Proteomic meta-analysis unveils new frontiers for biomarkers research in pancreatic carcinoma

Introduction
Pancreatic carcinoma (PC) is the sixth most common cause of cancer-related deaths in 2022, accounting for nearly 5% of all cancer deaths worldwide [1]. Currently, the causes of PC are not yet fully identified [2], but certain risk factors have been identified and are classified as non-modifiable (age, sex, blood group, family history, and genetic susceptibility, diabetes) and modifiable (intestinal microflora, smoking, alcohol, chronic pancreatitis, obesity, dietary factors, infection). At clinical presentations, patients typically present with jaundice due to invasion and obstruction of the common bile duct when the PC is located in the head of the pancreas. Alternatively, they may exhibit nonspecific symptoms such as back pain and weight loss. The current methods for establishing the diagnosis of PC include ultrasonography (US), endoscopic ultrasonography (EUS), endoscopic retrograde cholangiography (ERCP), computerized tomography (CT) or magnetic resonance imaging (MRI), with or without guided percutaneous fine-needle biopsy, ascites cytology or exploratory biopsy under laparoscopy or open surgery diagnosis [3]. Approximately 80-85% of patients are diagnosed with unresectable disease. This is largely due to the encasement of major mesenteric vessels or metastatic disease to the liver, peritoneum, or other distant sites. As a result, the 5-year survival rate is about 7.2% in the USA. [4]. Despite advances in diagnostic approaches, perioperative management, radiotherapy techniques, and systemic therapies for advanced disease, there is only modest incremental progress in patient outcomes [4]. Only 20% of patients with resectable diseases survive for 5 years after surgery, and most may still die from the disease afterward. The high resistance of PC to conventional chemotherapy with 5-fluorouracil represents one of the reasons for this poor prognosis [5]. Radiotherapy alone is largely ineffective. Gemcitabine monotherapy was demonstrated to improve median survival by just over 1 month, compared with 5-fluorouracil [6]. Within the last decade, there have been improvements in clinical outcomes with combination chemotherapies with gemcitabine/nab-paclitaxel and 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), providing median overall survival benefits of 1.8 and 4.3 months, respectively, compared with gemcitabine alone [7, 8]. Although FOLFIRINOX treatment resulted in a lower percentage of patients experiencing reduced quality of life, it also had increased toxicity and adverse events, thus preventing its administration to patients with multiple comorbidities [9]. Recent research on targeted therapy, immunotherapy, and microbial therapy has shown that these can be used in combination with traditional methods for the treatment of PC, such as surgery, chemotherapy, radiotherapy, and palliative care.
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of PC, and 80-90% of these tumors are invasive; this cancer is characterized by a progression from normal tissue to invasive lesions with specific morphological characteristics. The process starts when acinar cells transform into a ductal phenotype in response to specific stimuli, a phenomenon known as acinar-to-ductal metaplasia (ADM). When combined with an oncogenic “hit,” these cells progress to a pathogenic phenotype that eventually develops into Pancreatic Intraepithelial Neoplasia (PanIN). The development of the disease involves a transition from pre-invasive stage -which is graded on a three-tiered scale as PanIN-1 (PanIN1A and PanIN-1B subtypes), PanIN-2, and PanIN3- to the invasive PDAC stage. So, pre-invasive PanIN lesions progress from normal ductal epithelium through PanIN stages 1A, 1B, 2, and 3, ultimately advancing to stage 4 as invasive and/or metastatic PDAC. The process shows increasing nuclear atypia, loss of normal architecture and increased mitoses, and progresses with an increase in cellular invasiveness and branching of the basement membrane and extracellular matrix with metastasizing to distant organs [10]. The majority of PDACs are solid, firm, and infiltrative tumors with poorly defined margins. Microscopically, PDAC is characterized by neoplastic duct-like structures in a dense functional stroma composed of an extracellular matrix and extensive fibrosis (desmoplasia), which is the cause of resistance to therapies [11]. Desmoplastic stroma is characterized by the presence of cellular components, such as cancer-associated fibroblasts (CAFs), immune cells, and endothelial cells, and acellular components, such as collagens, laminin and cytokines in the extracellular matrix (ECM) [5], which is a physical barrier to the penetration of therapeutics [10]. The aggressive nature of PDAC is driven by the inflammatory process and an extensive stromal network in the tumor microenvironment (TME). Pancreatic stellate cells (PSCs) and cancer-associated fibroblasts (CAFs) play a crucial role in promoting the survival, growth, and proliferation of cancer cells. This, in turn, supports cancer metastasis and drug resistance [6]. PDAC tumor growth and progression are enhanced by a proinflammatory and immunosuppressive program characterized by the production of cytokines, growth factors, and other signaling molecules in the TME. Therefore, the current scientific and clinical challenge is targeting cancer cells and TME to induce a favorable therapeutic response [6]. Advancements in understanding the molecular mechanisms of PDAC in recent years have led to the identification of CA19-9 as the common serum tumor biomarker: this biomarker is considered the preferred choice for early screening; combining CA19-9 with CA125, CEA, and microRNAs leads to an increase of sensitivity, specificity, and accuracy by 84% compared to using CA19-9 alone [5]. Moreover, new potential biomarkers are identified in circulating cell-free DNA (CfDNA) and mutation-specific circulating cell-free tumor DNA (ctDNA), such as the K-RAS gene mutations in codon 12: there is an important correlation between the concentrations of these two factors and treatment response. About the presence of biomarkers, a repertoire of proteins in fibroblasts has been identified after the treatment with conditioned medium from MIA-PaCa-2 human pancreatic tumor cell line, such as ITGB3, TGFB1, and TGFB2 (involved in fibroblast movement and apoptosis) and SMAD3, STAT3, and BAG3 (involved in chemotaxis activation and cell adhesion) [12]. In recent years, proteomics based on mass spectrometry (MS) analysis have taken a central role in the investigation of potential biomarkers since they offer the opportunity to identify innovative molecular pathways and quantify the levels of differentially expressed proteins (DEPs) [13]. Particularly, functional proteomics turns out to be useful for combining the examination of protein activation, protein-protein interactions, and activated pathway analyses [14]; it can be used to classify the type of cancer, to study the prognostication and the prediction of response to therapy, and also to discover tool for targeted therapy. In contrast to genomic analysis, which defines potential gene products, proteomics reflects the actual protein expression in response to translational control and degradation or regulation of protein activity through post-translational modifications. It has been shown that protein expression data correlate better with drug sensitivity or resistance than data from other “omics” studies so these analyses can be used to study multifactorial pathologies, such as cancer [15].
