events and significant bleeding.
Perhaps the most notable trial that investigated PPIs with DAPT was the Clopidogrel and the Optimization of Gastrointestinal Events Trial (COGENT) by Bhatt et al. in 201043. The study involved 3,873 patients on clopidogrel and aspirin who were randomized to receive omeprazole or placebo43. The researchers demonstrated the prophylactic use of omeprazole to reduce the rate of upper gastrointestinal bleeding (hazard ratio (HR) 0.13, 95% confidence interval (CI) 0.03-0.56, P = 0.001) without significantly increasing the rates of cardiovascular events (HR 0.99, 95% CI 0.68–1.44, P = 0.96)43. A subgroup analysis of the COGENT trial by Vaduganathan et al. in 2016 found omeprazole to produce similar results even in high-risk cardiovascular subsets44.
Another large randomized controlled trial called the PRODIGY trial by Gargiulo et al. in 2016 involved 1970 patients who were randomized to 6- or 24-month DAPT with clopidogrel and aspirin at 30 days from index percutaneous coronary intervention (PCI)34. Seven hundred and thirty-eight patients were PPI users, with lansoprazole as the most common PPI at 90.1%34. Overall, there were no net clinical adverse events between PPI users and no PPI (12.9 vs 14.9%, adjusted HR 0.99, 95% CI 0.772–1.268, P = 0.93)34. There were no differences in composite all-cause death, myocardial infarction, and cerebrovascular accidents (9.2 vs. 11.5%, adjusted HR 1.051, 95% CI 0.788-1.400, P = 0.736) or bleeding rates (adjusted HR 0.996, 95% CI 0.672–1.474, P = 0.980)34. This study further justifies the safety of PPIs, especially lansoprazole, in combination with DAPT.
While clopidogrel and aspirin remain one of the most prescribed DAPT combinations, other studies have investigated the outcomes associated with PPIs and other P2Y12 agents. A subgroup analysis of the GLOBAL LEADERS trial by45 found that PPIs do not increase the rates of adverse cardiovascular events when combined with ticagrelor45. In 2020, Hagiwara et al. retrospectively found prasugrel to be associated with higher rates of hemorrhagic events compared to clopidogrel despite concomitant PPI therapy46.
While most studies aimed to evaluate the implications of concurrent PPI and DAPT therapy on major adverse cardiovascular events (MACE), some focused on other gastrointestinal outcomes. In 2015, Vardi et al. found that co-prescription of PPI and DAPT lowers mean pain intensity scores and non-pain symptoms related to dyspepsia47. Ahn et al. in 2014 and Hara et al. in 2018 found that PPIs reduce the rate of peptic ulcerations and small bowel mucosal injuries in patients on DAPT with clopidogrel and aspirin, respectively48,49. These studies further corroborate the already well-established prophylactic effects of PPIs on the gastrointestinal tract, even when co-prescribed with DAPT.
As seen in the studies investigating the pharmacokinetic and pharmacodynamic effects of PPIs on DAPT, attention has been paid to the choice of PPIs and their relation to adverse clinical outcomes when prescribed with DAPT. Studies by Ayub et al. in 2016 and Leonard in 2015 found that all PPIs produce similar rates of adverse cardiovascular events, strokes, and gastrointestinal bleeding in patients on DAPT50,51. However, in a systematic review with meta-analysis in 2022, Luo et al. also found esomeprazole to increase the risk of MACE compared to non-PPI; however, there were no significant differences in net clinical adverse events, all-cause mortality, or cardiac death between the two groups52. Ruiz et al. in 2018 found that only full adherence to any PPI therapy is effective at reducing the risk of gastrointestinal bleeding, suggesting that as-needed PPI or low-dose PPI should be avoided in patients on DAPT53. Interestingly, in 2022, Zhou et al. found that early initiation of PPI before discharge in patients who suffered acute coronary syndrome was associated with increased rates of gastrointestinal bleeding; however, further studies are needed to corroborate this finding54.
Identifying patients on DAPT who would benefit from or be harmed by concomitant PPI remains a significant challenge for clinicians55,56. Distinguishing risk factors that elevate an individual’s risk of gastrointestinal bleeding or DAPT failure should be prioritized. In 2021, Shi et al. found that PPI use in Asian patients was associated with lower rates of adverse clinical outcomes compared to use in Caucasian patients, suggesting that PPIs may be more beneficial in this population57. Advanced age, use of mechanical ventilation, tobacco users, renal insufficiency, or those with a history of peptic ulcer disease, Helicobacter pylori infections, or previous gastrointestinal bleeding also demonstrate a higher risk of gastrointestinal bleeding with DAPT and would benefit from PPI therapy58,59,60.
