Race and ethnicity matter! Moving Parkinson’s risk research towards diversity and inclusiveness
Introduction
Inclusion of ethno-racial variables in research design is vital to ensure generalizability of results and to characterize disproportionate effects of disease(s) across groups. Importantly, while differences found based on identification with a specific racial/ethnic group may or may not have a biological mechanism, distinctions may also be mediated by factors, including social determinants of health that modify susceptibility for disease in a specific population, such as differences in exposure to environmental risk modulators, inequities in accessing the healthcare system and/or variability of other social determinants1,2,3. As such, these differences can provide critical insight into potential mechanistic underpinnings, pathophysiology, and disease management (for a neuroscience review see Gilpin & Taffe4).
Ethnicity has been defined as groups linked by sociodemographic characteristics, including culture, diet, history, language, and religion, whereas race has been defined as a group of people with common physical characteristics5,6,7. While these are distinct constructs ideologically, it is well-documented that the distinction in the use of these terms in medical research has not been clear and has evolved over time8,9,10, as such, the current work includes research that employ race, ethnicity or ethno-racial categories. Importantly, race was previously thought to be a biologically-based variable; however, this definition has evolved (and been abused) to explain race as a social construct, stemming from historical, political and societal processes (see glossary of terms—Table 1)5,11,12,13,14. This distinction between race and ethnicity is consistent with the evidence that overall, race -when taken alone as a variable- does not define biological or genetic heterogeneity, as nucleotide sequences are shared by all humans at a rate of 99.6%–99.8%15,16. Furthermore, as genetic drift occurs, groups can share phenotypic characteristics based on geographic region, ethnicity, language, and religion. Thus, race should always be considered in parallel to social determinants of health8.
When considering disproportionate prevalence or risk in diverse populations, studies have not shown a definitive link between PD risk factors and race/ethnicity. However, some research has pointed to possible differences in the relative PD risk by population17,18,19,20,21,22. Despite existing research on specific risk factors and/or specific populations, there are few studies that examine potential links between race/ethnicity and PD risk in a large-scale, multifactorial design. One recent study conducted in the UK employed a primary East London cohort to examine PD risk factors across a diverse population (White, Black and South Asian-identified groups) and found that ethnicity and low socioeconomic status did not play a major role in PD risk23.
Validity, generalizability, and reproducibility are integral to the value of research reports to the greater scientific community, and in clinical practice available evidence may be critical for life-or-death decision-making. Central to determining the limitations and generalizability of our research findings is the inclusion of ethno-racial groups as variables. Unfortunately, ethno-racial participants are vastly underrepresented in many research fields. For instance, a systematic review of participants employed in Alzheimer’s disease neuroimaging research, quantified the inclusion of race/ethnicity data and found no representation of Asian American, Native Hawaiian/Pacific Islander, Multiracial, and American Indian/Alaska Native participants, with <10% representation of Black/African American participants24. Further, an evaluation of US clinical trials registered on clinicaltrials.gov conducted from 2000 to 2020 indicated that only 43% included race/ethnicity data25.
Disproportionate under-representation can negatively impact research outputs. It limits generalizability, and hinders our ability, as researchers, to address systematic biases, and, ultimately, prevents tailored initiatives, research, and future therapeutic interventions to address these shortcomings26. Scientific investigation requires identification of a knowledge gap prior to research design to address these very omissions. Data that are not representative of population diversity, therefore preclude effective identification of limitations in our research and our ability to address said limitations27.
To the best of our knowledge, the frequency of race/ethnicity inclusion in PD risk-related research has not been systematically evaluated. Furthermore, the extent to which these factors are employed (i.e. from a descriptor to inclusion as a target variable in the analyses), has not been studied. Thus, we conducted a literature review to assess the frequency of race/ethnicity inclusion in the PD field.
Results
To quantify and evaluate the extent to which ethno-racial factors were considered in PD risk research, we conducted a targeted literature review whose initial search string yielded 2713 articles. Based on screening and eligibility, 1142 articles were fully reviewed to assess inclusion of race/ethnicity (Fig. 1A). Within these, the majority of studies (59.3%) did not account for race/ethnicity (as a target population, descriptor, covariate or within analysis), and only 4.8% (n = 55) included the variables as an integral part of the analysis (subgroups, effect modification, interaction) (Fig. 1B). A detailed breakdown of the search strategy and studies classified with integral analysis can be found in the Methods, Table 2, Table 3 and Supplemental Data File.