In this study, we have gathered proteomic research from the past decade. We have analyzed protein biomarkers, categorizing them as tissue/cellular markers or circulating markers. Additionally, we performed a meta-analysis using the Ingenuity Pathway Analysis (IPA) bioinformatic tool to extract new and detailed information from the collected dataset.
Results
Distribution and analysis of protein biomarkers across biological fluids and tissues
Table 1 lists the protein markers, distinguishing them by biological fluid/cells/tissue of origin and their respective trend, biological matrix, tumor type, number of articles, PANTHER protein classification, and reference. We find proteomics and immunohistochemistry studies among the main assay types used in biomarker discovery. In particular, proteomic tools can be divided into antibody-based (including western blotting, enzyme-linked immunosorbent assay (ELISA), immunohistochemistry (IHC), and protein microarray) and non-antibody-based (including protein mass spectrometry-based technology) [14]. Interestingly, most modulated proteins resulted from studies conducted on biological fluids (74.2%), such as peripheral blood, plasma, serum, urine, and pancreatic juice [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. In comparison, only 25.8% of proteins are involved in studies conducted on cellular models or sections of tumor tissue. On the one hand, studying a tissue biomarker could be extremely useful in characterizing the tumor type, identifying tumor staging, or following the appropriate therapeutic strategy. According to “PANTHER Protein classification” (Fig. 1A), concerning circulating protein biomarkers, 20.9% of proteins are intracellular signaling molecules (ISMs), 9.0% of proteins are transfer/carrier proteins (T/CPs), and metabolite interconversion enzymes (MIEs), 7.5% correspond to cytoskeletal proteins (CPs). Considering cells/tissue proteins biomarkers listed in Table 1 and Fig. 1B, the most proteins found (29.2%) are related to metabolite interconversion enzymes (MIEs), 12.5% of proteins are cell adhesion molecules (CAMs), while 8.3% of proteins are related to protein-binding activity modulators (P-BAMs), transmembrane signal receptors (TSRs), calcium-binding proteins (C-BPs), and cytoskeletal proteins (CPs) [49,50,51,52,53]. Proteins involved in the mentioned protein classes are given in the “Panther Class” column of Table 1. All biomarkers used in this meta-analysis are related to pancreatic cancer, in particular fluid and tissue biomarkers linked with PC/PDAC cells or TME are provided in Table 1—column “tumor type”—with evidence of biomarkers related to PDAC, different stages of PC and PDAC, and TME, since the aggressive nature of PC/PDAC is driven by the inflammatory process and an extensive stromal network in the TME. Specifically, 59.57% of protein biomarkers are related to PC, biomarkers linked to PDAC represent 26.60% of the total, 10.64% of biomarkers describe particular stages of PC/PDAC and 3.19% of biomarkers are related to TME. The circulating biomarker mucin MUC5AC is upregulated in stage PanIN1A to late stage of PC; macrophage inhibitory cytokine-1 (MIC-1), alcohol dehydrogenase (ADH), carbohydrate antigen 19-9 (CA19-9), vitamin K-dependent protein Z (PROZ) and tumor necrosis factor receptor superfamily member 6b (TNFRSF6B) are upregulated circulating biomarkers related to the early stage of PC. On the other hand, plasma tissue factor pathway inhibitor (TFPI) and tenascin C (TNC-FN) are upregulated circulating biomarkers related to the early stage of PDAC (stage IA/IB/IIA, stage IIB); anterior gradient-2 (AGR2) and insulin-like growth factor-binding protein-3 (IGFBP3) are upregulated circulating biomarkers related respectively to stage PanIN3 of PDAC and invasive PDAC. Finally, thrombospondin-2 (THBS-2) and transforming growth factor-beta (TGF-β) are upregulated tissue biomarkers linked to the TME of PC. As reported in Table 1—column “# Articles”—it’s possible to notice that 62.77% of biomarkers are described in only one article mostly related to PC and PDAC but also to the early stage of PC, early-stages of PDAC (stage IA/IB/IIA, stage IIB) and TME; in particular tissue biomarkers as biglycan (BGN), pigment epithelium-derived factor (PEDF) and THBS-2 are related to the TME and the others to PC/PDAC. The 2.13% of biomarkers (CEA and CA19-A) are discussed in more than 20 articles—mostly related to PDAC and the early stage of PC- and 35.11% of biomarkers are described in a number of meta-analysis articles between two and seven related to PDAC, PC, stage PanIN1A and late stage of PC, stage PanIN3 and invasive stage of PDAC.
