Photovoltaic bioelectronics merging biology with new generation semiconductors and light in biophotovoltaics photobiomodulation and biosensing
Introduction
Understanding the interaction between light and biological materials has garnered significant interest due to its diverse applications in fields like medicine, biotechnology, biosensing, and bioelectronics. Light can stimulate cells either directly through naturally occurring opto-responsive proteins, such as opsins, or indirectly via non-biological materials, including thin films and nanoparticles (NPs). This interaction can be used to elicit cellular responses and has been foundational to the development of medical technologies like microscopes and X-rays. In particular, direct light stimulation can affect various tissues beyond the eye, such as the skin, blood vessels, and brain, through processes like melanogenesis, photorelaxation, and circadian rhythm regulation.
Semiconductors, being sensitive to light, can work as transducers for optical stimulation of biological entities, such as biomolecules, cells or tissues. Their properties can enable light-mediated stimulation, sensing, monitoring, and control of living systems. Currently, among semiconductors, silicon stands out as the most established technology for bioelectronics with notable applications such as photostimulation of neurons in mice1, artificial retina implantation in both humans2,3 (with clinical approval already achieved4) and animal models5 and implantable microdevice for deep tissue neuromodulation and measurement6. However, its inherent stiffness limits its widespread applicability, especially where flexibility is a requirement.
New generation semiconductors such as organic semiconductors (OSCs) share the advantages of traditional semiconductors in terms of electrical interaction with light, and polymer materials in terms of flexibility, while also offering advantages in the biological realm. OSCs show the unique capability to operate in biological aqueous electrolyte solutions conducting both electrons and ions7,8 thus bridging the typical transport mechanisms of biological systems, based on ionic transport, with the electron-based conduction of standard electronic devices. These advantages have led to their use in novel applications, including implantable artificial retinas in various animal models9,10, light stimulation of biological cells with OSC platforms11,12,13 and light-based biosensing applications14,15. OSCs, in their polymer form, can be deposited as films or even in small pixels with techniques such as ink jet printing and interfaced with biological systems16. Polymers have also been used in NP form, which are directly injectable either on the surface or inside living cells or tissues. Applications include retinas of mice17, light modulation of biological cells18 and biosensing and bio-detection (e.g. molecules, whole bacteria)19,20,21. Dye-sensitized solar cells (DSSCs), with their high sensitivity and electrical autonomy, are particularly promising for light-mediated biosensing applications22,23. Furthermore, a modified version of the DSSC architecture was the inspiration for developing organic semiconductor-based artificial retina devices16. Drawing inspiration from the human retina’s color perception and neuromorphic processing, even perovskite semiconductors were used for fabricating a narrowband power-free panchromatic imaging sensors based on R/G/B photodetectors without the need for complex optical filters24, or even R/G/B with the addition of either a broadband white perovskite sensor25 or a UV one26, and hemispherical nanowire array for biomimetic photosensing27,28. Although their stability in aqueous biological environments29,30,31, and potential toxicity remain challenging for widespread applicability and implantation, perovskites are known for their high efficiency in optoelectronics, offer exciting opportunities for future advancements in bio-photovoltaics and biosensing applications32,33,34.
This review summarizes, as schematized in Fig. 1, the recent research progress of the new generation semiconductor photovoltaics (PV) in (i) biomaterial incorporated photovoltaics, (ii) light mediated bio-applications, iii) light-based biosensing applications, and (iv) artificial retina devices. Differently from previous reviews on biosensors that predominantly concentrated on a specific type of semiconductor35,36,37 or on a single biosensing application38,39, our focus herein is on the light-mediated bio-applications of new generation semiconductors, i.e. OSCs, DSSCs, and perovskites. We explore the integration of biomaterials into these device stacks for improved performance, modulation, and potential use in vision applications, including artificial retinas.
The interaction of light with biomaterials and new generation semiconductors is a dynamic field of research with numerous applications in various domains, such as biosensing, bio-photovoltaics, and biomedicine.
We begin the review by providing an overview of the field of photovoltaic bioelectronics, followed by a discussion on the incorporation of biomaterials into various photovoltaic technologies including OSCs, perovskites, and DSSCs, aimed at enhancing or modulating the current-voltage performance under illumination. In biophotovoltaics, the incorporated biomaterials are mostly inert, so we explore the basics of interaction between light, biological systems and new generation semiconductors. This section specifically spotlights the use of light for sensing and photo-stimulation in biological systems, focusing on interaction and transduction mechanisms of OSCs with biological materials, along with testing approaches, biocompatibility, and illumination schemes. Additionally, we also examine the photobiomodulation of behavior of living systems with OSCs. Furthermore, we explore biosensing applications of new generation semiconductors (OSCs, perovskites, and DSSCs) in light based biosensing. The subsequent section delves into the application of new generation semiconductors particularly OSCs, and perovskites, in the field of artificial retinas and vision applications, before finishing with the outlook and conclusions.
Overview
Bioelectronics is the field focused on developing electronic interfaces designed to monitor or regulate biological processes40. Photovoltaic bioelectronics, where biology, semiconductor technology and current/voltage responses to light come together, holds transformative potential not only in the research and development arena but also for future clinical applications. Bio-integrated PV devices, such as self-powered implantable biosensors, offer new approaches to healthcare, including continuous health monitoring powered by harvesting light, as well as advanced therapies that rely on non-invasive light as trigger or modulation. In fact, implantable biosensors powered by photovoltaic cells could harness ambient light41 or body heat to continuously monitor vital metrics, such as glucose levels in diabetic patients or cardiac activity, eliminating the need for invasive procedures or battery replacements42. These devices could enable real-time feedback and early medical intervention, significantly enhancing patient care43. Additionally, light-mediated bio-response modulation presents opportunities for precise drug delivery and non-invasive therapies. Photovoltaic systems can be engineered to trigger targeted drug release in response to specific wavelengths of light, providing localized treatment with reduced systemic side effects, particularly in oncology44. Similarly, optogenetics-based neuromodulation, where light-responsive materials interact with neural tissues to control neuronal activity, could lead to innovative treatments for conditions like epilepsy or chronic pain45, without the need for invasive electrodes46.
A prime example of PV bioelectronics is represented by devices that aim to mimic, restore or augment vision. Various implantable systems have been developed for clinical studies in humans, including Argus II47, Alpha IMS48, PRIMA bionic system49, EPIRET350, IRIS V251, Suprachoroidal prosthesis52, and Subretinal prosthesis51. These implants aim to restore partial vision in patients with end-stage disease who are either completely blind or have light perception without the ability to localize it. Most of these devices include a light-capturing component, either an external camera or an intraocular photodiode array, which communicates to an electrode array to stimulate retinal neurons, primarily in the inner retina. By electrically activating the remaining neurons, the implants create a visual perception, partially substituting the lost photoreceptor function with artificial vision48. PRIMA bionic system49 and Alpha IMS48 stand out as using silicon for the photodiode arrays in one of the implants. The biocompatibility, electrical safety and targeted location of the artificial silicon retina within the subretinal space has been reported in animal studies3,53,54,55. Subsequently, the safety of a microphotodiode array implants has also been shown in human clinical trials2,56. OSCs, on the other hand, have been implanted in animals such as rats57 only. POLYRETINA consisting of 10,498 photovoltaic pixels with active components made of OSCs on a polydimethylsiloxane PDMS substrate distributed over an active area of 13 mm in diameter58 has been implanted in Göttingen minipigs. These devices have restored light-evoked cortical responses in blind animals at safe irradiance levels, indicating that these OSC materials hold the potential for achieving artificial vision in totally blind patients affected by retinitis pigmentosa in the future. More recently, OSCs in the form of polymer NP, have been subretinally injected into blind mice retinas (sometimes referred to as liquid retina prothesis) restoring visual activities for at least 8 months after single injection17,59. These innovations have the potential to deliver safer solutions, not requiring invasive surgical procedures. In the arena of vision, perovskite semiconductors have been used to develop narrowband power-free panchromatic image sensors24,25,26 and hemispherical retinas made of a high-density array of nanowires mimicking the photoreceptors on a human retina27,28. Rather than for restoring vision via implantation or injection, due to their current poor stability and cytotoxicity, this exciting perovskite research has focused instead on advanced biomimetic applications28.
Such advancements underscore the huge potential of photovoltaic bioelectronics to revolutionize diagnostics, continuous monitoring, and therapeutic interventions. However, as clearly apparent from the artificial retina examples, several challenges must be addressed for its broader adoption, particularly in understanding the interactions between new-generation semiconductors and biological materials. Ensuring long-term biocompatibility and stability, especially during in vivo testing60, is critical for developing reliable systems. Biocompatibility will confirm the ability of the device to perform its desired function when in contact with biological cells/tissues without producing any adverse effects on the latter. Conducting in vitro biocompatibility assessments on semiconductor surfaces is crucial for understanding cytotoxicity and the possibility of safe in vivo applications. Biocompatibility tests on silicon substrates with various in vitro cell cultures, such as C2C12 mouse myoblasts61, rat neuronal cells (B50)62, and mouse fibroblasts (SC-1)63, have shown that cell viability remains above 80%61 on the semiconductor surface even after 7 days, confirming the non-cytotoxic nature of silicon63. The biocompatibility of OSCs, using primary human retinal cell cultures by systematic measurement of both cell viability and morphological analysis of retinal ganglion cell neurite elongation over time, was investigated by Sherwood et al.64. Six materials were deemed promising candidates for novel retinal prostheses. The authors note that the vast majority of studies examining biocompatibility use animal tissue rather than human tissue given the difficulty of obtaining human samples. In addition, cell survival and death mechanisms of neurons are different to those of non-neuronal cell types65. Cell death and cell survival mechanisms can even vary between different types of neurons66, and selection of a biological tissue source which matches the target tissue of the proposed therapeutic application is critical for its effectiveness. Subretinal injections of polymer NPs in rat models did not trigger trophic or pro-inflammatory responses, further demonstrating the biocompatibility of polymer-based NPs17. Cytotoxicity studies on perovskites have been carried out using human lung adenocarcinoma epithelial cells (A549), SH-SY5Y neuroblastoma cells, and murine hippocampal neurons67. However, methylammonium lead iodide (MAPbI3) perovskites exhibited dose- and time-dependent toxicity in both SH-SY5Y and hippocampal neurons, causing plasma membrane damage and inducing cell death.
Even if the semiconductor shows in vitro biocompatibility, during in vivo testing interface between rigid semiconductor surfaces and soft biological tissues often creates mechanical mismatches, leading to fibrotic scarring and the formation of physical barriers that impair device functionality68. In case of OSCs, even though the semiconductor is soft, mechanical mismatches with the biological tissue can occur which can lead to adverse fibrotic scarring depending on the nature of the substrate10. To mitigate these effects while maintaining device functionality, advanced materials and design strategies are essential, with an emphasis on flexibility, bioinertness, softness, biocompatible substrates, and surface modifications that can enhance biocompatibility and reduce the risk of chronic immune reactions69. In addition to biocompatibility concerns of the semiconductor, the long-term stability of implanted devices in biological environments poses another significant barrier to the commercialization of new-generation semiconductors for in vivo applications.
Table 1 compares the properties of biotic living tissues/cells and the semiconductors they interface with, subject of this review: OSCs (in both film and NP form), perovskite, DSSCs, also silicon for comparison. The table clearly shows that certain physical properties, such as physical state, mechanical, and charge carriers types in OSC, more closely resemble those of biotic living tissues and cells compared to conventional semiconductors such as silicon. For example, Young’s modulus is many orders of magnitude smaller in OSCs compared to silicon, coming closer to that of living systems. OSCs are also able to conduct ions and their morphology can be textured to increase surface area and the adhesion/interaction with biological systems, offering distinct advantages over other semiconductors. DSSCs are especially apt at conducting both ions and charge carriers at their liquid/solid interfaces with high surface area resulting from the mesoporous nature of one of their key layers. Furthermore, they are able to operate in aqueous environment, even though stability over time should be investigated further. Although sharing similar fabrication processes to those of OSCs, perovskites have advantages related to the more efficient conversion of incident optical to electrical power (i.e. in performance). However, perovskites currently possess severe limitations due to the toxicity of their main formulations and lack of stability in aqueous media: these issues should be addressed, developing less toxic and more stable formulations in the future to unlock the potential of these new semiconductors for photovoltaic bioelectronics applications in vivo. Table 1 also provides some avenues for improving biocompatibility as well as the maturity and range of applications that have been developed for the different semiconductor devices as already highlighted at the beginning of this section.
Biophotovoltaics
Biomaterials in organic photovoltaics
The inherent chemical resemblance between OSCs and natural biological materials and compounds, owing to their shared conjugated carbon structures, underscores the potential for integrating biomaterials into OSC-based devices and materials for biomedical applications. This resemblance to biological molecules not only enhances the compatibility and versatility of OSCs but also unlocks new pathways for innovative biomedical and photovoltaic device applications70,71. The unique printability of OSCs, coupled with their ability to be finely tuned for optoelectronic properties through chemical design72 further strengthens their potential in these diverse applications.
Researchers are actively looking for bio-derived materials in the photovoltaic stack that can replace conventional materials to modulate and even improve the photo response of organic photovoltaics (OPV)73,74,75,76,77,78,79,80,81,82. Millions of years of evolution of living organisms have led to the design, synthesis, and utilization of materials with unique optoelectronic properties83. Biomaterials are naturally occurring84 and hence the incorporation of these materials in OPV can lead to more environmentally friendly devices. While the primary objective of this research has focused on identifying natural alternatives to synthetic materials in PV devices, the significance of studies in this domain extends far beyond. Through these investigations, valuable insights have emerged regarding the integration of biological materials with OSCs and the development of biohybrid devices aimed at modulating the photo response of photodiodes. Such advancements are fundamental for biosensing applications, underscoring the multifaceted impact of this research field. Plants, animals, and micro-organisms, are the main source of biomaterials that have been incorporated either in the active layer, charge transporting layers, substrates, light trapping layers, or electrodes of OPV85 as summarized in Table 2.
The biomaterials that are being utilized in the bulk of the active layer of OPV are proteins86,87,88,89,90,91,92,93,94, DNA95 and natural dyes96,97. Photosystem I (PSI) of the photosynthetic process that is able to convert photons into electrons with a nearly 100% quantum efficiency98, is stable and abundant and can be easily isolated and incorporated into the OPV device architecture as demonstrated by Das et al.79. PSI complex extracted from young spinach leaves was spin coated onto indium tin oxide (ITO) glass coated with the amino acid before a C60 layer was added on top by Ajeian’s86 group in 2017 for light harvesting, resulting in a power conversion efficiency (PCE) of 0.5%. It was demonstrated that DNA attached with fullerene, has a significant potential as key structural element as a structural scaffold in the bulk of the active layer. The devices reproducibly exhibited photovoltages of 670 mV95. Natural dyes have been explored as potential active materials in organic solar cells due to their low cost, renewable nature, and environmental friendliness. These dyes are typically extracted from plant materials or other natural sources and have been found to exhibit promising light-absorbing and electron-donating properties99,100. Chlorophyll, being one of the most abundant pigment which can be extracted from plants, has been coupled with P3HT97, and has shown to work as an electron acceptor in PV cells delivering a PCE of 1.48%.
Notable cases of incorporation of biomaterials for modifying the interlayers between electrodes and the semiconductor in OPV are illustrated in Fig. 2. Biomaterials such as amino acids101,102,103,104,105,106, DNA107,108,109,110,111, polypeptides112,113,114, polysaccharides115,116,117,118,119, and catecholamine120,121,122,123,124,125,126,127,128 can improve charge transport in OSCs. Amino acids are the building blocks of proteins that make them ideal for assembly since they can assemble, and form ordered structures. One of the most promising amino acids for use as an interlayer in OPV is arginine. In 2020, Li et al. successfully introduced L-arginine as an ETL by spin coating it on ITO in an inverted organic solar cell. The PCE improved by a factor of 4 compared to ITO-only devices, reaching a PCE of 9% as a result of improved work function and increased interface conductivity101. Recently in 2023, a group of researchers used L-cystine as ETL improving efficiency by 68% compared to the reference device129. DNA exhibits excellent thermal stability up to 140 °C in its solid form130 and easy tunability via chemical and physical treatments. Dagar et al. were the first to employ DNA nanolayers by spin coating these over ITO determining an enhancement of the electron extraction capabilities of the cells108. DNA led to strong improvements in rectifying behavior (by 2 orders of magnitude with rectifying ratios larger than 103) and in photovoltaic parameters like the open-circuit voltage (VOC from 0.39 to 0.73 V) and power conversion efficiencies (PCEs from ∼2 to ∼5%)108. When coupled with inorganic interlayers over ITO/ZnO-NPs107 power conversion efficiency improved from 7.2% to 8.5% due to a lowering of the work function caused by DNA and its capability of imprinting a different long range order on photoactive blend110. DNA has in fact been used as a structural scaffold for supramolecular chromophore assemblies95. Also, polypeptides, which are biodegradable polymers composed of amino acids, have been incorporated in OPV. α- poly-lysine was the first polypeptide used as electron extraction layer to improve charge transport in fullerene-free OPV113. When used as an overlayer in ITO/ZnO ETLs, the PCE improved from 7.23% to 8.32% due to the lowering of the work function by 0.06 eV resulting from dipole formation arising from the arrangements of amino groups of poly-lysine112. In 2020, researchers used carboxymethyl cellulose sodium as a co-modifying layer with ZnO for the transfer and collection of electrons in OPV and the best device delivered a PCE of 11.96%. Again, this interlayer lowered the work function of ITO116. The presence of functional groups in the bio-derived materials, and their tendency to assemble, can be exploited to improve energy level alignment, morphology of the photoactive layer, and dissociation of excitons in polarons. Among these biomaterials, overlayers of amino acids, DNA, and catecholamine are the most promising.