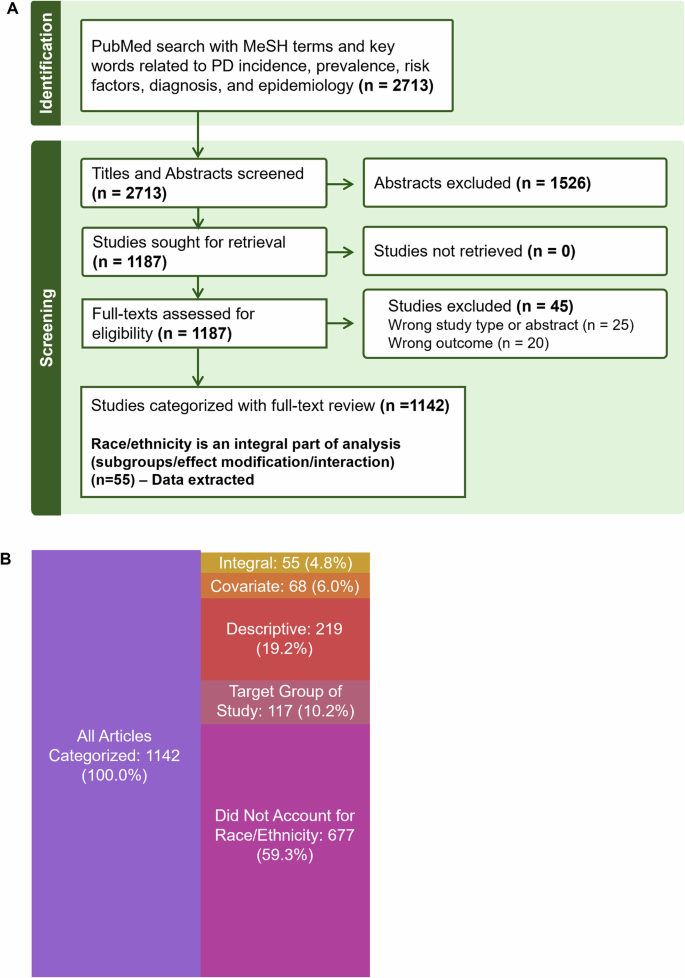
A Articles included in targeted literature review after screening and eligibility (n = 1142). B Following screening from a PubMed search, 1142 full texts of articles published between 2000 and 2024 were reviewed. Number of articles in each category describes how ethno-racial information was accounted for in the reviewed articles, where 40.7% of articles accounted for race/ethnicity. Integral analysis indicates comparing PD epidemiology with races/ethnicities as subgroups, with effect modification or interaction. Note: n = 6 articles not listed were categorized as Other, many of these specified collection of ethnicity data, and provided no further details. Table created using Biorender.com.
Representation/incidence/prevalence
Among the studies classified as including race/ethnicity as an integral part of analysis, 60% were based on North American data, 13% from Asia, 9% from Africa and 2% from Oceania/Australasia, a detailed breakdown of the studies is shown in Fig. 2A. Next, we examined trends over time and found a relatively similar number of studies were published in 5-year intervals between 2000–2024, suggesting no change in the rate of PD publications that incorporate race/ethnicity as an integral variable (Fig. 2B).
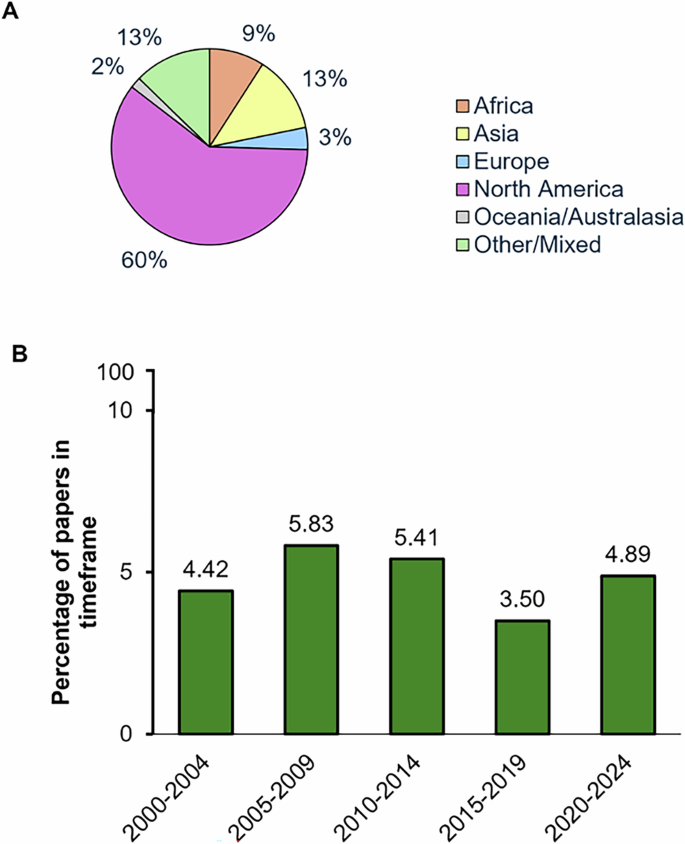
A Geographic distribution of studies that incorporated race/ethnicity as an integral part of analysis. B Percentage of studies that include race/ethnicity as an integral component that were published in each 5 year time interval between 2000 and 2024.