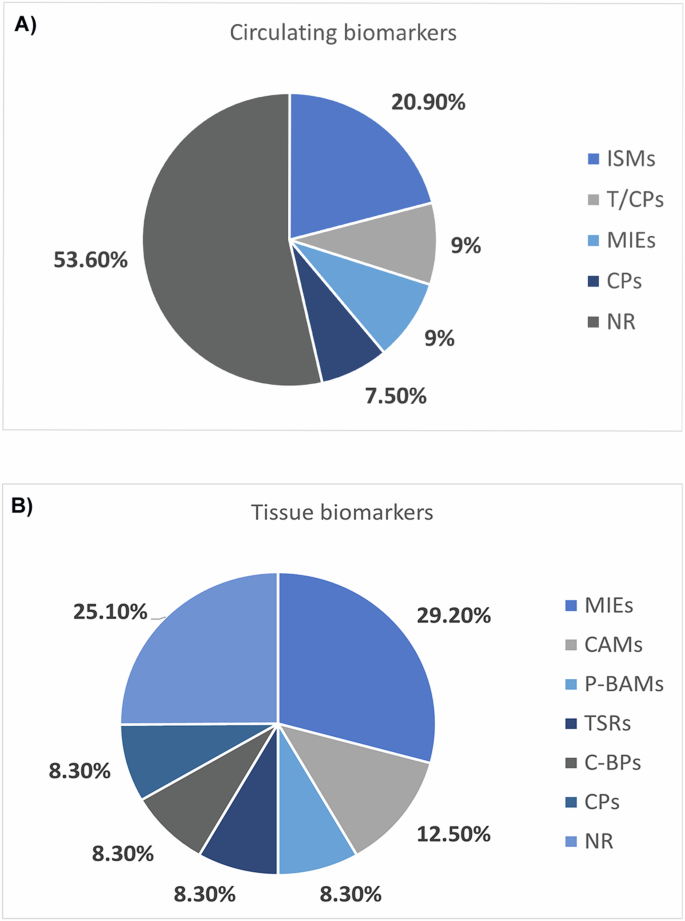
Protein classification according to PANTHER tool for circulating protein biomarkers (A) and cells/tissue protein biomarkers (B). Pie charts show the classification of circulating and tissue biomarkers according to their “Family and Protein Class”: these are grouped into intracellular signaling molecules (ISMs), transfer/carrier proteins (T/CPs), metabolite interconversion enzymes (MIEs), cytoskeletal proteins (CPs), cell adhesion molecules (CAMs), protein-binding activity modulators (P-BAMs), transmembrane signal receptors (TSRs), calcium-binding proteins (C-BPs), and non-relevant (NR) group. This classification was obtained by uploading—separately—the entire dataset of circulating and tissue biomarkers to “PANTHER Protein classification” by “supergrouping of protein families” way.
Functional analysis on fluid biomarkers unveils implications for cancer, inflammation, and lipid metabolism
In Fig. 2, we report the main pathways where our biomarkers could be involved, considering only the significant “canonical pathways” (−log10 (P value) >1.3). Among these, we found three interesting downregulated (z-score < −2) pathways involved in the inhibition of proliferation and inflammation: retinoic acid receptor (RAR) activation pathway, CDX gastrointestinal cancer signaling, and the adrenergic receptor signaling pathway. The complete list of canonical pathways obtained from the functional analysis on IPA is provided in Supplementary Table S1.
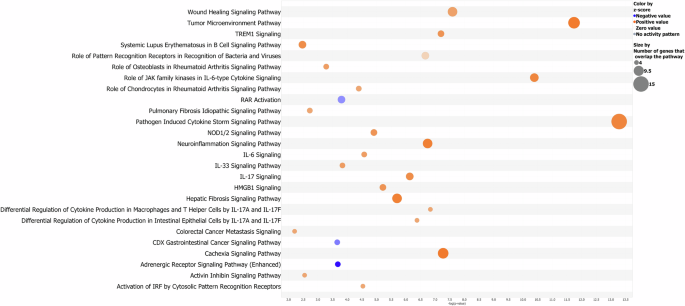
It is possible to notice how each pathway is characterized by a specific “−log10 (P value)” -only pathways with a value greater than 1.3 are shown and described in the text- and an absolute “z-score value”—regarding the modulation—greater than 2. As described in the legend, orange bubbles are indicative of significantly upregulated pathways (z-score greater than 2) and downregulated pathways (z-score lower than −2); the size of bubbles depends on the number of biomarkers that overlap the pathway. This bubble blot has been created using the values of “P value”, “z-score”, and “# of molecules” resulting from the functional analysis carried out by IPA software.
The “RAR activation” pathway leads to potent anti-proliferative and anti-inflammatory properties [54,55,56]; moreover, RARs are able to repress the activity of transcription factors such as AP-1, which is involved in cell proliferation and survival and in particular in the proliferation of cancer cells [55], and NF-kB, which regulates multiple aspects of innate and adaptive immune functions and serves as a pivotal mediator of inflammatory genes [56] and plays a critical role also in cell proliferation and survival [57]. The RAR pathway has been shown to be an important druggable pathway both in vitro test and clinical trial. The PI3K-alpha inhibitor “idelalisib” terminated the phase 1-clinical trial process in 2024 respectively for metastatic pancreatic adenocarcinoma (NCT03878524) and pancreatic ductal adenocarcinoma (NCT02468557); the PARP (poly ADP-ribose polymerase) inhibitor “niraparib” is in 2023 in the phase 2-clinical trial NCT05442749 for advanced PDAC.
Regarding the inhibition of the “CDX gastrointestinal cancer signaling”, knockouts of CDX genes exhibit increased colorectal cancer and CDX products present important roles in cancer progression and intestinal inflammation [58, 59]. In this scenario, i.e., the drug “ABBV-621” presents TNFSF10 as the target molecule within the “CDX gastrointestinal cancer signaling” and “adrenergic receptor signaling”, and it has completed the phase 1-clinical trial NCT03082209.