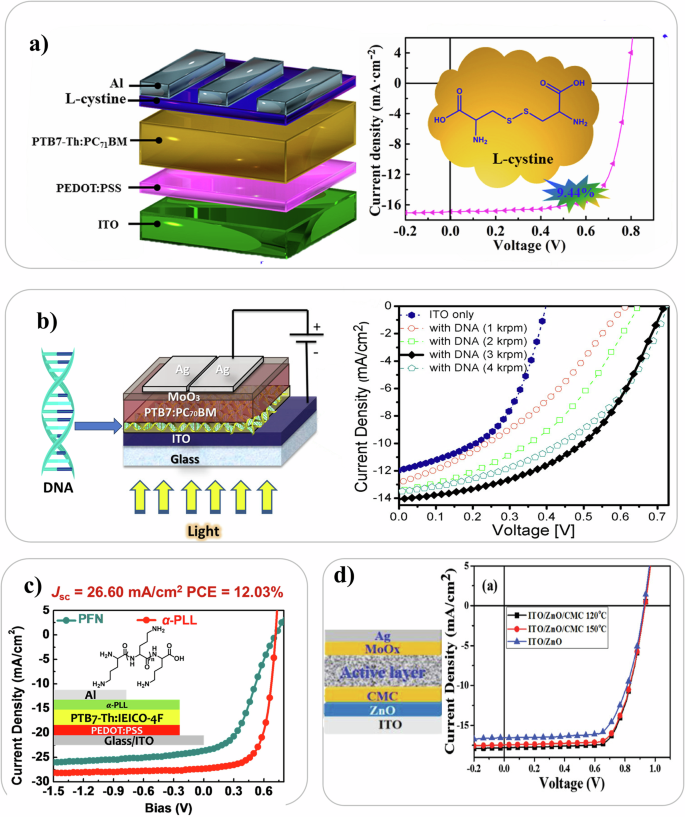
a Device geometry of OPV with L-cystine as ETL. The JV curve is displayed. Reproduced with permission from ref. 129. b The device structure of an organic solar cell incorporated with DNA nano layer and the device performance (JV curves) variation with respect to the spin speed of DNA layer deposition. Reproduced with permission from ref. 108. c JV curve comparison of an OPV device incorporated with Polyamino acid and Poly [(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9–dioctylfluorene)] (PFN) as electron extraction layer. Reproduced with permission from ref. 113. d Carboxymethyl-cellulose (CMC) based OPV device. The effect of different annealing temperature of ITO/ZnO/CMC on the JV curve compared with ITO/ZnO device. Reproduced under the terms of the Creative Commons Attribution CC BY 4.0116.
Biomaterials in perovskite photovoltaics
Perovskite solar cells (PSC) are new generation photovoltaic devices that use materials with a crystal structure similar to the mineral perovskite for harvesting light. They are one of the most promising PV technologies due to high photo conversion efficiency and low cost high-throughput fabrication processes. The solution processibility, band gap tuning, economic viability, and high efficiency are some of the qualities of the perovskites which make them desirable. Even though it is a highly promising and growing technology, there are still limited choices of perovskite materials, and their toxicity and instability remain challenging issues to be solved. A typical PSC comprises of five layers131,132 which are (i) transparent electrode, e.g., transparent conducting oxide (TCO)-coated glass/plastic, (ii) electron transport layer, e.g., a compact metal oxide layer (TiO2 or SnO2), (iii) a poly-crystalline metal halide perovskite layer as absorbing layer, (iv) an organic hole transport layer (HTL), and (v) a metallic top electrode.
Even though perovskite materials are highly sensitive to environmental/water-led degradation, there are some reports of incorporation of biomaterials133,134 in the PV device stack in the literature. Biomaterials have been introduced at the charge transport layer and semiconductor interfaces as well as in their bulk to improve both efficiency and stability as tabulated in Table 3. Amino acids135,136,137, DNA138, and cellulose derivatives139,140 have been used in the active semiconductor. Biomaterials such as amino acids135,141,142,143,144, proteins145, DNA146,147, catecholamine148, dopamine149,150,151,152,153, and polydopamines154,155,156,157 have been incorporated at the charge transport layer interfaces with perovskite. And recently, “reverse micelle” like bio-structures have also been explored in perovskites134. Molecules with ambipolar moieties and other types of bio-derived materials such as capsaicin, betulin and biopolymer heparin sodium (HS) salt, have been shown to be effective interlayers. These materials improved the perovskite’s morphology, defect passivation, and enhanced the charge extraction due to the modified transport layer/perovskite heterojunction158. These modifications are accompanied by simultaneous increase in device stability, which is essential for scaling up of this technology. Bio-derived materials with polar groups, such as amino acids and catecholamines, have been mostly implemented to mitigate intrinsic instability and to modify the TiO2/perovskite heterojunction interfaces, even enhancing charge extraction77,159. You et al. demonstrated that biopolymer heparin interlayers anchoring TiO2 and MAPbI3 enhanced trap passivation and device stability. The PCE improved from 17.20% to 20.10% when this interlayer was introduced in the device stack. Furthermore hysteresis was suppressed and the PCE maintained more than 80 percent of the initial value even after 70 days compared to 10 days in case of devices without the interlayer160 as shown in Fig. 3a. In 2021 a group of researchers used a non-toxic and sustainable forest based biomaterial called betulin161 in PSCs improving the PCE from 19.14% to 21.15%. Proteins have been investigated as potential charge transport layers in perovskite solar cells due to their unique electronic properties, biocompatibility, and low-cost fabrication methods. As depicted in Fig. 3b Das et al. in 2019 reported on how to enhance the efficiency of PSCs from 14.59% to 17.02% through protein functionalization of the TiO2 electrode145. Similar to OSCs, DNA has also been used in PSCs as an interlayer. In fact, in 2019 researchers showed it was possible to increase the PCE up to 20.63% as well as the stability of solar cells with DNA-modified perovskite layers (Fig. 3c)138. More recently, Wu et al. utilized perovskite semiconductors as a platform to investigate whether specific biomolecules can modulate the lattice structure. Their study revealed a unique mechanism for stabilizing the metastable perovskite lattice, achieving a PCE of over 22% (Fig. 3d). Through systematic experimentation with three tiers of biomolecules incorporated into the perovskite, they unveiled insights into a fundamental mechanism underlying the formation of a “reverse-micelle” structure. A comprehensive exploration of a diverse array of biomolecules uncovered guiding principles for the selection of suitable biomolecules, effectively extending the classic emulsion theory to these hybrid systems. Furthermore, this research offered a fresh perspective on engineering synthetic materials, showcasing the potential for leveraging biological principles to enhance semiconductor properties134.
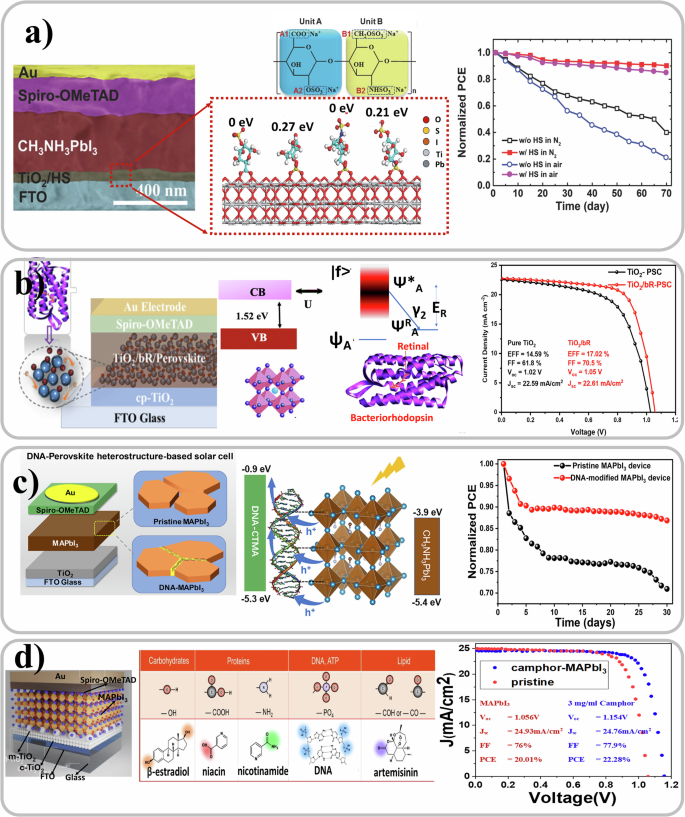
a Cross section of MAPbI3 perovskite solar cell with heparin sodium interlayer bridging TiO2 and perovskite layer. The functional groups attached to the TiO2 surface and chemical structure of heparin sodium salt with two repeating units are represented in the middle. The PCE plot shows that the solar cells with HS interlayer were more stable than the ones without. Reproduced with permission from ref. 160. b The image of bacteriorhodopsin functionalized TiO2 in the MAPbI3 solar cells stack is shown. This incorporation had improved the efficiency of the solar cell as shown in the JV graph. Reproduced with permission from ref. 145. c A DNA modified MAPbI3 perovskite solar cell with improved stability as shown in the PCE plot. Reproduced with permission from ref. 138. d Down-selection of biomolecules to assemble “reverse micelle” with perovskites. Reproduced with the terms of the Creative Commons Attribution CC BY 4.0 ref. 134.
Biomaterials have not only been incorporated at the interfaces of the solar cell stack but in the bulk of the perovskite film134. Hou et al. in 2019 demonstrated a core–shell heterostructure of perovskite wrapped by cetyltrimethylammonium chloride modified DNA to incorporate in the device stack. Such a design resulted in enhanced extraction and transport of holes in the bio-photovoltaic device and boosted the efficiency from 18.43% to 20.63%138. Amino acids have also been found to passivate the surface defects in perovskite materials, which can reduce non-radiative recombination and improve charge carrier lifetimes162. In 2020, Zhang et al. incorporated the amino acid L-lysine with two amino and one carboxyl groups as a chemical additive in the CsPbBr3 perovskite films to simultaneously anchor the uncoordinated Pb2+ (Cs+) and halogen ion defects. Furthermore grain size of CsPbBr3 perovskite is boosted from 688 nm to over 1000 nm as a result of the decreased nucleation rate and the sufficient growth of perovskite135. Amino acids have also been used to modify the surface of TiO2141,142 at the TiO2/MAPbI3 heterojunction improving cell performance. In 2023, Chen et al. reported the use an amino acid molecule to improve charge extraction of the ETL layer and the champion device had an efficiency of 24.71% and good stability163.
Biomaterials in dye-sensitized photovoltaics
DSSCs164,165,166,167,168 are an interesting solar cell technology because of their simple preparation methodology, low toxicity, and the presence of an electrolyte as core constituent. The semi flexibility169 and semi transparency170 of the technology allows for a wide range of applications. It consists of a mesoporous nanostructured wide band gap oxide semiconductor, usually TiO2, with a monolayer of photoabsorbing dye anchored to it, deposited on a transparent anode, a counter electrode with a catalytic layer, and an electrolyte solution that fills the device structure171,172,173,174,175 as shown in Fig. 4a. Electrolytes used in DSSCs, being one of their main components, can be in solid176,177,178, liquid179,180, or quasi-solid state181 forms.
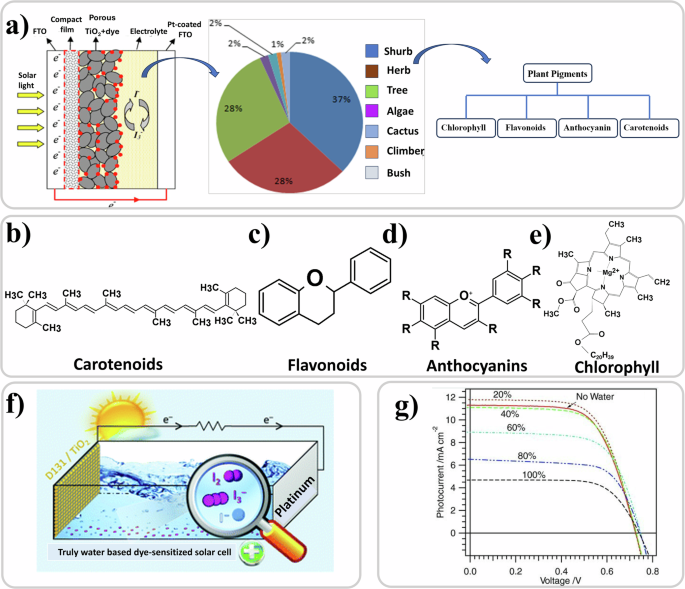
a The left part of the panel shows the structure of a typical DSSC, reproduced with permission from ref. 556, the middle part of the panel shows the pie chart of percentage of plant varieties used in DSSC applications, adapted with permission from ref. 185, and the right part of the panel shows the pigments from these plant varieties used as the dye184. Chemical structure of b Carotenoids172, c Flavanoids557, d Anthocyaninis172, and e Chlorophyll172. f The structure of a truly water based DSSC. Reproduced under the terms of the Creative Commons Attribution 3.0 Unported Licence558. g JV curves of the aqueous electrolyte-based DSSCs according to the content of water in the electrolyte. Reproduced with permission from ref. 227.
Since the first discovery182, much research has been focused in designing and synthesizing dyes and formulating electrolytes to maximize the conversion of photons into electrons and photovoltaic performance with certified records of 14.1% achieved183. Necessarily, devices with industrial potential rely on synthetic dyes and electrolytes based on organic solvents. Historically, related to the possibility of extracting a multitude of dyes from living systems with anchoring groups that can be easily attached to the mesoporous TiO2 layer, the literature on manufacturing DSSCs where the pigment is from natural living systems, plant-based in particular, has flourished184. A recent review study has shown that 37% of these pigments have been extracted from shrubs, followed by herbs and trees which contribute to more than 50% together185 (Fig. 4a). The main pigments (Fig. 4b–e) that have been extracted from these plant sources are chlorophyll186,187,188,189,190,191,192,193,194, anthocyanin15,186,187,189,195,196,197,198,199,200,201, betalains202,203,204, flavonoids186,205,206, anthraquinones205, rutin205,207, and carotenoids184. Natural dyes208,209, have also been derived from other sources such as bacteria210,211,212,213,214,215,216. Table 4 reports devices with natural dyes extracted from plants with an efficiency of at least 1%. As can be noted, the efficiencies from natural pigments are way off from those achieved with synthetic ones but the fascination remains of creating a photovoltaic device with natural biological components.
A significant push for selecting and investigating natural pigments is sustainability217. The same can be said for replacing conventional glass or plastic substrates with cellulose films218. Efforts in this direction on other constituent layers of the DSSC stack have mainly focused on the electrolyte which is generally based on organic solvents. Electrolytes have been modified with incorporation of biomaterials mainly for jellification, predominantly polysaccharides such as cellulose (in a standard liquid electrolyte containing 1-methyl-3- propylimidazolium iodide (MPII))219, starch (in a polymer electrolyte)220, chitosan (polymer electrolyte)221,222, gelatin223, and with ethyl cellulose, the latter being particularly stable224. Nanocellulose was also employed as an aerogel membrane for assisting in electrolyte filling. Nadia et al. used agar-based polymer electrolytes where they investigated the effect of sodium iodide and potassium iodide on ionic conductivity, the latter resulting in higher ionic conductivity225. Instead of the typical organic solvents used in electrolytes such as 3-methoxypropionitrile or acetonitrile226, the most eco-friendly ingredient for alternatives is water (Fig. 4f). Water-based DSSCs are usually assembled using solvent mixtures with varying percentages of water. Figure 4g shows that performance of the devices is reduced with increase in the content of water227. The lowering of efficiency and stability compared to conventional organic solvents is due to the dye desorption and the poor solubility of iodine in water226. Nevertheless, the fact that 100% H2O can be used in DSSC structures is an important factor in these architectures being used in biosensors or in artificial retina concepts as illustrated in future sections.
In summary, the integration of biomaterials in OPVs, PSCs, and DSSCs pursues similar objectives but employs distinct methodologies. In OPVs, biomaterials like proteins, DNA, and natural dyes have been shown to enhance light absorption, morphology, carrier transport and extraction, with notable efficiency improvements (with relative PCE increase of 20% for protein228, 18% for DNA229, and 15% natural dye101) with the highest reported efficiencies reaching around 18.35%76. In PSCs biomaterials have been mainly used for defect passivation not only at the layer interfaces but also at the grain boundaries and to improvement in charge extraction. Examples include perovskites modified with DNA, which boosted PCEs from 18.4% to 20.6%138, heparin sodium salt, raising efficiency from 17.2% to 20.1%160, and artemisinin affecting the colloidal-crystallization dynamics and boosting the PCE from 19.3% to 22.4%134. DSSCs, on the other hand, focus on the use of natural pigments like chlorophyll and anthocyanins, which are derived from plants and offer a sustainable alternative to synthetic dyes, but with lower efficiency (around 2% with highest reported of 4.6% with chlorphyll230). Biomaterials in DSSCs are also used to modify electrolytes, incorporating cellulose, starch, and chitosan to improve device stability. While all three technologies leverage the incorporation of biomaterials for improved performance and possible sustainability, PSCs have delivered the highest efficiencies and stabilization properties, OPVs the greater modulation of performance after incorporation, focusing more on environmental impact, and DSSCs presenting the highest number of publications using natural dyes (at the cost of lower efficiency).
Basics of the interaction of light, biological systems and new generation semiconductors
Light for sensing and photo-stimulation in biological systems
Light has always been a widely used tool for the control and stimulation of cells and living systems. For example, light was first used to control biological systems such as muscle tissue in 1891231, and ganglion cells of Aplysia californica in 1968232, while in 1971 selective stimulation of neurons via laser radiation was carried out233. Research has progressed tremendously since then234,235,236,237,238. The reason is that light can, depending on wavelength, selectively be absorbed, or propagated through different biological tissues and membranes. Thus, it exerts its effect without a direct physical contact that could damage the biological entity under investigation239,240,241. With respect to the standard approaches (e.g., electrical, chemical, and mechanical), light stimulation shows several advantages: it not only is a contactless probe, but also a probe that can be shaped in desired patterns to target specific areas. Its high selectivity and spatio-temporal resolution enables the targeting of even single cells and subcellular components242. In recent years, different techniques exploiting the interaction of light with biological systems have been demonstrated, showing an increasing trend towards the optical approach for stimulation and sensing of living systems. The methods for light interaction or light manipulation of biological systems are classified in two categories243:
-
i.