Clinicians also face the challenge of encouraging compliance with DAPT therapy to prevent the onset or recurrence of adverse cardiovascular events. PPI use has been associated with decreased rates of disruptions in DAPT adherence, as the most common reason for discontinuation is gastrointestinal bleeding61,62. Bleeding during DAPT has been associated with a significantly greater risk for all-cause mortality, stressing the need for co-prescription of gastroprotective agents like PPIs61,63.
Epidemiological studies have examined how the prescribing trend of PPI and DAPT has been affected by their surrounding controversy, especially following the United States Food and Drug Administration (FDA) and the European Medicines Agency advisory statements in 2010 recommending avoidance of concurrent omeprazole and clopidogrel therapy64,65. In 2018, a Cerner Health Facts® database study by Farhat et al. found that the prevalence of concurrent PPI and DAPT prescriptions in the United States steadily declined from 34.9% in 2008 to 16.4% in 201066. Concurrent omeprazole or esomeprazole use with DAPT continued to decrease to 0.8% by 201666. In 2014, a pharmacy database study by Kashour et al. found a statistically significant decrease in omeprazole prescriptions and increased ranitidine prescriptions with concurrent clopidogrel between May 2009 and May 2010, in response to the FDA advisory statement63. At that time, many patients were also taken off omeprazole with an active clopidogrel prescription, and a smaller number were transitioned from omeprazole to ranitidine63. The use of alternative PPIs also varies64. Despite this, the PPI re-prescription rate following a bleeding event within the first year of DAPT has been high67.
Discussion
In response to the mixed results demonstrated above, the American College of Cardiology (ACC) issued updated guidelines in 2020 (Table 3)5. It recognized the concern for reduced clopidogrel efficacy with concomitant PPI use, particularly omeprazole, while addressing the need for gastroprotective agents to mitigate the risk of gastrointestinal bleeding due to DAPT5. However, as these concerns have not correlated to increased adverse clinical outcomes, the ACC recommends initiating or continuing a PPI, including omeprazole, and avoiding other non-steroidal anti-inflammatory drugs when two or more antithrombotic agents are employed5. They also recommend clinicians have a low threshold for discontinuing the PPI if the regimen shifts towards oral anticoagulant monotherapy unless PPIs are prescribed for other indications5. In patients with chronic coronary disease treated with PCI, the current guidelines recommend treatment with DAPT for six months post-intervention, followed by single antiplatelet therapy to reduce major adverse cardiovascular and bleeding events3. Furthermore, select patients with chronic coronary disease treated with PCI who have completed a 1- to 3-month course of DAPT can be de-escalated to P2Y12 receptor monotherapy for at least 12 months to reduce bleeding risk3.
The European Society of Cardiology (ESC) takes a similar but more liberal approach to PPI use than the ACC (Table 3)6. In their updated 2023 guidelines for the management of acute coronary syndromes, the ESC acknowledges that PPIs inhibit CYP2C19, especially omeprazole and esomeprazole, and may reduce the pharmacokinetic response to clopidogrel6. However, as with the ACC, they recognize that no solid clinical evidence demonstrates an increased risk of adverse clinical outcomes6. They also note no interactions between PPIs and aspirin, prasugrel, or ticagrelor have been observed6. As such, concurrent PPI therapy is recommended with any anti-thrombotic regimen in patients at high risk for gastrointestinal bleeding, as classified by the Academic Research Consortium for High Bleeding Risk (Table 4)6,68. Patients with two or more of the following also should be prescribed PPI: 1) age ≥65 years; 2) dyspepsia; 3) gastroesophageal reflux disease; 4) helicobacter pylori infection; and/or 5) chronic alcohol use6.
As of February 2024, the American College of Gastroenterology and the Canadian Association of Gastroenterology have yet to offer explicit guidelines for using PPIs with concurrent DAPT64. These societies recognize the increased risk of gastrointestinal bleeding associated with antiplatelet medications in their 2022 guidelines on managing anticoagulants and antiplatelets during acute gastrointestinal bleeding and the peri-endoscopic period, but they do not offer prophylactic recommendations69.
The increase in gastrointestinal bleeding associated with DAPT is widely evident in literature, and the importance of the adjunctive use of gastroprotective agents is stressed. PPIs have demonstrated consistent superiority in preventing gastrointestinal bleeding due to DAPT without affecting the antiplatelet activity of aspirin alone. Studies have found potential interactions between PPIs and clopidogrel. Prasugrel or ticagrelor seem to avoid interaction with PPIs; however, their higher potency compared to clopidogrel should preclude their use in DAPT in patients with a high risk for bleeding.