Interestingly, the “adrenergic receptor signaling” pathway was found inactivated: this pathway is involved in the body’s fight or flight response since norepinephrine (NE) plays a role in mood and sleep regulation, and expression of behavior. Specifically, ADRA1-2 receptors have been found to influence cognitive functions such as working memory, attention, fear, and spatial learning, while ADRB1-2 receptors function in auditory fear, spatial reference, fear memory, and memory retrieval [60]. The inhibition of this pathway and its related implications could be interesting to study and monitor as co-symptomatology of PC. Regarding the pathway’ druggability, the drug “anakinra” targets IL-1A and IL-1B within the adrenergic receptor signaling and it’s in phase 2-clinical trial NCT04926467.
As for the many pathways predicted to be upregulated (z-score >2), they are involved in many macro-categories such as cancer signaling, cellular stress and injury, immune response signaling, neurotransmitters, and nervous system and other disease-signaling pathways (Fig. 2).
The engagement of pattern recognition receptors (PRRs) results in the activation of specific genes and subsequent triggering of innate immune responses; the NOD-like receptors are members of this family of molecules, helping activate innate immune responses to cellular stress and stress [61, 62]. NOD1 mutations increase susceptibility to inflammatory bowel disease, while NOD2 mutations have been associated with susceptibility to Crohn’s disease, Blau syndrome, and other intestinal innate immune defects. Aberrant NOD signaling has long been associated with a range of inflammatory disorders, and its inhibition could be beneficial in the treatment of disorders such as allergic asthma and type 2 diabetes mellitus [63]. Interestingly, the drug “ABBV-621” is again involved in the regulation of this pathway with TNFSF10 as the final therapeutic target. Moreover, as for the adrenergic receptor signaling, “anakinra” is also involved in NOD-like receptor regulation.
Moreover, within the “activin inhibin signaling” pathways, activin A activates the MAPK signaling pathway including P38 mitogen-activated protein kinase (p38-MAPK), further identified as an upregulated upstream regulator, mostly under inflammatory conditions and has a crucial effect on innate and adaptive immune responses and in the pathophysiology of human diseases such as cancer and fibrotic syndromes [64]. Again “anakinra” (NCT04926467) and “idelalisib” (NCT03878524) target this pathway regarding its druggability; moreover, i.e “ASN007” (NCT03415126) and “avutometinib” (NCT 03875820) as inhibitors of respectively MAPK1-3 and MEKs, are in phase 2 of both clinal trials.
“Cancer cachexia pathway” shows a systemic excess catabolism syndrome that decreases the quality of life, ability to tolerate treatment, and eventual survival; the wasting phenotype mostly occurs in skeletal muscle and adipose tissue, but other organs, such as brain, liver, pancreas, and heart, are also affected [65]. The activation of such pathways could be of interest for further investigation to deepen the understanding of PC comorbidities and responses to therapies, since loss of body weight, metabolic alterations such as glucose, protein and lipid metabolism, systemic inflammation, insulin resistance, and oxidative stress are pathophysiological changes involved in the progress of cancer cachexia [66]. It could also be interesting to investigate liver and lung function, as hepatic and pulmonary fibrosis pathways appear to be predicted upregulated.
The high mobility group-B1 (HMGB1) has a role in inflammatory responses and tumor metastasis: it’s regulated by the activation of inflammatory cells through binding of tumor necrosis factor (TNF), lipopolysaccharide (LPS), further identified as upregulated upstream regulators, and HMGB1 itself and it regulates the activation of MAPK pathways with again p38-MAPK. It also interacts with the extracellular matrix and membrane receptors, affecting cell motility and metastasis [67, 68]. So, targeting the HMGB1 or its receptors, could represent an important potential application in cancer therapeutics: i.e., “ASN007” (NCT03415126) in phase 2-clinical trial targets MAPL1-3 within the regulation of this pathway.
Regarding “inflammation and immune response”, triggering receptor expressed on myeloid cells 1 (TREM1) signaling results in the production of proinflammatory cytokines such as TNF and IL-6 [69]; moreover, inflammation and immune response are stimulated by IL-17, IL-33 and IL-6 signaling in a cytokine storm signaling pathway as well as cancer development [70]; in particular, IL-33 signaling results again in activation of MAPK signaling and p38-MAPK [71] and the transcription of the IL-6 gene is also stimulated by tumor necrosis factor (TNF), further identified as the upregulated upstream regulator. Additionally, inflammation at the neuronal level also appears to be activated with an excessive cell and tissue damage which results in the destruction of normal tissue and chronic inflammation that ultimately results in necrosis of glial cells and neurons [72, 73]; in this case as well, it could be important to further investigate the concomitant symptoms of PC since chronic neuroinflammation is closely related to chronic neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and has also been a focus of research into the pathology underlying psychiatric disorders like depression [74]. Globally, within the tumor microenvironment (TME) pathway, which comprises cancer cell, cytokine environment, extracellular matrix, immune cell subsets, wound healing signaling and other components, the pro-tumorigenic immune response plays a pivotal role in driving immune invasion; the tumor not only manages to escape from the host immune system, but it effectively benefits from infiltrating cells by modifying their functions to create microenvironment favorable for tumor progression [75]. For this reason, targeting the inflammatory pathways has become one of the primary objectives in pharmaceutical research on PC.
Regarding “disease and functions” analysis, the main categories of interest together with their significant z-scores and P values, are reported in Fig. 3: “cancer”, “cell signaling and movement”, “inflammatory response”, “free radical scavenging”, and “lipidic metabolism”. The complete list of diseases and biofunctions obtained from the functional analysis on IPA is provided in the Supplementary Table S2.