Endogenous light-sensitive methods: exploiting photoactive mediators that are naturally expressed by or located within the living systems, such as those in the opsin family—proteins that bind to light-reactive chemicals involved in vision, phototaxis, circadian rhythms, and other light-mediated responses in organisms244. For example, it is well known that light acts directly in the eye. Photoreceptive cells, namely cones and rods, respond to light differently depending on their structure and the opsins they carry (Fig. 5a). It is less known, however, that light also plays a direct role in the skin245,246 and in other unconventional tissues where specific opsins can respond to light247, such as in blood vessels248, in white adipose tissue249, and in the brain250,251. In these extraocular sites, the capability to detect light is not utilized for image capture but serves other functions, such as ultraviolet radiation-induced melanogenesis in the skin252, photorelaxation of smooth muscle cells in blood vessel walls248 and airways253, reduction in lipid content and adipokines secretion by adipocytes249, and regulation of circadian rhythms in the brain (Fig. 5b). Moreover, the setting of the circadian clock has also been attributed to specialized “intrinsically photosensitive” cells present in the eye, distinct from cones and rods, which are a subset of retinal ganglion cells254,255, and sphincter muscle cells of the iris that control pupillary constriction256,257. In addition to naturally occurring photo responsive molecules, advances in (opto)genetics (Fig. 5c) based on molecular biology and biotechnology258 have allowed the introduction of photo responsive molecules inside cells259,260. With these new approaches, degenerated retinal cells can partially recover their functions, enabling photo response261.
Fig. 5: Schematic illustration of the two different ways through which light can affect cell behavior. 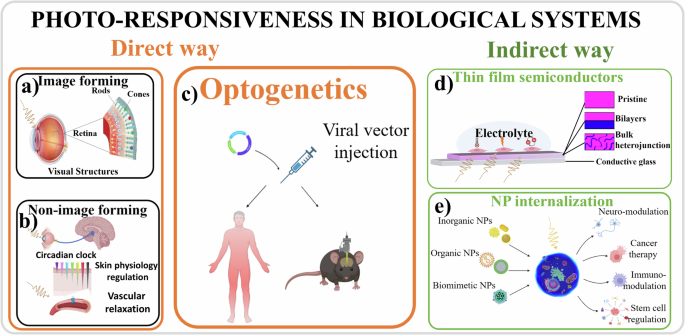
In the direct way, photo responsive proteins physiologically present in a cell, that are image-forming (a), and non-image forming (b), or transfected with a viral vector (c), can respond directly to light stimuli. In the indirect way, photo responsive materials present in the form of a thin film outside the cells (d) or in the form of NPs inside the cells (e), are responsible for absorbing light, transducing the excitation to the biological system thus affecting its response (created with BioRender.com).
-
ii.
Exogenous light-sensitive methods: converting the light excitation into electrical, chemical, or thermal stimuli associated with the presence of non-biological materials, either in close proximity (Fig. 5d), or integrated within the cell through the injection of NPs (Fig. 5e), enabling them to respond to incident light262. This stimulus can induce ionic or chemical changes in the cellular microenvironment, thus modulating the activity of responsive biomolecules such as voltage-gated ionic channels. The integration of these new materials with biological systems has enabled light to activate and deactivate biological processes in cells that were previously not photosensitive, thereby providing a degree of control over these processes through light. For instance, the proliferation rate of a neuroblastoma cell line cultured on polymer semiconductors was significantly influenced by periodic light pulses directed onto the system13. These approaches expand the possibility of controlling and modulating cellular responses, as optical stimulation addresses two important challenges: spatial resolution and response specificity. This ability of light to interact with biomaterials linked with non-biological materials has various applications, including modulation of the performance of photovoltaic (PV) devices, bioelectronics, and biosensing.
-
i.
Photobiomodulation is a well-established endogenous light stimulation technique for therapeutic targets263. By activating naturally expressed light-sensitive proteins (photons of light are absorbed by chromophore present within tissues) this technique induces beneficial effects on cells and tissues (e.g., wound healing and tissue regeneration264) by generating photochemical reactions employing low-power density lasers or light-emitting diodes (LEDs)265. Light-sensitive proteins present in biological systems are rhodopsin in the retina photoreceptor cells, and also other opsins outside the eye, such as phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. While photobiomodulation exploits the direct interaction of light radiation with cells and tissues, optogenetics is an endogenous optical approach based on the engineering design of light-sensitive proteins and photoactive molecules266. Although photosensitive complex, usually introduced in living cells through viral transfection, are necessary, the biological processes in response to light are generated by the cell itself. Optogenetics is a very promising approach. However, several ethical and safety issues related to the need of viral-gene transfer strongly limit in vivo therapeutic applications.
-
ii.
Due to the relatively limited existence and non-specific interaction between light and biological systems with notable exceptions such as the retina and photosynthetic units – research has focused on identifying and developing photosensitive transducers designed to interact optoelectronically with biological systems. Examples of these exogenous photoabsorbers267, either organic or inorganic, are micro and NPs268,269, quantum dots (QDs)270, and semiconductors271. Rigid semiconductor interfaces such as monolithic silicon-based devices have been recently used for high spatiotemporal resolution photostimulation. These innovations enhance the precision and control of light-based stimulation in biological tissues, with potential applications in neuroscience and medical therapies272. Despite ongoing efforts, concerns exist on the biocompatibility of inorganic exogenous materials used in photo-stimulation due to the intrinsic material stiffness, the possible cellular damage caused by the exposure to an excessive required light intensity and the necessity of an externally applied bias via electrical wiring which leads to tissue heating273,274 and cellular injury275,276. The new generation of thin-film semiconductors emerges as particularly promising due to their potential for interfacing with biological materials with good biocompatibility at both the mechanical and electronic levels277,278 (see previous section). These encouraging characteristics position these semiconductors as noteworthy subjects for detailed examination in the upcoming sections.
Interaction and transduction mechanisms between organic semiconductors and biological matter/cells
The fundamental component of the semiconductor’s interface with biological systems is the photoactive material acting as both absorber of light and transducer. OSCs have a broad range of physical and chemical features that can influence cell growth and function. The biological materials are very sensitive to the environment, therefore it is important to make sure of the fabrication of semiconductors in sterile conditions which can ensure the supporting/interfacing material do not adversely affect (but even improve, sustain, or enhance) biological standard behavior279. Surface properties of materials, such as roughness and surface-energy, can influence protein adsorption from a solution occurring within seconds. Adsorbed proteins consequently contribute to cell-substrate interactions280.
Biocompatibility is a crucial aspect to consider when working in bioelectronics, at macro-, micro-, and nano-scale levels. The forefront of bioelectronics based on OSCs is focused on the control of cell adhesion, growth, and differentiation as well as the stimulation and inhibition of bioelectrical signals. First, for each material used in bio-interfaces, it is vital to take into account the level of purity as well as the sterilization step to avoid potentially cytotoxic compounds and/or pathogens that can be hazardous for biological systems. Subsequently, the step of cell adhesion is an important issue that deserves consideration. In this context, not only is the type of material important, but also the characteristics of the surface, that is its topography (roughness, presence of pores, cavities), its chemical nature (surface energy and charge) and its physical characteristics (stiffness and wettability). Moreover, the material surface when utilized in vivo, is rapidly covered by adhesion proteins present in body fluids, like fibronectin and vitronectin that facilitate cell adhesion; for this reason, when working in vitro, it is necessary to mimic this adsorption step by pre-adsorbing adhesion molecules onto the material surface281.
Based on these considerations, OSCs have been proved highly suited for application in biological interfaces (at different levels of complexity, from animal cells and tissues to plant cells) because of their physical and chemical features282,283,284,285. Compare with inorganic photovoltaic material such as silicon, OSCs show soft nature, high efficiency of light absorption, tunable optical and electronic properties, and a superior capability of mixed, ionic and electronic, conductivity when interfaced with aqueous electrolyte as required in bio-hybrid interfaces286.
Biological systems primarily communicate through bioelectrical signals, which consist of ion movements (currents) and local variations in ion concentrations (potentials). In contrast, conventional electronics operate based on electron conduction. Thus, the fundamental divergence between these two domains lies in the nature of conductivity: extracellular and intracellular ionic conductivity in biology versus electronic conductivity in electronics. The interaction between OSCs and biological systems can function bidirectionally:
-
i.
As (bio)sensors, where a biological process or reaction transmits signals to an organic electronic device, thereby transducing the biological process into an electrical output.
-
ii.
As (bio)actuators, where an organic electronic device stimulates a biological process, facilitating the electrical stimulation of said process.
Conjugated polymer being inherently hydrophobic in nature, have to be prepared as NPs, such as P3HT-NPs, and have been employed in bio-photonic applications287. In 2016, a research team explored these NPs for interaction with Human Embryonic Kidney (HEK-293) cells. The NPs were observed to be internalized within the cytosol of live cells, demonstrating the potential of P3HT-NPs as light-sensitive actuators. Notably, the photophysical properties of the NPs were maintained without impairing the physiological functions of the cell18. In 2018, the same group used P3HT-NPs to modulate the intercellular Ca2+ dynamics of the same cellular line. With this method they opened the possibility of gene-less approach for studying cellular processes288. Recently, thiophene based core@shell NPs were used to photo-generate reactive oxygen species (ROS) without harmful singlet oxygen. It was showed that these NPs are fully biocompatible and can be used as exogenous photo-actuators, which can potentially operate through two different mechanisms based on charge capacitive effects and/or ROS generation inside living organisms289. Lately, porous semiconducting polymer nanoparticles have been designed and synthetized to be employed as intracellular wireless mediators for light-induced intracellular ROS production and modulation in vascular tissue290.
Concerning light-mediated exogenous methods with semiconductor thin films, they are based on the interaction of the photo-generated charges in the organic material and the living systems57. The basic idea is that the light-generated electrical charges in the film can modulate the membrane potential of a cell grown on top of it or can elicit some electrical activity in tissues placed on it. Unlike endogenous light stimulation where the focus is at the molecular scale, in exogenous stimulation the interface between the OSCs (mostly in the form of thin films) and the biological structures plays a crucial role in determining the excitation pathway. Most importantly, in light-mediated exogenous methods with bio-interfaces, OSCs show low production of heat, very specific absorption of photons because of the tuning of their optoelectronic properties, and a different range of charge transfer processes occurring with electrolytes that are useful for ionic mediated electric signaling. These interactions have been utilized, for example, in stimulation of retinal cells10 and in the modulation of ion channels in neuroblastoma cells13.
Several possible mechanisms are involved in the coupling process between optically excited OSCs and living systems. The general underlying principle is linked to the light-induced formation of long-lived excited states in the active photosensitive layer. These polaron states are responsible for interactions with the cells through the electrolytic environment present in the cleft, the narrow gap ( < 100 nm) separating the device from the cellular membrane. Immersed in electrolyte solution, OSCs convert absorbed light into heat via photothermal conversion, into electricity via photovoltaic (capacitive and reversible Faradaic) processes, and into chemical species via photocatalytic reactions271. In more detail, three different photo-stimulation mechanisms, at the OSCs/biological systems interface, proposed so far are the following242.
-
i.
Thermal coupling: light stimulation causes a local temperature to increase in the extracellular bath solution in proximity of the OSC layer. Photothermal OSCs mediated neuromodulation was demonstrated for inhibition or excitation of neuronal firing via temperature-dependent cellular membrane channels291.
-
ii.
Electrical coupling: light induces an electrical phenomenon, Faradaic and/or capacitive coupling, generated at the OSC/electrolyte interface. Upon light stimulation of the photoactive OSC layer, a local rearrangement of the charges is promoted in the ionic double Helmholtz layer present at the liquid/solid interface. This determines a sustained current and/or a variation of the capacitance of the electrical double layer compared to dark conditions. The carrier separation induces a change in the potential of the electrolyte solution. The capacitive coupling or reversible Faradaic process occurs at the OSC surface or within the bulk material. The capacitive coupling is characterized by the redistribution of charges causing the generation of local and transient electric field close to the polymer/electrolyte interface whereas the Faradaic coupling involves the electrochemical oxidation or reduction reaction caused by electron transfer between the polymer and the electrolyte292.
-
iii.
Photo-electrochemical coupling: this mechanism considers the possible redox reaction occurring at the interface. Under illumination, the OSC interface can be employed to trigger water reduction leading to the generation of gaseous hydrogen for energy production. The photoelectrochemical reaction promotes a local variation of extracellular and/or intracellular pH and the production of ROS, at a nontoxic concentration, and intracellular calcium (Ca2+) modulation288. Photocatalytic reactions have been observed under high intensity light exposure (AM 1.5 G, 100 mW/cm2)293, as well as during extended light stimulation.
The three processes usually coexist. However, the ideal scenario would be an exogenous optoelectrical stimulation via a capacitive coupling between semiconducting polymers and living cells294,295. The capacitive and Faradaic contribution of the electrical stimulation process depends on the materials, the electrolyte, and the device structure used that can favor the former or the latter mechanism296,297,298. Transient, photo-capacitive stimulation mechanism is considered one of the most biologically safe stimulation mechanism used to electrically stimulate cells and tissues299,300,301 because the electric field produced in this way stimulate the cells without causing any damage. Moreover, considering the interface with cells, targeting voltage-gated ion channels by a pure photo-capacitive stimulation is a preferred therapeutic approach as it avoids Faradaic charge injection302 through which ROS303, and changes in the pH value in the cellular environment304 occur which can cause damage to cells and tissues.
Testing approaches, biocompatibility, and illumination schemes
Significant research in the field aims to apply its methods in vivo. It should be considered that, due to the multiplicity of materials in use and the great variety of cells present in the human body, in vivo studies must be preceded always by in vitro studies that are based, as described above, on the evaluation of biocompatibility of the studied material compared to a material used as control, for example polystyrene, normally used for the fabrication of cell culture Petri dishes. Standardized protocols are crucial for evaluating the biocompatibility and stability of these devices, ensuring consistency and reliability across studies64. Equally important is the development of robust in vivo models that accurately replicate real physiological conditions. When conducting in vivo evaluations on animal models, in fact, a detailed description of the operating protocol is essential for reproducibility, along with the definition of the sample size that depends on the level of variability present in the system, to allow for a robust statistical analysis. In general, in vivo studies are particularly susceptible to pitfalls and biases due to the complexity of biological systems and individual variability, making data interpretation more challenging. Issues like small sample sizes, observer bias, and the difficulty in isolating specific effects further complicate outcomes. To overcome these challenges, the use of standardized protocols can minimize procedural variability, while rigorous statistical analysis ensures that sample sizes are sufficient and complex interactions are accounted for. Implementing appropriate control groups, blinding techniques, and automated data collection reduces bias, and replication of experiments across diverse models enhances the reliability of results. These strategies collectively lead to more accurate, reproducible, and generalizable findings in in vivo research. However, despite the complexity and economic cost of organizing in vivo experiments, they certainly add important information on: 1) long-term efficiency of the material which in vitro tests cannot completely reproduce due to the lack of a tissue microenvironment, and 2) possible inflammatory and/or immune reactions linked to the presence of a defense system that is impossible to reproduce in vitro since it is formed by various migratory cells present in the body (leukocytes, macrophages, mast cells, and lymphocytes). Therefore, due to the complexity of these issues, biocompatibility of materials and safety of implantable devices have to rely on detailed guidelines and regulations which are also continuously updated305,306. Moreover, for the specific application of photovoltaic bioelectronics one must also consider light as an important variable of the system. In fact, to study the stability under light, protocols should refer to the existing consensus statements for stability assessment of new generation photovoltaics also known as ISOS protocols307,308,309. These publications suggest a number of tests such as ISOS-D dark storage tests (the main ones being at normal environmental conditions and the damp heat test at high relative humidity of 85% and temperatures of 65 or 85 °C), ISOS-L light soaking tests (the main one at 1000 W/m2 under simulated AM1.5 G spectral irradiance), and ISOS-T thermal cycling tests (the main one from −40 to + 85 °C). The time required to drop to 80% of the initial efficiency of the PV cell, commonly denoted T80, is the main parameter reported serving as a figure of merit for solar cell stability. T80 of cells incorporating biological materials should be compared with controls without these, in order to understand their role in affecting positively or negatively device stability and the mechanisms underlying degradation.
According to ocular safety standards for ophthalmic applications the maximum permissible radiant power that could enter the pupil chronically or in a single short exposure (between 50 μs and 70 ms) at 532 nm is 146.17 mW310. The irradiance levels57 that have been generally used for in vitro retinal stimulation experiments span from around 1 mW/cm2 (~103 lx) to ~100 mW/cm2 (~105 lx)311,312,313 representing the optical power density range typically experienced in human photopic vision during daylight outdoors (1-100 ×103lx)16. Note that the light in homes and offices which is lower, is generally between 100–500 lx (10-1–100mW/cm2) as reported in a recent review on indoor photovoltaics314, and that the 100 mW/cm2 value is equivalent of looking straight at the sun which is known to damage the eye even after few seconds16,315,316. Compared to the PV cell, the artificial retina technology lacks a standardized protocol to carry out aging tests, to verify the long-term stability of the device in biological environments.
Photobiomodulation of the behavior of living systems with organic semiconductors
A recent new strategy for light-mediated exogenous control/stimulation of biological systems focuses on the use of OSCs. These have been used in the last decade for photo-stimulation of excitable and non-excitable cells, in the form of thin films, microstructured platforms, and NPs299,317,318. Their presence has induced neural differentiation, directed growth319, and enhanced tubulogenesis of endothelial colony-forming cells (ECFCs)320, and the correlated stimulation of pro-angiogenic Ca2+ signals in ECFCs through activation of Transient Receptor Potential Vanilloid 1 (TRPV1) channels useful for vascular regeneration and tissue repair in case of cardiovascular diseases321. Moreover, OSCs were also successfully employed for optoelectrical control of neurogenesis and neuromodulation322, and for sight restoration purposes since they have been shown to stimulate photoreceptors and retinal tissue both in vitro311 and in vivo59. OSCs can be used as pristine materials323 or as multilayer64 or mixed with others in a bulk heterojunction324 to further tune their capability for interaction with biological media as well as their optoelectronic properties. OSCs were also exploited as photosensitive and light-harvesting material to augment photosynthesis of chloroplasts325 and to regulate signaling (carbon dioxide uptake, oxygen release, etc.) in plants326. Here we will highlight the use of OSCs for light-modulation of biological systems, describing the biophysical interaction between OSCs-light-biosystems.