Evidence from the Antithrombotic Trialists’ Collaboration meta-analysis, CURE trial, and CAPRIE study indicates that aspirin is likely the main contributor to gastrointestinal bleeding in DAPT, albeit synergistically increased when combined with clopidogrel70,71,72,73. Aspirin-induced gastrointestinal bleeding likely results from a combination of direct mucosal damage and the systemic inhibition of protective prostaglandins due to COX-1 inhibition, compounded by its antiplatelet effects74,75,76. As such, the current ACC guidelines recommend de-escalation from DAPT to single antiplatelet therapy in patients with a high risk of gastrointestinal bleeding following 1- to 6-months of therapy following PCI, which may mitigate the risk of bleeding but also may predispose patients to adverse coronary events. Therefore, P2Y12 inhibitor monotherapy may be preferred in patients requiring long-term antiplatelet monotherapy who also have a high bleeding risk due to a potentially higher risk of gastrointestinal bleeding with aspirin; however, further studies are needed to directly compare bleeding outcomes of different antiplatelet monotherapy regimens in high-bleeding risk populations. Per the guidelines, PPI may not be necessary with antiplatelet monotherapy, especially if clopidogrel is the utilized agent.
One study above suggested aspirin may ameliorate the antiplatelet-suppressing impact of PPIs on P2Y12 receptor inhibitors, which could explain any increased MACE following de-escalation. Like with DAPT, studies have demonstrated a pharmacodynamic interaction between PPIs and clopidogrel monotherapy. However, information on the risk of major adverse cardiovascular or cerebrovascular events is conflicting and deserves more investigation77,78. Overall, the cardiovascular benefits of PPI with DAPT or P2Y12 receptor inhibitor monotherapy likely outweigh the risks of gastrointestinal bleeding.
With the popularity of omeprazole with DAPT declining, more attention to the pharmacodynamics and clinical outcomes of alternative PPIs have garnered interest. Studies have suggested that pantoprazole or lansoprazole could provide a better balance of bleeding prevention to P2Y12 receptor antagonist competition due to less of a CYP2C19 inhibitory effect than omeprazole or esomeprazole. Further prescribing consideration could be given to patients with specific CYP219 polymorphisms for a personalized medicine approach. The Clinical Pharmacogenetics Implementation Consortium provides guidelines from 2020 for adjusting PPI doses based on CYP2C19 genotype, such that CYP2C19 poor metabolizers should receive a reduced PPI dose to avoid potential toxicity and rapid or ultrarapid metabolizer hours should receive higher PPI doses due to potentially reduce efficacy (moderate levels of evidence)79. Dose adjustments for DAPT, particularly clopidogrel, in patients with alterations in CYP2C19 metabolism may also be warranted, such that poor metabolizers likely require quadrupling or doubling dosages in poor or intermittent metabolizers, respectively80,81,82. Prasugrel or ticagrelor may be more effective in these patients, as these agents do not rely on CYP2C19 for activation and provide more consistent platelet inhibition83. However, more research is needed to correlate clinical outcomes to varying combinations and dosing of PPIs and DAPT.
The ACC and ESC do not offer specific guidelines on alternative gastroprotective agents, such as H2RAs. However, they could be considered if clinicians wish to avoid any potential drug-drug interactions or adverse clinical outcomes associated with PPIs and DAPT. Studies comparing H2RAs and PPIs in patients on DAPT have demonstrated mixed results for cardiovascular and gastrointestinal bleeding outcomes, necessitating further research into them as possible alternatives for PPIs84,85.
In conclusion, the interaction between PPIs and DAPT presents a complex scenario with possible adverse clinically significant implications; however, the cardiovascular benefits appear to outweigh gastrointestinal bleeding risks in recent literature. Clinicians should carefully weigh the risks and benefits of their co-prescription, consider alternative gastroprotective strategies when appropriate, and be vigilant in de-prescribing DAPT or PPIs as indicated, relying on current guidelines, when applicable. Further research is warranted to elucidate the optimal management strategies for this clinically meaningful drug-drug interaction.
Methods
To identify relevant studies, a comprehensive search of the Pubmed/MEDLINE database was conducted to retrieve relevant articles from December 2013 to December 2023. A combination of Medical Subject Headings (MeSH) terms and text words related to PPIs and antiplatelet therapy were used. MeSH terms included “proton pump inhibitors,” “dual anti-platelet inhibitors,” “triple anti-platelet therapy,” “platelet aggregation inhibitors,” “aspirin,” “combination drug therapy,” “factor xa inhibitors,” “direct-acting anticoagulant,” and “vitamin K antagonist.” Only English-language literature involving human subjects was included, and a range of study types, including prospective cohort studies, experimental studies, population studies, meta-analyses, retrospective cohort studies, clinical trials, and observational studies, were eligible for inclusion. Only studies involving PPIs and DAPT, aspirin, or P2Y12 receptor inhibitors were included. After screening and data extraction by one reviewer (Fig. 1), the authors conducted a narrative synthesis of the studies.

Responses