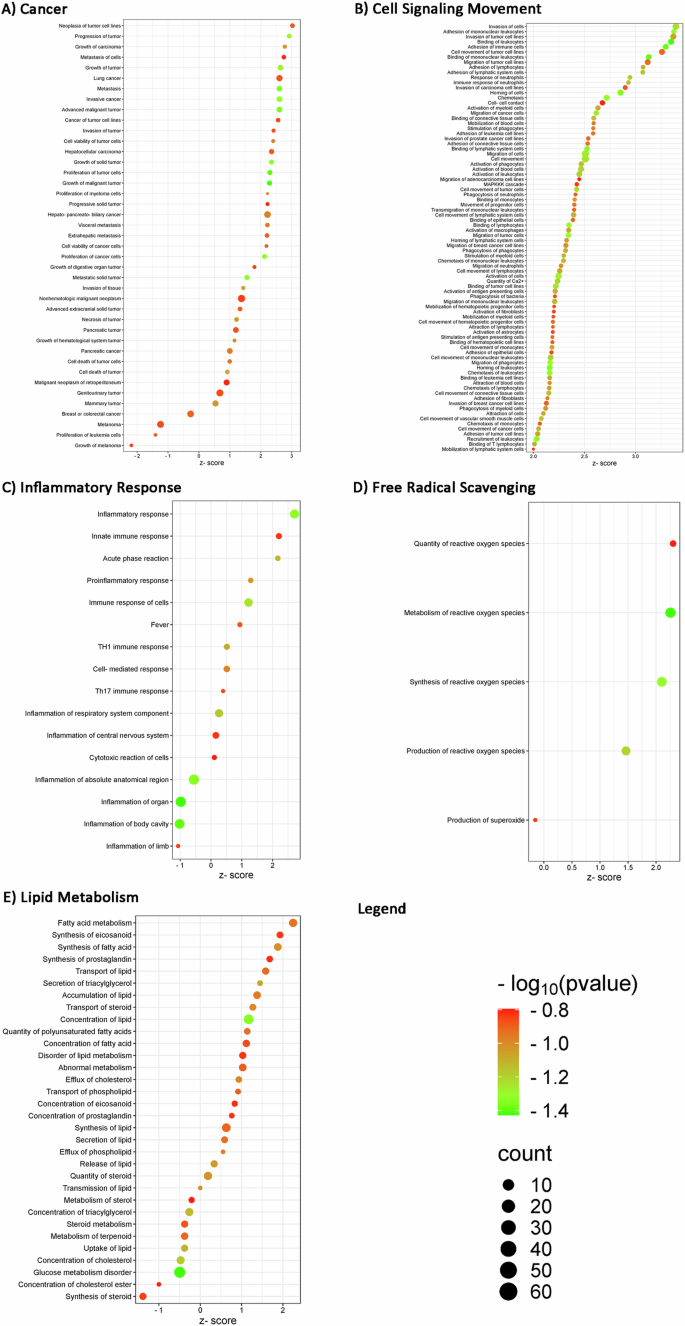
We displayed selected entities for each “Disease and Functions” category, also described in biological fluids meta-analysis: A cancer, B cell signaling movement, C inflammatory response, D free radical scavenging, and E lipid metabolism. The z-score value shows the modulation (upregulation for z-score greater than 2 and downregulation for z-score lower than −2) of each function with a significance of −log10 (P value) indicated by bubbles color (color scale of red and green). The size of bubbles depends on the number of biomarkers that overlap the described pathway. This bubble blot has been created using the values of “P value”, “z-score”, and “# of molecules” resulting from the functional analysis carried out by IPA software.
As expected, “cancer” is the first category we focused on, as proof of concept (Panel A). Among the functions predicted to be significantly upregulated (z-score >2), we found neoplasia of tumor cell lines, metastasis of cells, cancer of tumor cell lines, invasion of tumor, progressive solid tumor, and cell viability of cancer cells. “Pancreatic cancer” and “pancreatic tumor” functions have a z-score value of about 1, so they cannot be considered as predicted functions to be upregulated with a significant z-score, but the log10 (P value), on the other hand, is statistically significant. For the “cell signaling and movement” category (Panel B), since biomarkers in the circulating fluid are obviously also implicated in cell signaling, we could highlight how tumor cell movement and signaling is predicted to be activated by biomarkers such as migration and invasion of tumor cell lines, and cell-cell contact. In this context, we also find a remarkable activation of the immune and inflammatory response in the stimulation of phagocytes, monocytes, leukocytes, and lymphocytes. We can also observe a significant activation (z-score >2) of inflammatory response, innate immune response and acute phase reaction pathways (Panel C) as well as activation of reactive oxygen species’ quantity, metabolism, and synthesis (Panel D), confirming an important activation of inflammatory and immune responses, already identified in the “canonical pathways” analysis. As far as lipid metabolism is concerned, the bubble plot (Panel E) shows a significant activation (z-score >2) of fatty acid metabolism and a fair activation (z-score = 1.9) of eicosanoid synthesis; PC cells need lipids during some conditions such as rapid proliferation, metabolic stress, and many cellular activities; cells acquiring of fatty acids (FAs) and cholesterol depends on lipid uptake from the exogenous environment [76]. It’s clear that fatty acid translocase (CD36) is involved in metastasis initiation and proliferation [77]: large tumor size and reduced survival rate are associated with the low expression of CD36 [78]. Regarding cholesterol, his uptake dysregulation contributes to PC carcinogenesis but also cholesterol efflux is critical for cholesterol homeostasis since it contributes to diverse types of tumors. Moreover, despite lipogenesis takes place in hepatocytes and adipocytes, cancer cells can acquire lipids through de novo synthesis because of their high metabolic demand [76].