In 2014, Benfenati et al. grew primary neocortical astrocytes of rats on the top of a light-sensitive, organic P3HT:PCBM polymer films11. Patch clamp analysis showed that shining visible light on the system caused a significant depolarization of astroglial resting membrane potential. The effect was associated to an increase in whole-cell conductance at negative potentials. The ion channel likely to mediate the photo-transduction mechanism was identified as the ClC-2 chloride channel. Recently, Borrachero-Conejo et al. showed that pulsed infrared light can modulate astrocyte function through changes in intracellular Ca2+ and water dynamics, providing unique mechanistic insight into the effect of pulsed infrared laser light on astroglial cells, even without the presence of light mediating polymer films327. Thin films based on different OSCs (poly[2,1,3-benzothiadiazole-4,7-diyl[4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b’]dithiophene-2,6-diyl]] – PCPDTBT, rr-P3HT, poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylene-vinylene] – MEH-PPV, poly[9,9-dioctylfluorenyl-2,7-diyl] – PFO) characterized by different absorption spectra, morphology, light emission efficiency and charge transport properties have been investigated as photoactive bio-interfaces323. All the considered polymers, interfaced with human embryonic kidney cells (HEK-293), showed good biocompatibility and cell seeding properties, while electrochemical stability and cell photo-stimulation efficacy differ among them. In particular, the high band gap polymer (PFO), in the work from Vaquero and co-authors323, appeared not appropriate in establishing functional coupling with living cells due to phototoxicity with illumination of blue light. Electrophysiological measurements via patch-clamp under light stimuli (tens of mW/mm2, 200 ms, different wavelengths [see Table 5]) demonstrated that polymer-mediated (PCPDTBT, rr-P3HT, MEH-PPV, PFO) photoexcitation of in vitro HEK-293 cell cultures was possible. In fact, the well-known membrane potential variation with an initial depolarization when light was shone followed by a hyperpolarization effect when light was tuned off was observed323. Bioelectrical polymer light-mediated cell activity was recently linked to the effects on intracellular Ca2+ signaling triggered by OSCs (namely P3HT) upon excitation with light in human adipose-derived stem/stromal cells (hASC)317 which is shown in Fig. 6a. Aziz and co-authors showed that when cells were chronically exposed to light irradiation during the culture time, their responsivity, involving Ca2+ ion trafficking, increased, in comparison with cells kept in dark. This increase was attributed to the light-mediated polymer trigger effect on the Ca2+ ion influx from the extracellular medium, possibly due to the Ca2+ channels expressed on the plasma membrane. Ciocca and co-authors13 developed a compact bio-photo-electrolytic platform (Fig. 6c) based on P3HT thin films in contact with the electrolyte solution/medium. They demonstrated that when neuroblastoma cells were cultured on the OSC thin film and subjected to periodic light stimulation (1.26 mW/cm2, 30 min twice a day 10 h apart, LED with warm white spectra), an increase in intracellular Ca2+ level occurred. The intracellular Ca2+ increase was most likely the result of cell membrane Ca2+ channels being activated by the light stimulus transduced by the photoabsorbing P3HT layer. Moreover, on neuroblastoma cells cultured on P3HT under light-irradiation, proliferative activity was reduced by about 50% with respect to cells cultured in dark condition (either on standard glass or OSC thin films). These results demonstrating that the proliferation rates of cells interfaced with photoabsorbing polymers can be affected greatly by light stimuli, open up novel possibilities for more effective Photo-Dynamic Therapy (PDT) for cancer treatment and for in vitro cellular photo-manipulation in the fields of regenerative medicine and tissue engineering.
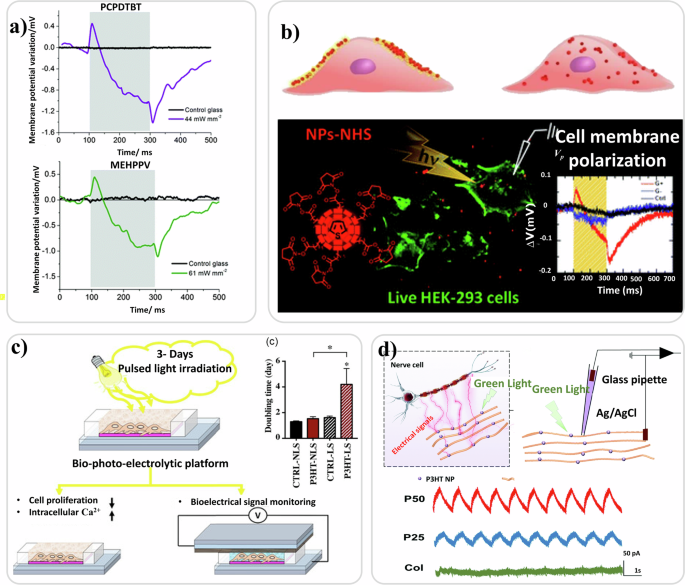
a Light modulation of membrane potential in HEK-293 cells cultured on PCPDTBT and MEHPPV polymers photo excited with light sources of 635 nm and 475 nm respectively. Reproduced under the terms of the Creative Commons Attribution 3.0 Unported Licence323. b Pictorial representation of NPs with pendant N-succinimidyl-ester groups docking on the surface of the cells and P3HT-NPs internalized by the cells. Engineered thiophene based NPs for phototransduction in live HEK-293 cells. Reproduced with permission from ref. 329. c Representation of polymer bio-photoelectric platform for electrical signal measurement and light modulation of cell proliferation and ion fluxes. The graph shows the cell proliferation evaluated during the 3 days of culture and expressed as cell doubling time. Reproduced under the terms of the Creative Commons Attribution CC BY 4.013. d Schematic representation of photoelectric effect-integrated PCL scaffold. Photocurrent curve measurement of the PCL -P3HT-Col scaffold and photocurrent curves of different scaffolds measured during green pulsed LS. Reproduced with permission from ref. 330.
In the form of 3D micro/nano scale bio-hybrid interfaces fabricated by self-assembling of nanofibers, electrospinning or lithographically patterned stripes, OSCs have been used for light-mediated electrical stimulation of neuron-like cells leading to neuronal differentiation and directed neurite outgrowth. Wu and co-authors328 demonstrated that neuron-like cells seeded on OSC thin films (namely P3HT) presenting specific structures and dimensions and cultured under green LED light irradiation (power 2 W) presented longer neuritis compared to those grown on a blank substrate. This cell behavior was mainly attributed to the photoconductive effect of P3HT, that is an increase of charge production by the semiconductor under light illumination that influence cell growth depending on the topographical features of the substrate itself. Moreover, it was directly proportional to the intracellular Ca2+ levels that was verified by monitoring the changes of Fluo-4 AM, a fluorescent Ca2+ indicator. The intracellular Ca2+ level increased for cells plated on P3HT thin film when illuminated by a green LED for 30 min328.
Polythiophene NPs, synthesized as poly(3-octylthiophenes) functionalized with the amine-reactive N-hydroxysuccinimidyl ester group (NHS), were used as photoactive materials inducing phototransduction and polarization of cell membrane under light irradiation. In this work, authors also showed that NPs-NHS attached to the cell membrane, while P3HT-NPs were internalized by the cell329 as shown in Fig. 6b. It has been challenging to provide electrical signals to nerve cells non-invasive/wireless mode, accompanied by the construction of a biomimetic cell microenvironment for supporting nerve cell survival and functional expression. P3HT-NPs were used as photoelectric material to fabricate a self-powered bioactive oriented scaffold for the wireless-light control of nerve cellular behavior in 2022330 as shown in Fig. 6d. A pioneering work recently introduced a 3D bio-printed light-sensitive cell scaffold for bio-photonic applications, based on P3HT-NPs|hydrogel novel bio-ink. The light-sensitive cell scaffold can be used for light control and modulation of cellular activities with several applications in neural engineering and regenerative medicine331. These studies have shown not just that bio-interfaces based on OSCs do not negatively affect biological functions, but that their physico-chemical and structural features can be exploited to sustain and potentially enhance bioactivities.
Biosensing using new generation semiconductors and light
Biosensing, defined by the precise detection of specific bio-compounds or bioelectrical activity, plays a critical role in understanding complex biological processes within living systems. It has found wide-ranging applications in fields such as biomedicine, pharmaceutical drug discovery, food safety, defense, security, environmental monitoring, and basic science332. A biosensor, the core of this technology, integrates a biological element with a physicochemical detector to produce measurable signals in response to target molecules333. These detectors are typically made from a variety of materials, both inorganic and organic, and can be conductive, insulating, or semiconducting334. The primary function of this union is to quantify signals generated by specific biological interactions335.
Key considerations in biosensing are detection specificity and sensitivity336,337. The detection limit in biosensing varies significantly based on the measurement principle, which is broadly categorized into optical and electrical methods. Optical methods, which include fluorescence detection and chemiluminescence with labels, offer relatively high detection sensitivity338. In contrast, electrical methods rely on the amount of electrical current generated from electron transfer between electrodes and molecules in electrochemical measurements, typically exhibiting lower sensitivity compared to optical methods339.
Biosensors, categorized based on their physicochemical transduction mechanisms into distinct groups such as electrochemical340,341, thermal342, piezoelectric343 and optical344 devices, offer diverse capabilities for precise detection in various applications. In optical biosensors, modulation of the optical transduction—through changes in absorbance345, fluorescence346, or refractive index347—regulates the optoelectronic response of the device348. This modulated light signal generates electric output, which is then utilized for biosensing tasks349. For the subject of this review, light-mediated biosensing is especially interesting, and refers to the utilization of light to detect biological processes, leveraging the electric response of the device through photoelectrochemical reactions.
Among the possibilities for biosensing are devices based on semiconducting materials, such as silicon350 or gallium arsenide351, which serve as transducer to detect the biological analytes. There are several types of semiconductor-based biosensors, including field-effect transistor biosensors, electrochemical biosensors, and optical biosensors. Recently, new generation semiconductors such as OSCs that can be deposited in thin film form, and as NPs19,20,21 are being researched to be used for biosensing applications352,353. OSCs, especially solution processed conjugated polymers, have attracted great interest in bioelectronics, due to their outstanding electrical and optical features and their significant bio-interfacing capabilities. Bioelectronics based on OSCs is a rapidly growing field354,355,356,357,358,359,360 that bridges the gap between biology and electronics361. The number of papers published annually on the topic, identified by the keyword ‘organic bioelectronics,’ has increased from one paper in 2002 to 153 in 2023359.
Although detection techniques rooted in photo-electrochemistry have been documented since the 1980s, the concept of “photo-electrochemical (PEC) biosensing” is relatively recent. PEC biosensing is fundamental across various fields, including healthcare, environmental monitoring, food safety, biomedical research, security, and defense. Its paramount significance is within biomedical applications, particularly in healthcare for medical diagnosis. PEC biosensing is crucial for disease monitoring, drug discovery, identifying pathogenic microorganisms, and detecting markers indicative of various health conditions in bodily fluids335,362. PEC biosensing harnesses light to prompt electron transfer between photo-electrochemically active species and electrodes. It operates by tracking changes in the resulting photocurrent363,364 or photovoltage signals induced by biological interactions between recognition elements (such as enzymes, nucleic acids, antibodies) and their targets. This process converts bioanalytical data (like target analyte concentration) into changes in photocurrent or photovoltage339,365.
The major components of PEC biosensors include the transducer, target analytes, receptors or biorecognition elements, the electrolyte or electrolyte solution, the light source, and the electrodes. The choice and quality of the transducer are crucial. The transducer’s function is to transform the biorecognition event into a quantifiable signal, usually an electrical signal, reflecting the presence of the chemical or biological target. Transducers in PEC biosensors encompass a range of materials, classified as follows: a) inorganic semiconductors, including titanium dioxide (TiO2), cadmium sulfide (CdS), zinc oxide (ZnO), cadmium telluride (CdTe), and bismuth sulfide (Bi2S3); b) OSCs, such as phthalocyanine complexes, graphitic carbon nitride (g-C3N4), porphyrin and its derivatives, and polymers such as phenylenevinylene (PPV), poly(thiophene), and various conducting polymers; c) hybrid semiconductors, which combine inorganic semiconductors with differing band gaps or merge organic and inorganic semiconductors, as well as integrate diverse metal or carbon nanomaterials with inorganic/organic semiconductors366. This section will concentrate only on the application of new generation semiconductors for light-based biosensing, first OSCs, then perovskites, and DSSCs providing some significant examples.
Biosensing with organic semiconductors and light
OSCs are among the most promising materials for light-modulated biosensing applications due to their high biocompatibility, tunable absorption, biofunctionalization capabilities, and adjustable electronic properties367,368,369. When integrated into biosensing platforms, OSCs interact effectively with biological analytes, converting these interactions into measurable electrical or optical signals. These signals can be modulated by light to enhance detection capabilities. In light-modulated biosensing, OSCs are commonly used as active layers in devices such as photodetectors and field-effect transistors. By introducing biological recognition elements like enzymes, antibodies, or DNA probes onto the surface of the OSC, specific interactions with target analytes can be achieved. When exposed to specific wavelengths of light, these interactions induce changes in the electronic or optical properties of the OSC, resulting in a detectable signal370,371.
Recently, PEC biosensors with OSCs have garnered significant interest, particularly in enzymatic analysis372, immunoassay14, and DNA sensing373. Among these, PEC DNA biosensors are notable for their ability to provide sensitive, selective, and rapid detection of DNA sequences. A DNA biosensor typically includes a probe DNA as the biorecognition element and an electrode as the signal transducer, with the biorecognition event based on complementary DNA base pairing374. Feng Yan and coworkers in 2018 introduced a new type of organic transistor, known as organic photo-electrochemical transistor (OPECT), to create a groundbreaking DNA sensor with exceptional performance. OPECT is a combination of organic electrochemical transistor and a photo-electrochemical (PEC) gate375. Figure 7a displays the schematic representation of the device configuration of an OPECT. Here, poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) served as the active layer of the device, while ITO glass, modified with CdS quantum dots (QDs), acted as the gate electrode, phosphate-buffered saline (PBS) along with 0.1 m ascorbic acid (AA) served as the electrolyte. Upon exposure to 420 nm light (intensity: 0.2 mW cm−2), CdS QDs absorbed photons exceeding their bandgap energy, prompting the generation of electron-hole pairs as electrons transition from the valence band (VB) to the conduction band (CB). The presence of AA, a potent electron donor, effectively suppressed electron-hole recombination, ensuring stable electron transfer to the ITO gate. The detection capability of this DNA sensor reached an impressive limit of 1 × 10−15 M, surpassing traditional PEC bioanalysis by several orders of magnitude. OPECT, as an innovative biosensor, holds promise for a wide array of biosensing applications including enzymatic sensors, immunosensors, and cell-based biosensors375.
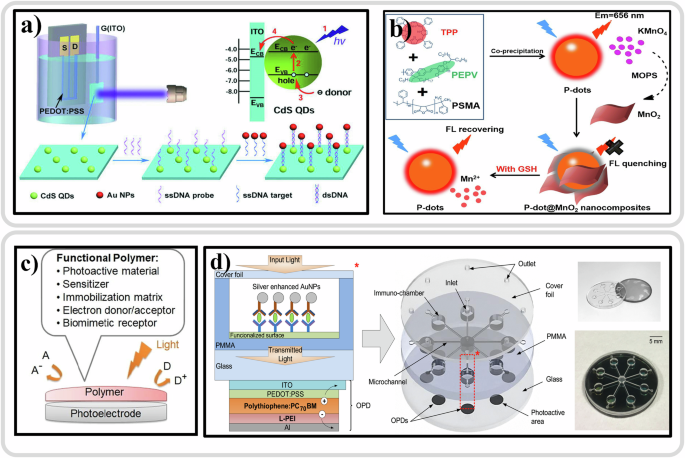
a Schematics of an OPECT-based biosensor, the charge transfer between CdS QDs and ITO gate electrode and their fabrication method. Reproduced with permission from ref. 375. b Schematic representation of the fabrication of the Pdot@MnO2 nanocomposite-based sensing platform for GSH detection. Reproduced with permission from ref. 388. c Illustration of functional polymers in photoelectrochemical biosensing. Reproduced with permission from ref. 366. d Layout of the optical microfluidic biosensor with L-PEI modified polythiophene-C70 OPDs, includes a schematic overview of the biosensor including layout details for the OPD, tridimensional scheme, and photographs of device layers. Reproduced with permission from ref. 378.
Conjugated polymers, characterized by π-conjugated semiconducting backbones, offer enhanced sensitivity in biosensing376. Functional conjugated polymers play a multifaceted role in PEC biosensing, enabling sensitive, selective, and reliable detection of biomolecules for various applications (Fig. 7c)366. Functional polymers can act as sensitizers in PEC biosensors, enhancing the absorption of light in the visible or near-infrared range. These polymers can be tailored to absorb specific wavelengths of light, thereby improving the efficiency of photoconversion processes377. Dong et al. developed an optical microfluidic biosensor utilizing polythiophene-C70 bulk heterojunction for the photoactive layer in organic photodetectors (OPDs) for detecting clinically relevant salivary biomarkers378. The schematic figure of the device architecture of the optical microfluidic biosensor with linear polyethylenimine-modified polythiophene-C70 OPDs is shown in Fig. 7d. Biosensing was carried out with additional testing using human saliva samples, and its outcomes were compared with conventional absorbance assays, namely Enzyme-linked immunosorbent assay (ELISA). Furthermore, this biosensor holds the potential for detecting various protein biomarkers using different antibodies, thereby serving in diagnostics and screening for a range of diseases.