Insights biomarker pathways explore inflammation and tissue dynamics
When looking at well-characterized metabolic and cell signaling pathways, also known as “canonical pathways”, our analysis showed two significant canonical pathways (z-score >1 and a −log10 (P value) >1.3). These pathways are “macrophage alternative activation”, which plays a crucial role in innate and adaptive immunity and the inflammatory response, and the “S100 family signaling”, which is linked to cancer signaling pathways. In particular, the immune function is the primary role of S100 proteins, which also are important regulators of macrophage inflammation [79]; cell stress and injury and inflammation promote proinflammatory signaling pathways in turn related to cell differentiation, inflammation, migration, cell survival, proliferation, and tissue repair [80]. The complete list of canonical pathways is provided in Supplementary Table S3. Second, we again focused on disease and function identification to point out some downstream effects expected to increase or decrease, according to IPA prediction. The complete list of diseases and biofunctions obtained from the functional analysis on IPA is provided in Supplementary Table S4. So, Fig. 4 shows the “disease and functions” for each category of interest: in this case, it’s not possible to find the “cancer” category, as a proof of concept in fluid biomarkers, since some pathways are described in a significant way but without any prediction regarding pathways regulation (not significant z-score). However, we find some downstream effects linked to cell viability and migration of tumor cell lines as significantly upregulated (z-score >2), such as cell viability, aggregation of cells, migration of cells, cell movement of tumor cell lines, migration of tumor cell lines, invasion of carcinoma cell lines, cell movement of carcinoma cell lines, migration of carcinoma cell lines and invasion of tumor cell lines (Fig. 4A). The bubble plot in Fig. 4B shows a significant activation of the inflammatory response (z-score >2) and a significant inhibition of organismal death (z-score < −2). The predicted activation of the inflammatory response and the inhibition of cell death would, however, seem to confirm the tumor trend, although in a less evident way than in the fluid biomarkers’ analysis. Since the IPA research is based on data published in the literature, there is probably less information on tissue studies; this could explain the lower number of correlations found in tissue biomarkers compared to fluids. In this context, it would, therefore, be interesting to further research on tissue biomarkers in PC.
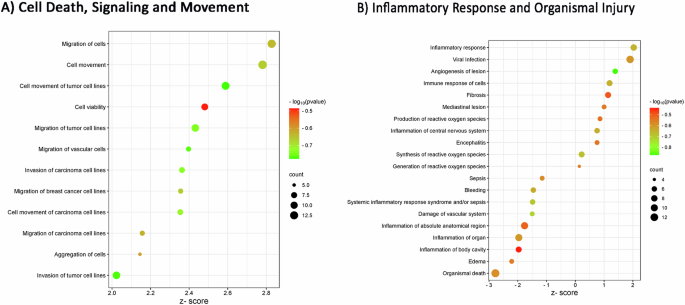
We displayed selected entities for each “Disease and Functions” category, also described in tissue biomarkers meta-analysis: A cell death, signaling, and movement and B inflammatory response and organismal injury. The z-score value shows the modulation (upregulation for z-score greater than 2 and downregulation for z-score lower than −2) of each function with a significance of −log10 (P value) indicated by bubble colors (color scale of red and green). The size of bubbles depends on the number of biomarkers that overlap the described pathway. This bubble blot has been created using the values of “P value”, “z-score”, and “# of molecules” resulting from the functional analysis carried out by IPA software.
Exploring upstream regulators to understand transcriptional control and biomarker expression patterns
In the functional enrichment analysis conducted using IPA software, we have identified a cascade of upstream transcriptional regulators that can account for the changes in protein biomarker expression observed in our dataset. This information can provide insights into the biological activities taking place in the analyzed fluids and tissues. IPA makes it easy to take this result even further by examining what biological processes, pathways, and diseases the transcriptional regulators and their targets may control, and how these upstream molecules may regulate one another. The complete lists of upstream regulators obtained from the functional analysis on IPA are both provided in the Supplementary Tables S5 and S6. The description of “upstream regulators” is accompanied by their predicted regulation, generated by bioinformatics software and quantified through the “z-score” value. Since the upstream analysis aim is to predict some biological regulators above our biomarkers, it may not be affected by the original fluid or tissue; so, we then focused on upstream regulators that are shared between fluid and tissue biomarkers functional analysis with a significant z-score value (z-score > 2 or < −2): we identified seven upstreams that meet these conditions, 470 unique fluid upstreams and 21 unique tissue upstreams (the complete lists are in the Supplementary Tables S7–9). In the following tables (Tables 2 and 3), we list and tabulate the shared seven upstream regulators with their respective information about molecular type, predicted activation state, and activation z-score in both functional analyses; they present the same trend in both functional analyses performed on fluid and tissue protein biomarkers.
LPS is predicted to be upregulated and, in turn, increases the stimulation and release of TNF [81, 82] and the activation of P38-MAPK [83] and angiotensinogen (AGT) [84]. It shows a role in the expression of pancreatic cancer cell lines [85], activation and phosphorylation of tumor cell lines [86], and proliferation of pancreatic cancer cell lines [87]. The second upregulated upstream is the TNF, which is involved in the production and release of reactive oxygen species as a confirmation of the disease and biofunction predicted to be upregulated as downstream [88, 89]. It’s also regulated by P38-MAPK [90], which is the third upregulated upstream and also has a role in the production of reactive oxygen species [91]. AGT, as again upregulated upstream, regulates TNF [92] and presents a positive expression in pancreatic cancer cell lines [93]. Moreover, its product angiotensin II protein, increases the quantity of reactive oxygen species, continuing to confirm our disease and function effects analysis [94]. Tetradecanoylphorbol acetate is a drug used in cancer treatment [95] and regulates TNF [96] and P38- MAPK [97]. Its predicted upregulation may be a proof of concept since we are studying protein biomarkers involved in cancer research. The last two upstream regulators are predicted to be downregulated in our original fluids and tissues: the downregulation of human miR-34a-5p is associated with pancreatic cancer in humans [98]. Overall, as another proof of concept, it is related to pancreatic cancer cell lines since in cell culture, human miR-34a-5p mature microRNA increases the arrest in cell cycle progression of MIA-PaCa-2 cells in cell culture [99] and decreases the viability of MIA-PaCa-2 cells [100]. miR-423-5p is regulated by LPS, which increases its expression in cultured THP-1 macrophage cells [101]; according to Volinia et al. [102], it seems to be involved in breast cancer disease so it could be interesting to study its role and its regulation also in PC.
Discussion
Pancreatic cancer is considered a highly fatal malignancy with an estimated overall 5-year survival rate of patients of 7.2% at the time of diagnosis. The efforts of the clinical community in implementing diagnostic approaches and perioperative management collide with the high resistance of PC to conventional chemotherapy treatments, and this represents one of the reasons for this poor prognosis. To date, the “gold standard” for the diagnosis of PC is the fine-needle biopsy performed with image techniques or under direct visualization at surgery. However, as widely discussed in this study, in recent years, much progress has been made in the field of biomarker discovery. In fact, current proteomic techniques aim to identify and quantify a high number of proteins from different biological samples, trying to provide the most complete view of the metabolic pathways or pathological processes implicated in the onset and development of PC, with the final purpose of investigating potential new pharmacological targets. In this study, we have collected protein biomarkers from the last ten years studies, distinguishing between tissue/cellular markers and circulating markers and performing a meta-analysis through the IPA bioinformatic tool with the aim to provide new and detailed information from published data. Our meta-analysis revealed that data literature, deriving from various biological fluids as well as cells and tissues, are consistent with each other and primarily focused on the description of functions related to cancer and cell migration, as expected. On the other hand, some new evidence has emerged regarding inflammation, activation of free radical production, and lipids metabolism, conditions that could be further explored in the assessment of the onset and progression of PC. It is possible to notice, also as a proof of concept, that we find in fluid biomarkers functional analysis all pathways related to cancer and inflammation signaling as significantly activated and those related to anti-proliferative and anti-inflammatory functions as significantly inhibited pathways. Moreover, according to “canonical pathway” analysis, it is interesting to notice that recent studies have investigated the correlation between PC onset and progression and the regulation of lipid metabolism, found to be modulated also in the “Diseases and functions” analysis; furthermore, our results obtained from fluid functional analysis fully correspond to the literature data on lipid metabolism, better described in the Results section. In this scenario, research on potential therapeutic drugs aims to identify future PC treatment targeting FA synthesis, cholesterol synthesis, lipid catabolism, and transcriptional regulators of lipid metabolism. Globally, this targeted therapy represents a novel and potentially effective strategy for PC treatment, but since it is still necessary to investigate some unclear aspects, further understanding and research are required.
Interestingly, the study of upstream regulators has highlighted some factors already known in the context of PC, such as LPS, AGT, tetradecanoylphorbol acetate, miR-34a-5p, and miR-423-5p. At the same time, TNF and p38-MAPK, which are involved in oxidative stress scavenging, have not been directly linked to PC. All these findings could represent new starting points for both the characterization of cancer progression and the study of potential diagnostic biomarkers, allowing a more complete description of clinically relevant information. Moreover, as fully described in the results, all the mentioned pathways, mostly inflammation, exhibit significant druggability, which provides a future outlook on the development of additional therapeutic approaches in pancreatic cancer (PC). It’s important to note that integrating a large number of biological components with their interactions and environmental relationships, provides the opportunity to reach an in-depth description of the pathological condition in PC and to define correlations between concomitant symptoms and tumor genesis and progression. This meta-analysis offers valuable insights into specific pathways implicated in prognosis, and druggability. Despite our challenging approach, this meta-analysis provides a more holistic perspective on existing data. One of the primary benefits of our work is the ability to synthesize and contextualize a wide array of literature data. Individual studies contribute meaningful information, yet when analyzed collectively, they unveil new evidence regarding the pathways involved in various biological processes, such as inflammation. By integrating findings from diverse studies, we hope to stimulate further investigation into the roles of these pathways, thereby contributing to the advancement of knowledge in the field of proteomics and its applications in pre-clinical and clinical research. Among the significant pathways identified, inflammation, activation of free radical production and lipids metabolism, are the most important to pursue in scientific research. Understanding their relationship with PC and determining whether their alterations are a cause or consequence of its onset, could further reveal their potential role in early detection or treatment monitoring. In conclusion, our work represents a strategy to combine results from different studies on various PC biological samples in a more comprehensive way.
Materials and methods
Biomarkers identification and evaluation workflow
To deepen proteomics features and studies in PC, we performed a systematic review of the literature with meta-analysis. In Fig. 5, we summarized the inclusion and exclusion criteria used for the literature search. We selected article type such as meta-analysis, randomized controlled trial, review, and systematic review on the PubMed website from the past decade, searching the keywords “pancreatic cancer”, “PDAC”, “drug” and “biomarkers” (combined with each other using the Boolean operator -AND -OR), obtaining 5156 eligible papers. We then narrowed our search even further by inserting -AND “proteomics”, “immunohistochemistry” as new keywords, resulting in 102 articles. Subsequently, we focused on those that specifically addressed tissue and circulating biomarkers related to diagnosis, prognosis, and overall survival. Following these research parameters, we obtained a total number of 94 PC protein biomarkers (n. 11 downregulated biomarkers and n. 83 upregulated) to perform the meta-analysis. Finally, we have classified our biomarkers by “PANTHER—(Protein ANalysis THrough Evolutionary Relationships)—protein classification” basing on their “Family and Protein Class” (supergrouping of protein families). The core of PANTHER is a comprehensive, annotated “library” of gene family phylogenetic trees. All nodes in the tree have persistent identifiers that are maintained between versions of PANTHER, providing a stable substrate for annotations of protein properties like subfamily and function. Each phylogenetic tree is used to annotate each protein member of the family.