Researchers have recently developed several distinct fluorescent probes with specific recognition capabilities using various methodologies. The compact nature of these OSC probes facilitates cellular penetration, while their remarkable diffusion characteristics enhance imaging resolution370,371,379. Nonetheless, challenges persist in the advancement of OSC probes in biosensing applications. Primarily, probes such as matrices380, metal-organic frameworks (MOFs)381,382,383, polymers384, etc. often exhibit inadequate photostability, limiting their longevity during imaging processes385. Nevertheless, OSC NPs, renowned for their exceptional qualities, find extensive applications in biosensing. At present, there is considerable interest in OSC NPs, a novel and promising class of fluorescent nanomaterials. These NPs, comprising organic semiconducting π-conjugated polymers, offer desirable attributes including excellent chemical stability, customizable surface properties, and low cytotoxicity386,387. Zheng et al. have recently devised a fluorescence nanoprobe utilizing OSC NPs and dopamine-melanin nanosystems for rapid and specific detection of Glutathione (GSH). Their design integrates NIR OSC NPs with MnO2 nanosheets. Initially, they introduced a NIR-emitting dye, tetraphenylporphyrin (TPP), into poly[(9,9′-dioctyl-2,7-divinylene-fluorenylene)-alt-2-methoxy-5-(2-ethyl-hexyloxy)-1,4-phenylene] (PEPV) chains to form compact spherical P-dots via the nanoprecipitation method as shown in Fig. 7b. Subsequently, MnO2 nanosheets were grown in situ on the surface of NIR-emissive OSC NPs to produce functionalized OSC NPs@MnO2 nanocomposites, resulting in fluorescence quenching. Furthermore, the authors evaluate the potential of OSC NPs@MnO2 as fluorescence imaging probes for monitoring cellular GSH levels388.
Biosensing with perovskite semiconductors and light
Extensive efforts have been made to develop biosensing platforms based on organic semiconductors (biosensing with organic semiconductors and light) and dye-sensitized semiconductors (biosensing with dye-sensitized films and light), owing to their good biocompatibility. Recently, halide perovskites have introduced a novel avenue for biosensing, as their optoelectronic properties exhibit sensitivity to bio-related small molecules, including hydrogen sulfide (H2S), ammonia, ammonium persulfate, and volatile organic compounds. Many halide perovskite materials have found application in colorimetric389, fluorescence390, electrochemical391, and PEC biosensors392, leveraging their high absorption coefficient, high ionic conductivity, low excitation band energy, high photoluminescence quantum yields, and superior charge carrier mobility393,394.
Chen and co-workers designed and developed a new device to isolate H2S from an aqueous solution34. By utilizing CsPbBr3 quantum dots (QDs) as the detection probe, a novel fluorescent sensor for swift H2S detection has been developed. Detection of H2S holds significant importance due to its high toxicity as a gas pollutant and its role as an endogenous gaseous signaling molecule involved in various physiological processes. Achieving high selectivity and specificity in H2S detection is crucial. Figure 8a illustrates the operational principles of the proposed fluorescent sensor for detecting H2S. H2S, being a liposoluble gas, dissolves readily in water as well as petroleum solvents. On the other hand, CsPbBr3 QDs are highly unstable in aqueous solutions, but exhibit robust fluorescence in n-hexane solutions. When the H2S sample is introduced into the phosphoric acid solution, nearly all of the H2S migrates from the aqueous solution into the n-hexane solution to interact with the perovskite quantum dots. Various concentrations of H2S were dissolved in phosphoric acid to release gaseous H2S, which was then individually introduced into the CsPbBr3 QDs solution (Fig. 8b). The corresponding changes in fluorescence intensity were recorded. Figure 8c illustrates that only H2S induced notable fluorescence quenching in CsPbBr3 QDs, while other compounds scarcely affected fluorescence, indicating minimal interference from these substances in H2S detection. These findings strongly support the proposed method’s potential for selective H2S detection34.
a Schematic Mechanism for H2S Detection via CsPbBr3 QDs. Reproduced with permission from ref. 34. b Fluorescence spectra of CsPbBr3 QDs upon the addition of different concentrations (top to bottom was 0–100 μM) of H2S. Reproduced with permission from ref. 34, c Change of fluorescence intensity of CsPbBr3 QDs in the presence of H2S (100 μM) and other interfering agents (1 mM). Reproduced with permission from ref. 34. d Molecularly imprinted polymer-modified perovskite PEC sensor for salicylic acid detection, and their photocurrent generation mechanism. Reproduced with permission from ref. 395. e Photocurrent of the MIPs/MAPbI3/ITO PEC to variable concentrations of SA. Reproduced with permission from ref. 395.
Yang et al. presented the synthesis of a dual-functional molecularly imprinted polymer (MIP)-modified organometal lead halide perovskite (MAPbI3) and its application in PEC bioanalysis of salicylic acid (SA)395. The MIP precursors were synthesized via thermally initiated free radical polymerization using SA as the template, methacrylic acid (MAA) as the monomers, ethylene glycol dimethacrylate (EGDMA) as the cross-linker, and azobisisobutyronitile (AIBN) as the initiator. The MIPs/perovskite composite was prepared by incorporating MIPs precursor solution as polymer additives during the deposition of MAPbI3 film on conductive glass substrates as shown in Fig. 8d. This method enabled control over crystal growth and morphology while reducing film defect density. Upon template removal, specific imprinting sites for SA were retained on the MIPs/perovskite film. The developed MIPs/ MAPbI3 sensor demonstrated the ability to detect various concentrations of SA, with Fig. 8e depicting a decrease in photocurrent with SA concentration. During PEC detection, SA occupied the imprinting sites through shape and hydrogen bond recognition, resulting in steric hindrances that impeded electron transfer between the electrode and O2, thereby influencing photocurrent response. This work highlights the synthesis of dual-functional MIPs-modified MAPbI3 for PEC bioanalysis.
Based on the foregoing discussion, it is evident that greater emphasis should be placed on enhancing material stability and device efficiencies. In conclusion, current research efforts on halide perovskites predominantly concentrate on solar cells, LEDs, lasers, and photodetectors. However, given the remarkable properties of perovskites, their application potential should be broadened to encompass a broader range of fields, such as photocatalytic hydrogen evolution, heavy metal detection, biosensing, and other PEC applications396.
Perovskite nanocrystals have emerged as highly promising candidates for luminescent biosensing, offering significant potential for novel applications in food product analysis. Notably, recent work by Li et al.397 had provided a comprehensive examination of perovskite nanocrystals as luminogens for high-performance biosensing of foodborne hazards. This research underscored the versatility and utility of perovskite materials in diverse applications. For instance, various biosensing approaches have been proposed, including direct fluorescent biosensors (e.g., utilizing fluorescent quenching biosensors to detect H2S based on the escape of H2S from phosphoric acid solution to n-hexane solution to quench the fluorescence of CsPbBr3 QDs34), Enzyme- and nanozyme-based biosensors (e.g., involving electron-transfer processes between H2O2 and CsPbBr3@Cu nanohybrid398), immunosensors (e.g., uniformly spraying perovskite nanocrystals399 on the test line by linking with BSA, followed by interaction with AuNPs in the presence of CEA to decrease the fluorescent intensity of CsPbBr3 perovskite nanocrystals), and electrochemiluminescent (ECL) biosensors (e.g., employing a modifying strategy of CsPbBr3 QDs film with high quality on an electrode, resulting in violent and stable ECL emission with ultranarrow emission spectral bandwidth, thus improving ECL intensity compared to conventional ECL biosensors400). These innovative approaches highlight the promise of utilizing perovskite nanomaterials for biosensing applications.
Biosensing with dye-sensitized films and light
The photoelectrochemical processes occurring in DSSCs primarily consist of photo excited electrons transfer from the excited dye molecules to the conduction band of n-type semiconductors, along with the oxidation/reduction reactions at the solid/liquid interface401. Dye-sensitized solar cells (DSSCs) as a type of photoelectrochemical device, have outperformed the conventional electrochemical cell in biosensing due to the electrical autonomy and high sensitivity features402,403 making them a promising technology for biosensing applications22. A modified version of this architecture is being utilized in some organic-based artificial retina devices16. Whereas organic photovoltaics (OPV) and DSSCs started to come to the fore in the 80 s and 90 s, halide perovskite photovoltaic technology has been developed in the last 15 years becoming one of the most promising fields in solar energy harvesting404,405 and other optoelectronic406,407,408 applications due to their high efficiency409,410,411 and low-cost fabrication processes412. Despite the benefits of perovskites, biosensing application of these materials remain a challenge due to their poor structural stability especially in the presence of water, natural environment for living systems29,30,31, and the presence of potentially-toxic lead in the most common formulations. Their application in the biosensor field33,413 is less developed compared to the aforementioned semiconductors. Nevertheless, growing literature is being published where natural biomaterials are being incorporated in or at the interface with the perovskite414,415, demonstrating an exciting potential for researching bio-photovoltaics.
The photocurrent response of DSSC is electrolyte concentration-dependent, which is sensitive to the interfacial electrochemical interactions between the dye and the redox electrolyte. Therefore, it can serve as an experimental platform for detecting biocatalytic events, where the biochemical reaction will be converted to electrical signals by the interactions between semiconductor/catalytic materials and the electrolyte-like bio-solutions with the assistant of illumination. Compared with other types of photovoltaic devices, the DSSC system holds the merits in biosensing due to the following characteristics: (i) the electrolyte-like bio-solution can readily replace the traditional electrolyte solution in the DSSC cell structure, making it easier to construct the device; (ii) the bulk liquid electrolyte system associated with biorecognition can potentially provide compatible biological environments for embedding biomaterials either in the electrolyte itself or at the interface between dye and electrolyte; (iii) the biosensing component can also be loaded as the photosensitive dye at the front electrode, meanwhile keeping the traditional electrolyte material at the counter electrode, thus providing a versatilely designable platform to monitor the bioelectrical response.
Owing to the exclusive features of DSSC configuration, various biosensing devices were developed either merging the biorecognition with counter electrode or photo-anode. Sales and co-authors have developed a simple chemistry strategy to synthesize the orientational carcinoembryonic antigen (CEA) on the modified conductive substrate and directly used bio-modified electrode as the CE for DSSC (Fig. 9a). As a result, the biosensing device displayed a linear response against CEA concentration, ranging from 5 pg/mL to 15 ng/mL23. To improve the photocurrent and sensitivity of the biosensor, Sales and co-authors further combined the biosensing element with the polypyrrole (PPy) CE by embedding a cancer biomarker, carcinoembryonic antigen (CEA), as illustrated in Fig. 9b, where the bio-complexed CE works as the biomolecule recognition, and the photoelectrical response of DSSC acts as the electrical reader22. Since biocatalytic events could affect the catalytic process of the CE and control the photovoltaic performance, the response of DSSC can be an indicator for detecting bio-behavior. Based on this configuration, Moreira et al.416 optimized the CE by using imprinted polyaniline as electrocatalytic material and aniline as monomer for integrating the CEA. As a result, the hybrid system exhibited a linear trend between the power and log CEA concentration and showed a high sensitivity to concentrations lower than 0.025 ng mL-1. This concept can be extended to other biorecognition elements when using appropriate CE materials. Thus, the bio-complexed CE concept could represent a path for integrating biomolecules in self-powered systems.
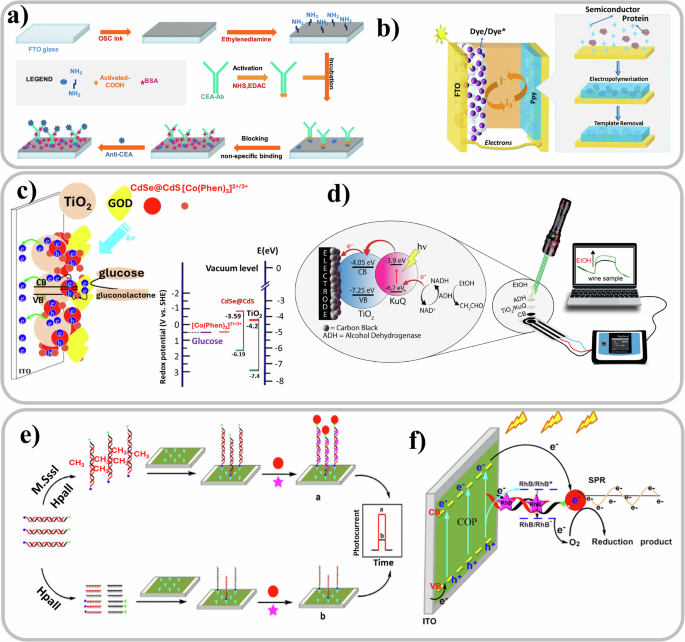
a Synthesis route of orientational carcinoembryonic antigen for merging in DSSC configuration. Reproduced with permission from ref. 23. b Schematic of DSSC merged with an imprinted layer of PPy assembled by electro-polymerization. Reproduced under the terms of Creative Commons Attribution 4.0 International License22. c Schematic of photocurrent generation and electron transfer route of the GOD/CdSe@CdS QDs/TiO2 nanocomposite photo-anode. Reproduced with permission from ref. 417. d Scheme of the carbon black based photo-anode and the involved electron transfer in the photoelectrochemical sensing system. Reproduced with permission from ref. 418. e Synthesis route of the porous COP onto an ITO substrate and integration in DSSC-based biosensor. Reproduced with permission from ref. 419. f Schematic of charge carrier transfer from the COP film in biosensing device. Reproduced with permission from ref. 419.
Apart from utilizing the electrochemical reaction at the counter electrode as the base of sensing, photo excited electron transfer at the photo-anode can also be exploited for biosensing. As shown in Fig. 9c, Zheng et al.417 fabricated a TiO2/CdSe@CdS QDs nanocomposite as the photo-anode and incorporated [cobalt(o-phen)3]2+/3+ redox couple and glucose oxidase (GOD) within the mesoporous layer. Biosensing is based on the enzyme catalytic oxidation reaction of GOD, where FADH2 groups in GOD are oxidized by [Co(Phen)3]3+ ions. The associated variations of the photocurrent can be used to detect the glucose-involved interactions. This work demonstrated the potential to broaden the scope of the TiO2/CdSe@CdS nanocomposite photo-anodes for biosensing. Another path is to use carbon material, which is a promising candidate to accommodate the biomaterials in designing biosensing devices. Mazzaracchio et al.418 reported a photoelectrochemical biosensor by using carbon black and mesoporous TiO2 as the photo-anode, which was sensitized by KuQ dye, for detecting β-Nicotinamide adenine dinucleotide (NADH), as showed in Fig. 9d. The photocurrent of the device was dependent on the concentration of the NAD+/NADH redox species, with high sensitivity with the detection limit equal to 0.062 mM, demonstrating the potential application in white wine samples. In addition to inorganic photo-anode, Cui et al.419 combined the benefits of covalent organic polymers (COPs) and metal nanoparticle-induced surface plasmon resonance (SPR) effect, and developed a dye-sensitized and Au nanoparticles plasmon-enhanced electrochemical biosensor. The electrochemical biosensor is composed of an in situ synthesized p-type COP film embedded with biotin- and HS-labeled double-stranded DNA (dsDNA) probes with the M.SssI MTase recognition sequence, as shown in Fig. 9e. The embedded dsDNA not only enhanced the interaction with the Au NPs but also the coupling with the dye-sensitizer rhodamine B (RhB) to improve the photoelectric response. The involved electron transfer path is illustrated in Fig. 9f. Upon illumination, the photo excited electrons are transferred from COPs to the Au NPs due to the enhanced charge separation induced by SPR effect; meanwhile, the electron can also transfer from the valence band of the COP film to the excited-state RhB* to facilitate photocurrent generation in the biosensor. This type of configuration can screen the potential inhibitors and has the advantage of detecting M.SssI MTase in serum with high sensitivity.
Artificial retina and vision applications
The direct interplay between light and biological cells in the retina results in the generation of electrical signals, which are then processed in the brain to allow vision. This makes the field of vision one of the most intuitive areas of research that merges the principles of light interaction and photosensitive materials. Even though vision gives an unparalleled sensing experience of nature, many people across the world are legally blind due to visual impairments like retinitis pigmentosa (RP)420 and age-related macular degeneration (AMD)421. These diseases cause blindness due to the loss of retinal cells422 and damage to macula area423, respectively. When the optical nervous system and other optical parts in the eye are intact, it has been possible to interface photovoltaic materials to the retina, sub-retinally424 or epiretinally312, to restore partial vision to the patient. Researchers have estimated that a retinal prosthesis should have 500 pixels distributed in the central area of approximately 5 mm in diameter to be useful in daily life and provide adequate mobility skills425,426,427,428. It has also been shown that it is possible to excite the photoreceptors by external stimuli like electric pulses429 and heat430. Vision-inspired photodetectors are used to develop mobile artificial vision for imaging and data processing devices431. These devices are designed to detect light and convert it into electrical signals432. These photodetectors have many potential applications, including medical imaging, security systems, and autonomous vehicles. A new line of research has emerged in the last decade, which uses materials whose optoelectronic behavior can be tuned via compositional and structural design, such as perovskite and organic semiconductors that aim to mimic the function of vision. In this section, we will explore the developments in this field.
The natural human retina
The eyes are arguably one of the most important sensing organs for a great number of species on this planet. For example, in human beings, over 80% of the information in the brain is acquired through the vision system433. Each eye contains millions of nerve fibers used for information pre-processing, transmission, and post-processing. Through parallel processing, the brain can simultaneously receive and analyze neuro-electric signals from around one million nerve fibers, through which high-speed vision processing and object recognition are executed every time someone opens their eyes. Figure 10a shows the complete structure of the human eye with a lens27, a vitreous humor (a clear gel filling the spherical space between the lens and the retina), and a hemispherical retinal layer (consisting of a multi-layer hierarchical structure) as illustrated in Fig. 10b434. The cells that transduce light signals into electrical signals are called photoreceptors. On the front side of the retina, there is a multi-layer neuromorphic network consisting of various types of neuronal cells (e.g., ganglion, bipolar cells) that are responsible for the pre-processing of neuro-signals derived from the photoreceptive cells435, after which the signals are transmitted through the optic nerve to the brain436. The photoreceptive density in the foveal region of the retina is roughly about 10 million/cm2 with an average pitch distance of 3 µm, far better corresponding to the resolution of state-of-the-art CCD/CMOS image sensors437,438.