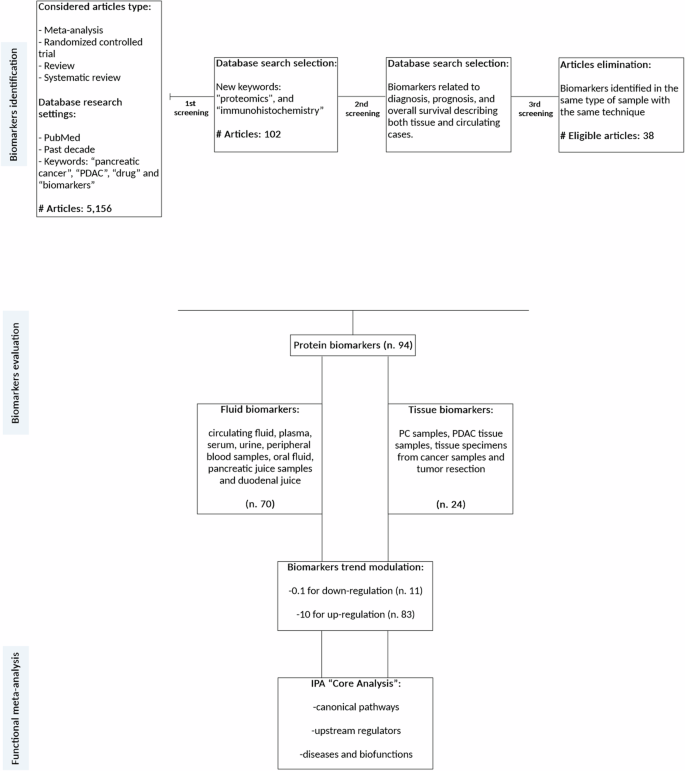
We performed a selection process in order to considerer only eligible articles, according to our inclusion and exclusion criteria, for the further analysis of circulating and tissue biomarkers.
Functional meta-analysis by Ingenuity Pathway Analysis
As can be seen from Table 1, our selected biomarkers (n. 94) have different origins, so we decided to perform the subsequent functional enrichment analysis through “Ingenuity Pathway Analysis” software (IPA, Qiagen, Hilden, Germany) and categorize the biomarkers based on their origin, since they have distinct functional implications: fluid biomarkers (n. 70) and tissue biomarkers (n. 24). Biomarkers found in plasma, serum, urine, peripheral blood, oral fluid (OF), pancreatic juice (PJ), and duodenal juice (DJ) were categorized as “fluid biomarkers.” Biomarkers identified in pancreatic cancer (PC) samples, pancreatic ductal adenocarcinoma (PDAC) tissue samples, tissue specimens from cancer, and tumor resection were categorized as “tissue biomarkers”. The list of all biomarkers and related fluids/tissues of origin is provided in Table 1, respectively with their trend, biological matrix, tumor type, number of articles, PANTHER protein classification, and reference. Subsequently, we used IPA “Core Analysis” to map statistically protein biomarkers to their functional annotation such as “canonical pathways” analysis, “upstream regulators” analysis and “disease and function effects” networks. As reported in Table 1, we have evaluated the single regulation of each molecular biomarker in the PC fluid or tissue compared with healthy controls. Biomarkers from fluid and tissue studies were uploaded on IPA software as two different matrices composed by protein biomarkers and their up or downregulation trend, as reported in our previous studies [13] (Fig. 5). It is important to notice the inherent variability among different proteomic methodologies: the differences in extraction techniques, instruments, and data processing lead to limitations in the comparability of results. So that, the measurements of protein concentrations in proteomics are often expressed as abundance ratios rather than absolute concentrations. In our analysis, biomarkers were classified based only on their modulation status, either upregulated or downregulated, acknowledging the challenge of standardizing these measurements across studies. As described in Claudia Rossi et al. in 2022, we used the same methodology to assess the protein expression of each biomarker: the quantitative data (fold change) from each study were standardized by converting downregulation values to 0.1 and upregulation values to 10, reflecting the observed trends. IPA tool returns the results by assigning to each pathway “upstream” and “downstream,” a value of −log10 (P value) and a z-score value. The −log10 (P value) measures the statistical overlap between the protein dataset and the function categories; the significance is attributed to −log10 (P value) greater than 1.3. Instead, the predicted regulation of each “canonical pathways”, “upstream regulators” and “downstream” network effects is inferred by the z-score generated by IPA system (z-scores ≥2.0 means that a molecule or pathway is activated, whereas z-scores ≤ −2.0 means the inhibition of target molecules or pathways). First, we focused on well-characterized metabolic and cell signaling pathways defined as “canonical pathways”. We next studied “diseases and functions,” which allows us to describe and predict the effect of molecular changes in our dataset on biological processes and disease or toxicological functions, trying to predict whether these phenomena are activated or inhibited. Regarding the analysis of “upstream regulators”, IPA can identify the cascade of upstream transcriptional regulators that would cause the observed modulations in biomarker expression.
Responses