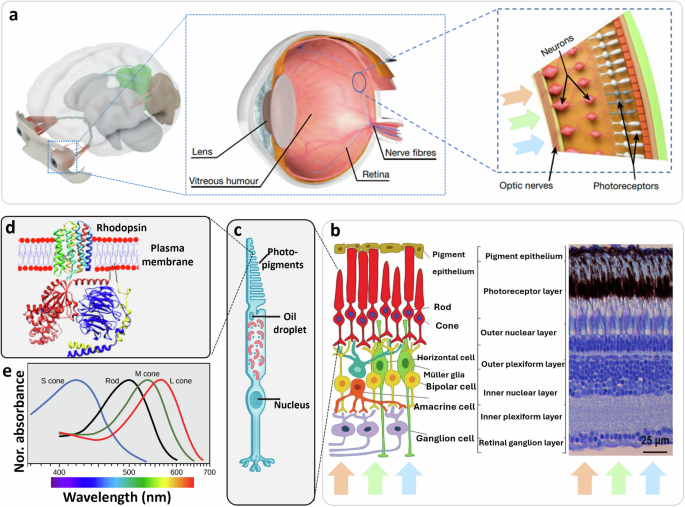
a Human visual system consists of two eye bulbs, each of which has complex optical configuration including lens, vitreous humor, retina, nerve fibers, and a film of retinal cells at the back side of the eye. Reproduced with permission from ref. 27. b Retina structure: cross-section photomicrograph through the retina, displaying the different cellular and synaptic retinal layers; and a corresponding diagram, showing the six types of neuronal cells and the two supporting cells. Reproduced under the terms of the Creative Commons Attribution CC BY-NC-ND 3.0434. c Schematic showing a cone retina cell. Reproduced under the terms of the Creative Commons Attribution CC BY 4.0 ref. 442. d Schematic showing the photoreceptor of Rhodopsin, an integral cell membrane protein. Reproduced under the terms of the Creative Commons Attribution CC BY 4.0 ref. 442. e Spectral responsive regions of the human photosensitive cells (cones and rod cells). Reproduced under the terms of the Creative Commons Attribution CC BY 4.0 ref. 442.
In the human eye, there are two main types of photoreceptors, i.e., rod cells and cone cells. Cones are specialized neurons that are responsible for color sensation (trichromatic vision in humans), while rods are responsible for night vision and are exquisitely adapted to transform light stimuli into electrical signals that modulate neurotransmitter release at the synapse439. In fact, a rod cell is extraordinarily sensitive, being able to sense a single photon of light440, while cones are up to 100-fold less sensitive than rods441. Cone cells are responsible for color vision and are realized by three types of pigments in the cone segment (Fig. 10c)442, i.e., S-cones (short wavelength or blue light absorption), M-cones (medium wavelength or green light absorption), and L-cones (long wavelength or red-light absorption). Red, green, and blue light photosensing comes from three different opsins at the membrane in the cone segment of these retinal cells (Fig. 10d)442. Figure 10e shows the color-visual spectrum of the three cone cells, ranging from long to short wavelength light as well as the photosensitive region of the rod cell442. Through the combination of using RGB cone cells and modulation by the neuro-cells, humans can have sensitive perception of color able to distinguish approximately 500 levels of brightness, 200 different hues, and around 2 million distinct colors443.
Artificial retina with organic semiconductors
Retinal prostheses that mimic the functions of the light-sensitive tissue are intended to restore some visual function in patients suffering from diseases of the eye444. Many studies are being carried out in the field of artificial vision and electrical retinal stimulation from 1755445,446,447,448,449,450,451,452,453,454,455,456,457,458. Organic semiconductor materials have found their application in the field of retinal cell stimulation from 2011299 and other vision applications thanks to their unique optoelectronic properties and being able to be integrated seamless onto any substrate, even flexible ones459,460. As mentioned in earlier sections, OSCs have shown excellent ability to photostimulate neurons12,299,461,462 and explanted retinas57,311 and it has been experimentally proven that implanted organic thin films can impart light-sensitivity to blind retinas in vivo10,463. Most importantly, since they are biocompatible271,464,465,466,467,468 and because of their soft nature64, OSCs are potentially very suitable for retinal prosthesis and may provide new functionalities compared to earlier devices based on silicon or rigid metallic electrodes requiring external power supply and adapters10,324,446,455,456,457,469,470. Some of the challenges of OSCs face before being applied in vivo to humans for artificial retina purposes are:
-
i.
withstand the biological environment present in the human body
-
ii.
interface with neurons in a way that does not cause damage or inflammation271
-
iii.
delamination of OSCs in the presence of human body electrolytes471
-
iv.
phototoxicity caused by the OSCs323.
The physiological activities, as explained before, in any living organism are mediated by water-based electrolytes and ions. OSCs can form stable interfaces with water which opens a door for incorporation of electronics in biological systems467. In 2011, Gautam et al. developed a photosensor with single layer polymer device which can be used as a tricolor sensor. OSC in this device was interfacing with aqueous medium and there was a similarity of photo response from this device to that of natural vision system472. Inspired by this, Ciocca et al.16 reported an artificial retina model device resulting from the amalgamation of different set-ups from various fields, such as electrophysiology, OSCs, and DSSCs (where biological electrolytes replace synthetic ones uses in solar cells). In this architecture, the semiconducting photosensitiser was interfaced with extracellular fluid electrolyte and sandwiched with a Pt-based counter electrode. Specifically, P3HT, poly(9,9-di-n-octylfluorenyl-2,7-diyl) (PFO), and blend of P3HT:PCBM with absorption spectra that mimic the M cone, S cone and rods, respectively, were ink jet printed as color sensing components in concentric arrays (Fig. 11a). Based on a similar configuration, Skhunov et al.473 used organic small p-conjugated donor–acceptor (D–A) molecules with various optical bandgaps, including TPA-T-C(O)H (blue), N(Ph-2T-DCV-Hex)3 (green), TPA-BTZ-Rh-Et (red) and N(Ph-T-CNA-EtHex)3 (rods) to mimic S, M, L cones and rod cells, respectively (Fig. 11b), to realize full-color light response. To improve the solid/liquid interfacial charge transfer by 20 times, a ZnO interlayer was deposited on the ITO electrode surface. Likewise, inkjet printing was employed to deposit 20~35-micron diameter pixels473. These diameters would meet the resolution requirements for restoring 20/100 vision. Lately, poly(triarylamine) PTAA and indenofluorene-phenanthrene copolymer PIFPA conjugated polymers were also successfully employed in an artificial retinal model for human S cones mimicking474.
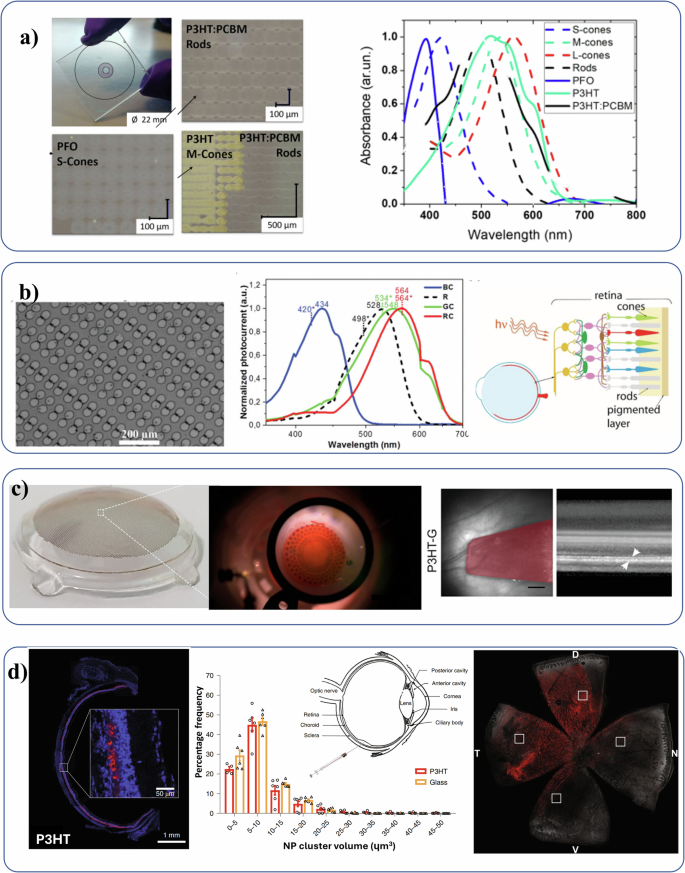
a Inkjet printed radial symmetric artifical retina model developed by Ciocca et al. The pixels of P3HT, PFO, and P3HT:PCBM are showed. On the right, the absorbance spectra of human photoreceptors (dashed lines: Rods, S-Cones, M-Cones, L-Cones) compared with those of polymers used, reproduced with the Creative Commons Attribution 4.0 International License with ref. 16. b Left to right: scanning electron microscope (SEM) image of inkjet printed, red absorbing organic pixels in this work, at 375X magnification, typical normalized photocurrent spectra of thin films spin coated on glass/ITO/ZnO substrates, interfaced with an electrolyte (PBS). Spectra corresponding to three materials (TPA-T-C(O)H (BC), N(Ph-2T-DCV-Hex)3 (GC), TPA-BTZ-Rh-Et (RC)), and schematic of an eye, and retina cross-section showing neurons connected to rods and cones, reproduced with the Creative Commons Attributon-NonCommercial 3.0 Unprotected Licence with ref. 473. c Left to right: Picture of the high-density POLYRETINA prosthesis with 10,498 photovoltaic pixels324, picture of POLYRETINA placed in epiretinal configuration312, and image of the retina P3HT-G (magenta) implanted in a rat showing the sagittal view of the prosthetic placement at 30 DPI (left; scale bar, 1 mm), and the respective OCT images showing the transversal section of the retinas and the device positioned subretinally (right, white arrowheads; scale bar, 200 µm). Reproduced under the terms of the Creative Commons Attribution CC BY-NC-ND 4.0 with ref. 460. d Left to right:full equatorial reconstruction of dystrophic retinas from RCS rats injected with either endogenously fluorescent P3HT-NPs17, showing the tangential diffusion of NPs (red) in the whole subretinal space, percentage frequency distribution of the volume of P3HT and glass NP clusters (5-μm bins) at 30 DPI17, and whole-mount retina from 13/15-month old RCS rat were analyzed by confocal z-stack scans to localize P3HT-NPs, reproduced under Creative Commons Attribution 4.0 International License with ref. 59.
Understanding semiconductor interaction with neurons and explanted retina were required before initiating the development of devices for implantation. Ghezzi et al. developed a hybrid bioorganic interface for neuronal photoactivation in 2011. The device interfaced a network of primary neurons with an organic blend and carried out the optical stimulation measurement with patch clamp set up. This work showed that primary neurons can be grown on OSCs without affecting the optoelectronic properties of the latter and biological functionality of the neuron network. It was also clear that action potential can be triggered in a spatially selective manner with short pulses of light299. Later in 2013 they were able to measure with patch clamp the neuron firing in explanted retina of blind rat placed on P3HT coated ITO glass57. Similarly in the same year Vini Gautam311 and others developed a polymer optoelectronic interface on an multielectrode array which provided visual cues to an explanted blind chick retina. The photoelectric signals initiated by the OSC thin film evoked neuronal activity with features resembling the natural response of the retina to light stimulation. These results suggested the possibility of developing OSC based artificial retinal implants.
The possibility of eliciting signals from cells interfaced with OSC thin films demonstrated using multielectrode array (MEA) or electrophysiological tools like patch clamps, and the viability of retinal tissue cultures on these films, has spurred researchers to explore other avenues for fabrication of artificial retina systems, which can be implanted. Two types of approach64 for providing OSCs-based artificial vision are being studied:
-
i.
interface polymer films460,475 or pixels324 made of different polymers with malfunctioning retinas for mono or poly-chromatic photostimulation (the implanted devices of these sort are represented in Fig. 11c);
-
ii.
injection of conjugated polymer NPs476,477 in the subretinal area of the eye to restore lost functionalities to the retina.
Of the first type Antognazza et al. used retinal devices with OSC over silk substrates which they implanted into the subretinal space of rats in 2016. From this study they were able to characterize the prosthesis for implantation and demonstrate OSC based flexible retinal device implanted in non-dystrophic eyes, was highly biocompatible, and suitable as retinal prosthesis in case of photoreceptor degeneration478. Later in the next year they implanted similar device in a rat model of degenerative blindness and from the electrophysiological and behavioral analysis, the rescue of the visual function was confirmed10. In 2018 a foldable and wide-field epiretinal prosthesis called POLYRETINA was developed312 and it was demonstrated in 2021 that this device was able to restore high-resolution response equivalent to the pixel pitch of 120 µm in blind324. Later in 2022, the device was implanted to restore light responses in blind Göttingen minipigs58. Implantation of retinal prosthesis based on OSCs in pig eye can help translate this technology to use in human eyes9.
Of the second type, Lanzani and researchers17,477 were able to implement nanoparticle-based retinal prosthesis, which is directly injected onto the subretinal region, and by controlling the shape and size of the nanoparticle, they can obtain the desired photostimulation of the retinal cells(Fig. 11d). The injected P3HT-NPs which spread out over the entire subretinal space mediated a light evoked stimulation of retinal neurons and rescued the visual function17. Recently, these NPs restored vision in advanced stage retinitis pigmentosa rats and reinstated physiological signals at the cortical level59. In 2019, Ma et al. were able to design ocular injectable photoreceptor binding upconversion NPs and these materials were found safe and enabled NIR light-sensation to the mouse retina with negligible side effects479. In 2024, Wang et al. developed nanocomposite composed of ultra-small conjugated NP and ferroelectric P(VDF-TrFE) exhibiting excellent photo-pyroelectric response under visible and infrared light, with a peak-to-peak voltage exceeding 90 V. The ultra-thin retinal prostheses (∼ 20 µm) constructed from this nanocomposite could directly stimulate nerve cells, restore the visual function of blind rats, and endow them with infrared light-sensitivity480.
In the last two years the interest in artificial retina and synaptic devices based on OSCs and other novel materials for vision-based applications has been growing rapidly481,482,483,484,485,486,487,488,489. Askew et al. demonstrated conjugated polymer photocapacitor devices immersed in electrolyte eliciting a photovoltage of ≈−40 mV for 15–39 µW mm−2 using blue, green and red polymer photoreceptor candidates. Photoresponses were improved by introducing polymer donor/acceptor molecules reaching between −70 mV and −200 mV474,490. New polymer materials can not only mimic photoreceptor cones but can also be more efficient in the transduction proposes compared to the workhorse P3HT490. Zhu et al. developed flexible composite films of ferroelectric BiFeO3-BaTiO3 particles and ferroelectric poly(vinyldene difluoride-trifluoroethylene) with a strong photoelectric response from visible to infrared. Blind rats implanted with the flexible composite artificial retinas displayed light-responsive behavior491. Francia et al. engineered a hybrid retinal prosthesis consisting of a dual P3HT and graphene layer onto a flexible substrate, and subretinally implanted in vivo in rats460. Implanted dystrophic rats restored visual performances at both subcortical and cortical levels in response to light stimuli, in the absence of marked inflammatory responses. Moreover, the analysis of the physical-mechanical properties after prolonged permanence in the eye showed excellent biocompatibility and robustness of the device. Yang et al. detailed the performance of subretinally implanted gold-nanoparticle-coated titania nanowire arrays in mice and monkeys in ex vivo retinas (as determined by patch-clamp recording of retinal ganglion cells)492. In blind mice, the arrays allowed for the detection of drifting gratings and flashing objects at light-intensity thresholds of 15.70–18.09 μW mm–2 and offered visual acuities of 0.3–0.4 cycles per degree, as determined by recordings of visually evoked potentials and optomotor-response tests. In monkeys, the arrays were stable for 54 weeks.
Artificial retinas are not only intended to be implanted in the eye, but much research concentrates in mimicking the operation of human vision using artificial materials (with no interfacing to biological systems or aqueous media). These devices can be in the form of photodiodes493 but also other types of forms (e.g. wires) and devices (e.g. thin film transistors494,495). Wang et al. demonstrated an artificial visual neuron employing polymer heterojunctions, wherein on of the films induces a charge trapping effect496. The photogenerated charge trapping and detrapping processes at the interface were shown to emulate synapse-like behavior generating excitatory postsynaptic currents akin to biological visual perception curves. Integrated in a circuit, the systyme executed motion recognition on a machine cart preventing collisions with high-speed obstacles. Sun et al. described a retinomorphic neuron photoreceptor system based on Au nanoparticle decorated ITO fiber, an electrochromic device as a light filter, and a spike-generation unit that mimicked the biological structure and processing functions of retinal neurons for light-sensing and signal transduction including pupillary light reflexes497. Luo et al. combined neuromorphic principles with retinal and ionoelastomer engineering, to engineer a self-driven hemispherical retinomorphic eye with elastomeric retina made of ionogel heterojunction as photoreceptors498. The receptor driven by photothermoelectric effect showed photoperception with broadband light detection (365 to 970 nm), wide field-of-view (180°) and photosynaptic (paired-pulse facilitation index of 153%) behaviors for biosimilar visual learning. Trung et al. developed a bio-inspired artificial retina based on a fibrous photonic artificial synapse formed by organic–inorganic heterojunctions on a single fiber and operated without external power by modulating the capture and release of photo-generated carriers and the photo-gating effect at barriers formed by organic–inorganic heterojunctions499.
Zhang et al. developed retina-inspired optoelectronic synaptic transistors that present broadband responses covering ultraviolet, visible, and near-infrared regions, which leveraged the wide-range photoresponsive charge trapping layer and the heterostructure formed between PbS quantum dots and organic semiconductors (pentacene and PMMA)500. The optoelectronic synaptic transistors displayed optical programming and electrical erasing features, allowing the simulation of typical synaptic functions under a wide wavelength ranges. Zhang et al. presented a dual-gated thin-film-transistor artificial optoelectronic neuronic device based on TIPS-pentacene intentionally tailoring its optical bandgap to 1.6 eV that replicated various photon-triggered synaptic characteristics, performing visual sensing, memory processing, and other functions with low power consumption501. Gao et al.502 exploited solution-processed poly(amic acid) dielectrics and organic semiconductors to fabricated transistor arrays for photodetection across ultraviolet to near-infrared spectral bands. The successful execution of flexible imaging, Pavlovian classical conditioning reflex associative learning, coupled with a 93.9% accuracy in handwritten numeral recognition, corroborated the viability of these high-mobility, low-power organic neuromorphic synaptic transistor arrays. Carboxyl groups were introduced in polymer dielectrics induce effective charge transfer at the semiconductor/dielectric interfaces improving image sensing503.
Artificial retina and vision applications with perovskite semiconductors
Halide perovskites have been recognized as excellent semiconducting light-sensing materials due to many merits such as direct bandgap nature allowing sufficient light absorption with thicknesses in the hundreds of nm range, high defect tolerance allowing efficient photocarrier collection, and structural/chemical modulation that makes it possible to tune the spectral response range from UV to NIR regions. As explained in earlier sections many perovskites degrade in water environments and may not be directly biocompatible, replacing natural retinal cell sensors with synthetic perovskites requires proper material engineering to counteract the intrinsic instability in aqueous environments, and achieve greater biocompatibility.
A direct mimetic strategy is to use the halide perovskite as a light sensor to convert light signal into electricity, where the electrical potential can stimulate the neural cells in the neuromorphic network in front of the retinal film in the eye (Fig. 12b). For example, Lee et al.504 reported an artificially intelligent (AI) photonic synapse using arrays of perovskite nano-cones, which were grown from a nano-template of self-assembled block copolymer (BCP). They employed a field effect transistor (FET) structure with a floating gate. These perovskite arrays can electrically induce charge (de)trapping and photo-carrier generation and exhibit versatile synaptic functionalities (mimetic of the nervous system), such as long-term potentiation and paired-pulse facilitation. Briefly, as shown in Fig. 12a, the BCP/perovskite nanocrystal bilayer films worked as the floating gate of a FET (bottom gate and top contact structure). In general, such a three-terminal FET design can be more advantageous than two-terminal alternatives because of higher operational stability and lower batch-to-batch variability505. Basically, in our brain synapses are contacts which enable communication via transfer of signals between two neurons. They can strengthen or weaken over time in response to an increase or decrease of their activity. This ability to change strength is referred to as synaptic plasticity, and it is fundamental to the processes of learning and data storage (memory). In this study, signal transmission is concurrent with the synaptic weight modulation by the FET gate. The concurrent learning refers to a mechanism akin to Hebbian learning, where an increase in synaptic strength occurs when the pre-synaptic neuron (sending neuron) and post-synaptic neuron (receiving neuron) are activated concurrently. In neuromorphic systems, a FET could be used to mimic this synaptic behavior. The gate of a FET can modulate the current flow, acting much like a synapse modulating signal transmission between neurons. The statement implies that the artificial synapse can adjust its strength (i.e., the FET gate can modulate the current flow) while it is transmitting signals, thereby mimicking the concurrent learning observed in living brains506. Based on these, through a perovskite photocarrier generation upon light stimuli and the use of this photonically modulated signal transmission as a gate to modulate the electrical conductance in the drain and source channel, a photonic synapse can be conceptualized. Furthermore, by means of modulating the spatial distribution of the perovskite nanocrystals on the BCP templates, the photonic synaptic behavior can emulate the human retina functions, with a biomimetic structural design (Fig. 12a). Intelligent functionalities, similar to those found in a natural nervous system, e.g., paired-pulse-facilitation (PPF), long-term potentiation/long-term depression (LTP/LTD), and learning-experience behavior, can be realized from a 60 × 12 array device of perovskite light sensors504. This approach that designs multiple pixel photoreceptive devices on limited area with distributed dispersion of the devices can become a new route to design light-sensitive e-skin and intelligent visual perception. In contrast to organic semiconductors, because of low exciton binding energies, light absorption in perovskites can directly lead to the presence of free charge carriers of higher electrical potential energy, so that photogenerated signals are greater. These can be used as the electrical source for further transport, storage, and process, in a similar way to that of digital cameras. Following this concept, Gu et al.27 presented an artificial eye, consisting of a hemispherical retina made from perovskite nanowire arrays working as light sensors to mimic retina cells. Figure 12b shows the cross-sectional SEM images of the FAPbI3 nanowires in a porous aluminum oxide membrane (PAM) as well as the working mechanism schematic of an individual pixel device under an external bias voltage of -3 V27. Briefly, upon light excitation, the perovskite nanowires generated free electrons and holes which could be extracted and routed towards the corresponding electrodes. However, it is interesting to mention that although each nanowire works as an individual device like a single retinal cell, it remains questionable that it seems all the nanowire devices share the same liquid electrolyte (1-butyl3-methylimidazolium iodide, i.e., BMIMI) in the vitreous humor to form a closed electric loop and functionalize the device. Hence, the signal readout from each pixel device cannot occur at the same time. This may open the area for further structural optimization to completely mimic the retinal system in the human eye. Nevertheless, this work presents a valuable artificial eye to duplicate the delicate natural visual system from each component in the eye. In 2023, Long et al. extended this line of bionic eye technology and its capabilities in color vision, optical adaptivity, and energy efficiency by comprising tunable liquid crystal optics, and a hemispherical neuromorphic retina with filter-free color vision, enabled by wavelength-dependent bidirectional synaptic photo-response in a metal-oxide nanotube/perovskite nanowire hybrid structure. Moreover, by tuning the color selectivity with bias, the device could reconstruct full-color images28.
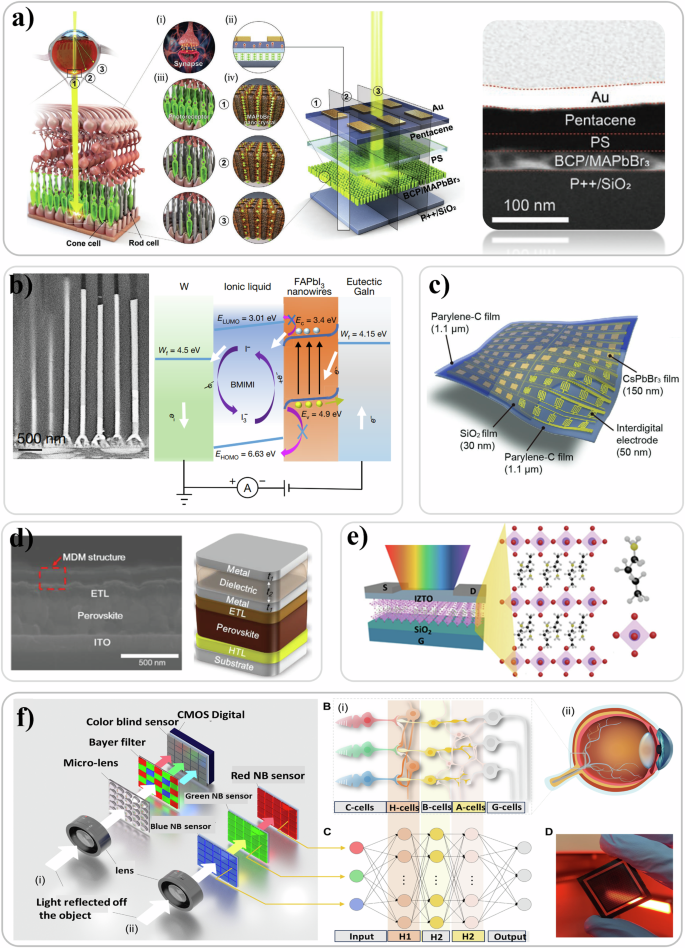
a Device and schematic of the human vision system containing: (i) synapse and (iii) photoreceptor, as well as the corresponding (ii) & (iv) device mimicking (i) and (iii). A cross-sectional SEM showing the device configuration. Reproduced with permission from ref. 504. b Cross-sectional SEM micrograph showing FAPbI3 nanowire crystal in the aluminum oxide membrane micro mold through a vapor deposition. Band diagram and working principle of the nanowire device. Reproduced with permission from ref. 27. c Structural configuration of an ultrathin photodetector arrays. Reproduced with permission from ref. 507. d Cross-sectional SEM micrograph and device configuration of a band tunable microcavity integrated on a perovskite photoreceptor. Reproduced with permission from ref. 508. e Schematic of the optical synapse device made of 2D perovskites. Reproduced with permission from ref. 511. f Illustration of a retina-inspired narrowband perovskite photodetector (PD) system for panchromatic imaging. It includes schematic designs of two configurations: typical parallel and stacking layouts of the panchromatic imaging sensor. Inspired by the neural network architecture of the human eye, a three-layer neuromorphic algorithm is depicted for signal processing and image reconstruction. Finally, a photo of a prototype perovskite NB PD array developed in this work is shown, demonstrating the practical implementation of the design. Reproduced with permission from ref. 24.
Moving forward, if their moisture instability can be solved, perovskite can become attractive for attaching a membrane like array device matrices to degenerated retinal cells to directly photo-sense light and convert light information into potential stimuli to the synapses of neural cells. This however will require a certain flexibility of the retinal-like matrix device and many experimental efforts to physically photo-excite neural cells through the perovskite light sensor. Recently, Wu et al.507 reported an ultrathin perovskite photodetector array (with a thickness of 2.4 μm) using relative more stable CsPbBr3 perovskite and waterproof parylene-C as an encapsulation protector. As shown in Fig. 12c, the device consisted of 10 × 10 individual pixels, and each contained five functional layers of (i) parylene-C substrate, (ii) Au film, (iii) SiO2 film, (iv) CsPbBr3 film, and (v) another parylene-C encapsulation layer. This configuration was able to detect light images and convert them into electrical current maps (i.e., imaging functionality).
The perspective of functional and structural mimetic systems, using synthetic materials and devices to duplicate the ability of human photoreceptors to distinguish color, can be of great interest. The modern digital camera utilizes a color filter to obtain red, green, and blue light for the specific wavelength detection of a CCD/CMOS silicon photodetector. This color filter can also be redesigned using a microcavity configuration. For example, Tsai et al.508 reported a band tunable microcavity structure integrated with a perovskite absorber to realize the color detection function. Briefly, Fig. 12d shows the metal/dielectric/metal (MDM) microcavity structure. MDMs are complementary to dielectric/metal/dielectric (DMD) structures509, where the thicknesses and materials of each layer can be engineered in order to obtain maximum broadband transmission, which has been used to develop highly anti-reflective and transparent electrodes, even flexible ones510. Differently from MDMs, DMDs can lead to a designated narrow-band transmission. Fundamentally, the metal layers (thickness of t1) control the cavity strength and the spectral FWHM of the light, and the dielectric layers (thickness of t2) determine the optical path of the cavity and consequently the peak wavelength of transmission. Eventually, the group successfully obtained artificial photoreceptors showing a high detectivity of 1013 Jones, large linear dynamic range of 154 dB, and a short response time of 580 ns, with certain spectral selectivity509. In designing a real device, additional factors must be considered, such as the structural continuity and conductivity of the metal layers, the lateral uniformity and dispersion, the selection of materials with appropriate optical properties, the optical interactions, and the interference between different materials, and the overall manufacturability.
Previous studies have used perovskites as light sensors to replicate the functions of photoreceptor cells of the retina. This is a promising step towards using man-made materials to mimic natural body systems, which could eventually help replace the faulty body parts for medical reasons (despite the drawbacks of current perovskite materials, future perovskite type materials with enhanced stability and biocompatibility are needed). The idea of using these synthetic materials to recreate the smart functionalities of natural vision systems is also exciting and filled with potential. For example, Park et al.511 reported a synaptic transistor that not only could detect light information but also possess capability of long-term visual memory. Figure 12e shows the device structure, composed of 2D layered perovskites (BA2PbBr4) deposited on indium zinc tin oxide (IZTO), through a sequential vapor deposition technique. By applying short pulse optical signals to the device, a transient current signal spike is obtained. This can be used to mimic the short-term plasticity (STP), which is a temporary dynamic modulation of the synaptic weight (at seconds and minutes time scale, in comparison to long-term plasticity (LTP) that lasts days to years scale512). For optical pulses of short duration <50 ms, drain currents increased upon the optical excitation in the perovskite. The spikes were similar to the neural spike transmitted in neuron systems (e.g., brain). In parallel, another type of neuron behavior is the paired-pulse facilitation (PPF), which is a synaptic plasticity of short-term and activity-dependence, and it is commonly present in general synapses using chemical neuron-transmitting mechanism. The term “short-term” refers to the temporal aspect of these changes. In the context of PPF, synaptic plasticity is not permanent but lasts for a short duration, which can range from milliseconds to a few minutes. These changes are generally quickly reversible and depend on the recent history of presynaptic activity or postsynaptic demand. “Activity-dependence” indicates that the changes in synaptic strength are directly related to the level or intensity of neuronal activity. When neurons are more active, the synapse (the junction between two neurons) becomes more effective at transmitting signals. This activity-dependent plasticity is a fundamental property of the nervous system and is critical for many processes, including learning and memory. The signature of PPF is the enhanced amplitude of the subsequent quickly evoked excitatory postsynaptic potentials (EPSPs513). The larger the time interval between two synaptic spikes, the lower the amplitude of second postsynaptic spike, process used in nature to recognize and memorize information514. The time interval between two synaptic spikes can indeed affect the strength or amplitude of the second postsynaptic spike. This is due to the transient nature of synaptic plasticity. When the time interval between two spikes is large, the effect of the first spike might have already worn off by the time the second spike arrives, which could result in a smaller postsynaptic response. This mechanism can be seen as a type of synaptic memory, where the history of synaptic activity influences current synaptic function. Many species in the natural world use this mechanism for information processing, recognition, and memory, as data storage. By adjusting the strength of synaptic connections based on the timing of spikes, the nervous system can encode and store information. By applying pulsed-light stimuli at different time intervals, current spikes can be observed. They can be used to obtain the PPF performance of the artificial device.
The retina, a pivotal component of the human visual system, assumes the critical task of receiving incoming light, converting it into neural signals, and subsequently transmitting these signals to the brain for visual interpretation. Its cone cells (long, medium, and small cones with absorption peak at red, green, and blue (R/G/B) respectively) function as innate narrowband photodetectors (PDs), finely attuned to discern R/G/B lights. Numerous studies have endeavored to replicate the light sensing capabilities of retinal cells496, aiming to discern light color515, intensity516, and other attributes517. However, while much research has focused on the optical-to-electrical conversion aspect of retinal mimetics, the intelligence inherent in signal processing has often received less attention499. In reality, the retina’s intricate multilayer neuron-network orchestrates neuromorphic preprocessing of signals before their transmission to the brain, thus showcasing inherent intelligence. Recently, Hou et al.24, drawing inspiration from this sophisticated biological mechanism, proposed a narrowband (NB) imaging sensor. As seen in Fig. 12f, this sensor integrates R/G/B perovskite NB sensor arrays, mimicking the function of R/G/B photoreceptors, with a neuromorphic algorithm emulating intermediate neural networks, thereby achieving high-fidelity panchromatic imaging. This approach leverages perovskite “intrinsic” NB PDs, obviating the need for complex optical filter arrays commonly employed in commercial sensors. Moreover, by adopting an asymmetric device configuration, photocurrent collection is facilitated without external bias, thereby enabling a power-free photodetection feature. These findings signify a promising design paradigm for the development of efficient and intelligent panchromatic imaging systems.
In 2023, Liu et al. developed a high-resolution perovskite-based color camera using a set of narrowband red, green, blue, and broadband white perovskite photodetectors as imaging sensors that enabled high-resolution color images (up to 256 × 256 pixels) in diffuse mode. The narrowband RGB perovskite photodetectors mimicked long-, medium-, and short-wavelength cones cells whereas the broadband white perovskite photodetector with better detectivity mimicked rod cells for improving weak-light imaging ability25. Vijjapu et al. developed metal-insulator-metal type capacitors based on hybrid nanocomposites of perovskites (methyl-ammonium lead bromide) and the ferroelectric terpolymer (polyvinylidene fluoride trifluoroethylene-chlorofluoroethylene). These capacitors exhibited photosensitive capacitive behavior in the visible range that mimiked the spectral sensitivity curve of human photopic vision. The hybrid nanocomposite was stable in ambient air for 129 weeks as a result of encapsulation with a hydrophobic polymer. The functionality of the proposed photoreceptor was validated to recognize handwritten digits datasets using an unsupervised trained spiking neural network with 72% recognition accuracy518. In 2024, Park et al. presented an artificial vision system that capitalizes on certain interesting aspects of avian vision. The system was composed of an artificial Gaussian-shaped fovea that could magnify and focus an object similarly to a zoom lens and a vertically stacked perovskite photodetector array that could detect R, G, B, and UV light without filters optimized with theoretical simulations. The artificial vision system was able to successfully identify colored and mixed-color objects and detects remote objects making it particularly promising for uncrewed aerial vehicles for detecting target objects and their motion26.
In summary, when comparing OSCs and perovskite-based semiconductors for artificial retina applications, each technology offers unique advantages and faces specific challenges regarding biocompatibility, efficiency, and practical use in biomedical settings. OSCs possess suitable biocompatibility and mechanical flexibility, which allows them to interface with the soft tissues of the retina more effectively compared to rigid photosensors. This property makes them particularly suitable for integration in biological environments. Additionally, the chemical tunability of OSCs offers a broad spectrum of optoelectronic properties, which can be tailored to mimic photoreceptor responses of cones and rods, making them particularly suited for application in artificial retinas. Notably, artificial retinas based on OSCs have already been successfully implanted in animals, demonstrating their viability in real-world applications. OSCs are susceptible to long term degradation when exposed to environmental factors like light and moisture, raising concerns about their durability. Long term in vitro, in vivo, and clinical studies will help gauge their potential for long term operation. OSC in their thin film or patterned form have been implanted in animal retinas surgically. Instead, more recently, OSCs in their nanoparticle form have been injected in retinal tissues leading to successful restoration of some vision capabilities in rodents with promising stability, without the need of the more invasive surgical procedures of semiconductors on substrates.
Perovskite semiconductors boast significantly higher efficiency in light conversion to electrical signals, making them attractive for low-light environments, such as those encountered in retinal implants. Perovskites can also be engineered for tunable bandgaps, enhancing their ability to mimic natural photoreception in the eye. Exciting new research has delved in mimicking the response of the eye in image sensors. Despite these strengths, perovskites face significant obstacles related to stability and biocompatibility if this semiconductor aims to be interfaced with biological systems or be implanted. The most widespread perovskite compositions degrade rapidly in the presence of moisture and oxygen, and lead-based perovskites pose toxicity concerns that complicate their clinical viability. As a result, perovskite-based technologies currently remain limited to image sensor prototypes. Therefore, while perovskites offer excellent optoelectronic properties, significant research is needed to enhance their long-term stability and reduce their cytotoxicity to broaden their applicability to biological systems.
Outlook for photovoltaic bioelectronics
To create a more detailed roadmap for future research in photovoltaic bioelectronics, one needs to address several key research questions and strategies. A critical area to concentrate on is enhancing the stability, and efficacy of bioelectronics devices in physiological environments. A fundamental question here is: how can the long-term stability of the photovoltaic materials, such as perovskites and organic semiconductors, be improved in bio-fluids (without sacrificing efficacy in the transduction process)? Research strategies must be twofold: improve the inherent stability of the semiconductors in aqueous electrolytes, by compositional design as well as functionalization, and exploring new encapsulation techniques to protect photovoltaic materials from degradation caused by moisture, oxygen, and ionic species present in biological environments. Developing bio-compatible and impermeable coatings or multi-layered barrier materials could help shield these devices. This has to be achieved whilst maintaining both mechanical flexibility and softness as well as the desired optical (e.g. transparency) and electronic (e.g. adequate interfacial charge accumulation or transfer) properties. Additionally, exploring self-healing materials or dynamic interfaces that repair or adapt to minor damage could extend the lifetime of devices, particularly for implantable devices subject to mechanical stress or biochemical interactions. Utilizing bio-derived or bio-inspired materials for functionalization or as interfacial layers might be an avenue of exciting research. Efficiency is another important challenge, especially for light-harvesting systems designed for biological applications. A pertinent question here is: what modifications in material composition or architecture can optimize energy conversion efficiency or the efficacy of the transduction process of the photogenerated signal towards the biological system in low-light or fluctuating light conditions, such as those within the human body? Strategies may include optimizing light absorption and charge carrier mobility in organic semiconductors or hybrid perovskites by tuning the material’s bandgap to match biological light absorption spectra. Innovations in nanostructured surfaces could further enhance light trapping and efficiency. Artificial retinas can be taken as example that enhancing both performance and biocompatibility over time can become enabling for the implementation of successful biomedical applications. The technology must continue to advance to ensure both safe implantation (or injection) and stable operation so that the restoration of vision can be maintained over sufficient time. Enhancing the selection of biocompatible, stable next-generation semiconductors, combined with advanced printing, patterning, functionalization, or injection techniques, will enable better image resolution, possibly even color vision, and restore enough visual perception to those patients with retinal dystrophies to carry out the widest possible range of tasks.
At the microscale there is also much to delve into and understand. The biophysics of the transduction process at the interface between the semiconductors and the biological systems, including the electrolytic media and living cells, upon photoexcitation, is still not well explained. Researchers can use ultra-fast and/or impedance spectroscopy to delve deeper in the bio/semiconductor interfacial interactions which happen at different time-scales (photoexcitation, charge generation, relaxation, charge separation, generation of space charge, trapping, ionic motion, ionic/charge accumulation). Advanced imaging techniques like super-resolution microscopy (SRM) could be used to study the nanoscale interactions between biomaterials and semiconductor interfaces. SRM goes beyond the limitations of conventional microscopy, offering nanometric resolution, multi-color imaging, and the ability to detect single molecules519. This powerful capability can allow scientists to visualize complex cellular structures in greater detail, providing a clearer understanding of how biomaterials interact with semiconductor surfaces. Deeper insight into biomaterial-semiconductor interactions could drive significant advancements in the field of photovoltaic bioelectronics. By optimizing these interfaces, researchers can improve the performance of technologies such as photovoltaic energy harvesting, biosensors, and artificial retinas, ultimately leading to more efficient and effective bioelectronic devices.
Computational modeling and simulation will also serve as indispensable tools in advancing and accelerating the development of bio-integrated photovoltaic systems by simulating their performance under various physiological conditions, including different ionic and electronic transport properties, tissue conductivity, temperature fluctuations, fluid dynamics, and the effects of mechanical stresses on device performance, helping to deliver reliable predictive capabilities of device behavior in vitro and in vivo. Finite element analysis (FEA), for example, enables the modeling of how organic photovoltaic semiconductors or perovskites interact with biological environments, including the effects of mechanical strain on device stability and performance. Moreover, FEA modeling approach is also valuable for optimizing the light absorption, charge transport, and photothermal response of photovoltaic materials when applied in biomedical contexts such as implantable biosensors or neuronal stimulation devices. Furthermore, the use of multiphysics simulations, which integrate electrical, thermal, and mechanical effects, can inform the researcher and help guide in the design of materials and interfaces that both maximize efficiency and biocompatibility. Incorporating computational models will also enable rapid prototyping, reducing reliance on time-intensive experimental studies, allowing for faster iteration cycles in device design. Additionally, machine learning algorithms can be leveraged to analyze large datasets generated from these simulations, enabling the identification of optimal material properties and device architectures. By predicting and mitigating potential issues before clinical trials, computational modeling will significantly accelerate the transition from laboratory research to real-world medical applications.
Beyond the technical challenges, the development of photovoltaic bioelectronics necessitates careful consideration of the associated ethical and societal implications. While this technology offers significant potential for therapeutic applications, particularly through the modulation of biological responses via bio-integrated devices, it is important to acknowledge the complex issues related to human enhancement that could emerge. For example, although these advancements may improve health outcomes and monitoring, they could also blur the distinction between therapeutic interventions and human enhancement, raising ethical questions about fairness, accessibility, and the potential social implications of human augmentation. Such concerns underscore the need for responsible stewardship in guiding the development of these technologies. Furthermore, the continuous monitoring and collection of biological data through bio-integrated devices could introduce potential risks to privacy and data security. Questions surrounding the ownership, control, and potential misuse of this sensitive data must be addressed, particularly in the context of ensuring that individuals’ privacy rights are protected. Additionally, the long-term biological effects of embedding electronic devices within human tissues are not fully understood. While early results have demonstrated biocompatibility, more research is required to assess the risks of chronic exposure to electronic stimulation or foreign materials, particularly over extended periods. Thus, as we look toward the future of photovoltaic bioelectronics, it is critical to remain mindful of these ethical dimensions.
Conclusions
This review has analyzed the merging of new-generation photovoltaic technologies based on organic, perovskite, and dye-sensitized semiconductors with biological systems, a branch of research we term photovoltaic bioelectronics. Advancement in fabrication techniques and tailoring of material properties of these semiconductors, together with the incorporation of biomaterials have opened new horizons: energy harvesting devices in bio-photovoltaics, modulation of the light-induced response in bio-hybrid devices using light as stimulus, novel concepts in biosensing, and mimicking photoreceptor cell functionality with artificial retina and vision models.
Very good compatibility between biological and some of the new generation semiconductor materials designs has enabled the inclusion of various biomaterials in the stack of multilayer PV devices. For example, the incorporation of biomaterials such as DNA in organic and perovskite solar cells, either as interlayers or in semiconductor films, has already been successful in improving the performance and stability of devices. Figure 13a demonstrates that there has been an increase in interest in the field of bio-photovoltaics for the last 10 years, especially in those concerning perovskite and organic semiconductors (data were collected from SCOPUS using keywords such as perovskite AND biomaterial etc. for each of the new generation PV). It is also evident that the replacement of synthetic dyes in DSSCs with natural ones such as plant-derived pigments (the most used, with notable performance, being chlorophyll) continues to be the most studied arena in bio-photovoltaics, a line of research driven by the fascination, and potential eco-friendliness, of being able to use biological dyes to produce electricity. Significant improvements in device performance and stability have to be attained for these materials to be used in power generation. For example, the highest PCE reported for conventional OSC (19.2%), PSC (26.1%), and DSSC (14.1%)183 are higher than the highest PCEs of cells (OSC 18.35%, PSC 22.63% and DSSC 4.6%, see Tables 2, 3, 4) that incorporate biomaterials. NPs of carbon and silver derived from biological sources like plant extracts have been documented for their utilization in the production of solar cells520,521.
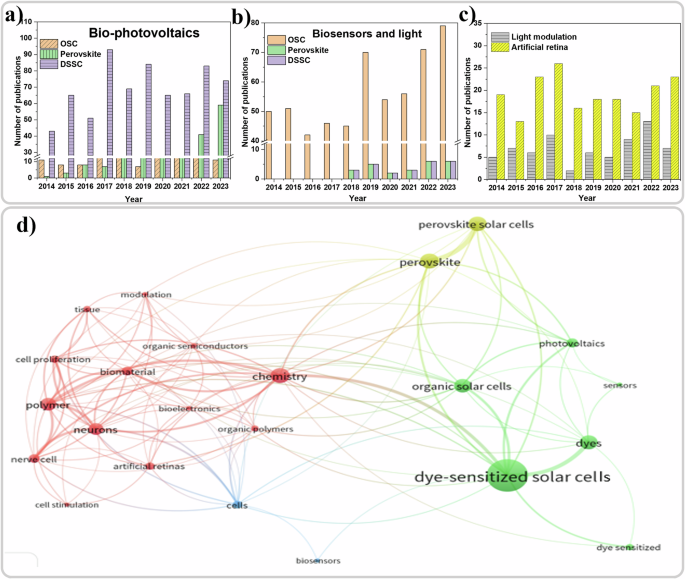
a Bar chart showing the comparison of number of papers published in each of the new generation PV technologies per each year for the last 10 years in the field of bio-photovoltaics. b Bar chart showing the number of publications per each year for the last 10 years in the field of light-based biosensors based on new generation semiconductors. c Comparison of number of publications per year for the last 10 years of the entire artificial retina research field and light modulation of biological cells/tissue/processes with OSCs. d Visualization of 23 keyword characters in this review from 1353 reference collected from SCOPUS using VOSviewer software.
The incorporation of biomaterials into new generation semiconductors like organic semiconductors, perovskites and dye-sensitized materials has led to significant advancements across the photovoltaic and the electronics field in which the biomaterials show their benefits in enhancing functionality, improving stability and introducing novel mechanisms of performance optimization522. For organic semiconductors, biomaterials like proteins, and enzymes are incorporated to create bio-electronic interfaces. This has been particularly useful in the development of organic bio-sensors and neural interfaces523, where the biocompatibility of the materials is essential for applications such as monitoring brain activity or detecting biological signals in real time. In this way, biomaterials incorporated into organic semiconductors provide enhanced biocompatibility, which is also crucial for in vivo applications such as drug delivery systems, bio-integrated circuits, or even artificial tissues. By functionalizing organic semiconductors with bio-recognition elements (e.g., antibodies or enzymes), the newly developed materials can detect various biomolecules, which enhances their application in medical diagnostics. For perovskite semiconductors, biomolecules like DNA and lipid can help regulate the crystallization process and reduce the formation of defects at grain boundaries, enhancing both the material’s device performance and environmental stability. Additionally, the incorporation of biomolecules has expanded the application of perovskites in bio-sensing524. Due to the affinity of biological molecules for specific target analytes, bio-functionalized perovskites can be tailored for use in sensitive detection systems for biological molecules, making them promising candidates for bio-sensors in healthcare or environmental monitoring. For dye-sensitized materials, biomaterials such as chlorophyll, anthocyanins (from fruits and plants), and other natural pigments can be used as natural dye-sensitizers. These biomolecules are non-toxic, biodegradable, and readily available, offering a sustainable alternative to synthetic dyes. Furthermore, the integration of biomaterials into dye-sensitized materials has broadened the scope of these materials beyond conventional solar energy harvesting applications. Bio-sensitized solar cells are being explored for photosynthetic energy harvesting525, where biomolecules from natural photosystems (such as Photosystem I and II) are incorporated directly into the cell to mimic the photosynthetic process, thereby generating energy in a biohybrid system.
Nevertheless, these numbers are promising, especially for OSCs and PSCs and even more so the fact that biomaterials can modulate the photo response of PV architectures, which can be the basis for new biosensing or bio-mimicking devices. In fact, the number of papers in the field of biosensing using light and new generation PV materials (Fig. 13b) has reached a maximum last year (i.e. over 80 papers in 2023). It is clear that light-based biosensing is dominated by organic semiconductors due to their excellent biocompatibility, versatile chemical design, and the possibility of tailoring their optoelectronic properties to the application. Examples of materials that have been sensed include DNA, proteins, glutathione etc. Nevertheless, both dye-sensitized and perovskite-based systems have been gaining ground in the last 5 or 6 years suggesting further discoveries await in the future. The possibility of incorporating biorecognition elements within the counter electrodes or photo-anodes in the DSSC configuration, along with having a biological electrolyte, is a strong benefit for biosensing applications for this type of PV architecture. Instead, for perovskite technology, lead toxicity and water instability represent the main challenges that need to be overcome for it to be used effectively in biosensing applications.
Light, being a contactless probe, has been utilized not only in new generation semiconductors-based biosensing applications, but also to stimulate and modulate biological behavior in spectrum, intensity, and time. The relatively limited and non-specific interactions between light and biological systems have been overcome by using exogenous new generation semiconductors that can absorb the light and transduce or elicit responses in or from biological systems to which they are interfaced to. These interfacing semiconductors have enabled modulation and control of the biological behavior and activities of cells through making light shine, usually in pulses, on the semiconductor/biological system. Excitation323, intercellular Ca2+ level variation, and proliferation rate control of cells13 are some of the reported artificial biological processes affected and modulated by semiconductors under light stimulation. Other than pristine semiconductor thin films, NPs were also reported to be attached or internalized (depending on the NPs)329 to the nerve cells for wireless-light control of cellular behavior330. The fact that light-sensitive semiconductors generally do not negatively affect biological functions promotes their application for sustaining and enhancing bioactivities. Conversely, some OSCs are known to cause photo-toxicity, and this property could effectively been used for cancer treatment526. Nevertheless, it is very clear from Fig. 13c that new generation semiconductors such as OSCs are an interesting group of materials to be used in the light modulation of biological processes and the field is growing.
The field of artificial retina is interesting due to its potential high impact on society. Improvements have been achieved in this field and it has grown from crude big implants based on metal electrodes that needed supplementary tools such as batteries, processors, and software towards self-powering photovoltaic semiconductor artificial retina devices that require no maintenance after implantation in the animal models. The choice of semiconductors for artificial retina applications is significantly influenced by their flexibility, biocompatibility and integration with biological systems. OSCs are particularly favored due to their flexibility, biocompatibility and proven ability to stimulate biological cells, making them well-suited for seamless integration into biological environments as mentioned in the previous sections. This research has gone through tremendous upgrade from in vitro devices demonstrating the stimulation blind retina placed on thin film OSCs299, to implantable artificial retina such as Polyretina324 and further into injectable polymer NPs59 that reduced the surgical difficulties in animal models. Even though the leap of technology from crude devices to injectable NPs is impressive, there needs to be a lot of improvements that must be made before one can use this technology for treating retinal dystrophies routinely in humans. In contrast, perovskite semiconductors, while exhibiting remarkable performance in photovoltaics, face considerable challenges in the realm of bioelectronics. Their instability and limited biocompatibility pose significant hurdles for their application in biological environments. Despite their promising advancements in optoelectronics, perovskites have never been developed into implantable devices, rather been used as retinal-neuromorphic devices527. Thus, while perovskites show potential, substantial improvements are needed to address their stability and biocompatibility issues before they can be effectively utilized in artificial retinas within living systems.
So far, the most interesting PV materials used for artificial retina applications are mostly based on OSCs. Once the instability of perovskite under moisture and the lead toxicity are resolved, this will not only benefit the field of solar cells but also in the field of vision. It is evident from Fig. 13c that the use of OSCs for light modulation has grown a lot in the last 10 years along with artificial retina applications.
With the data visualization with 23 specific key words as shown in Fig. 13d (using a total reference 1353 collected from SCOPUS with 30 specific words such as “dye-sensitized-solar-cells AND plant-pigments”), certain conclusions about this new field of photovoltaic bioelectronics can be gathered. The size of both the label and the circle are determined by the weight, which represents the importance of the key word. The thickness of the lines represents how strongly the key words are linked, and the closer two key words are to each other, the stronger is their relatedness. “Dye-sensitized solar cells” having the biggest circle and label among the other key words suggest the highest occurrence of this key word in the references used for the data visualization. This points to the large amount of research that has been carried out in the field of DSSC. By comparing the Fig. 13a & 13d, it is clear that the major contribution to the bio-photovoltaics is from DSSC, which is evident from the large number of biological pigments being used as the dye. The cluster of words in the red color and their thick connections to key words such as polymers and organic semiconductors points to the contribution of OSC in the field of biosensing, modulation, and artificial retina research. The comparatively smaller circle and lesser number connections of key words such as artificial retina implies that the field of artificial retina using the new generation PV are quite recent applications compared to solar cells, light modulation or biosensing. The less connected perovskite solar cells compared to the key words representing OSC and DSSC implies that the PSC is a relatively new field in this arena.
Although great improvements have been made in this field of photovoltaic bioelectronics, there are still some challenges that need to be addressed. It is evident from this review that the incorporation of biomaterials in the new generation PVs could lead the field into greener technology, but further improvements in the stability and PCE are necessary. It is noteworthy that biological materials, such as aquatic algae, have been used as light-harvesting components in solar cells, replacing conventional semiconductors to produce bioelectricity528. Although the generated photocurrent was smaller compared to industrial counterparts, this research opened the door to completely green photovoltaic technology for energy harvesting529, as well as other aspects of photovoltaic bioelectronics.
Better optimization of new generation PV for light-based bio-application is needed in terms of making it biocompatible and compact for implantation, maybe through incorporation inside or on the cells surface rather than implanting films. In vivo application of these semiconductor materials for light modulation of biological functions needs to be addressed and has to be expanded towards general cells, tissues and organs (other than neurons and neuron-like cells). Finally, an artificial retina technology that is fully biocompatible, surgery free, and highly stable is still a line of research that could mean restoring sight to millions of people who are visually impaired.
Responses