Among the 55 studies, many assessed representation across diverse groups (n = 19), specifically ten studies that showed a higher incidence/prevalence of PD in White versus African American or black populations28,29,30,31,32,33,34,35,36,37; and two studies indicated race differences in age of onset and age of diagnosis with black individuals showing a younger age of onset38 and a later age of diagnosis39. In addition, there were three studies which found no association of ethnicity or differences in frequency/incidence/prevalence across race or ethnicity23,40,41.
Risk for PD
Findings from studies (n = 17) that examined PD risk factors by race/ethnicity included (1) lifestyle factors or prior disease morbidity such as cancer42, gout43, restless leg syndrome44, alcohol consumption, Type 2 diabetes, hypertension, high cholesterol, head injury, high/low weight, constipation, hypotension, erectile dysfunction, insomnia, dizziness, anxiety, fatigue, depression, memory symptoms, neck pain, shoulder pain, rigidity, balance difficulties, tremor and epilepsy23; (2) environmental exposures such as PM245, selenium46, air pollutants, noise, road proximity47, metal emissions48, smoking49; (3) past pharmacotherapy including beta 2 agonists50; metformin and sulfonylureas51, and calcineurin inhibitors52. Importantly, when examining the results from these studies overall, the lack of overlap in risk factors examined, populations compared, and outcome variables, in addition to the shear low number of studies overall (17/1142), make generalized conclusions near impossible. Moreover, some studies show differences in risk variables by race/ethnicity, sometimes these are simply a difference in magnitude of association as opposed to directional differences in risk37 .
Sex/gender and race
While many of the studies that examined race as an integral variable included both male and female subjects, only three studies included data that stratified race/ethnicity variables by sex. Interestingly, all three of these studies28,29,30 indicated similar findings such that males showed greater incidence and/or prevalence compared to females regardless of race/ethnicity.
Genetics and race
Of the studies that examined genetics as a risk factor for PD by race/ethnicity (n = 18), all of them compared specific risk gene mutation frequency in sub-populations, with six studies focused on LRRK2 variants in specific populations53,54,55,56,57,58, including notably higher frequency of LRRK2 G2019S mutations in Non-Basque (versus Basque populations)56, Ashkenazi Jewish populations versus non-Ashkenazi Jewish57, and no G2019S mutations were found in a Yemenite population53.
Prognosis and social determinants of health
Only one study examined prognosis by race/ethnicity, which showed a higher risk of death for Black PD patients compared to White PD patients and a lower risk of death for Hispanic and Asian PD patients59. Furthermore, none of the articles assessed race-social determinant of health stratified groups, though many adjusted for factors such as education. For instance, Yacoubian et al. and Dahodwala et al. adjusted for social determinants of health and found no impact on the results indicating higher PD prevalence in White populations compared with African Americans33,34.
Discussion
Highlighting the striking lack of representation in the scientific literature regarding PD risk, our targeted literature review found only 4.8% of the 1142 studies related to PD risk published in 2000–2024 included race/ethnicity as an integral part of analysis, and only 40.7% studies considered race/ethnicity in any form. While a few studies identified in our review indicated reasons for exclusion of these factors, some rationales listed to omit race/ethnicity in the results included small sample size60 or data unavailability61. Thus, despite the inclusion of ethno-racial considerations in some forms, few studies examined the role of race/ethnicity on risk or the impact with social determinants of health. The limited literature on these topics identifies an evidence gap that needs to be filled, along with a call for greater awareness to study these concepts. Importantly, Robbins et al.62 identified that a PubMed search for racism and Parkinson’s only identified 2 records62, further demonstrating a gap to understand and address existing disparities in PD research.
Based on our review, specific effects observed included an increased incidence, risk or prevalence of PD in White individuals compared to Black individuals28,29,30,31,32,33,34,35,36,37. Despite this we did find a few studies that found no differences, and an earlier study (pre-dating the inclusion criteria of the current review) showing that African-American individuals had a higher incidence of PD compared to White individuals in Manhattan, New York63. Importantly, it was later highlighted that the Manhattan study’s population may not have been representative of the general public30, nevertheless, understanding why differential findings may be found across regions, may, in and of itself point to important risk modifiers.
It is also important to note that accurate baseline counts of PD diagnosis would necessitate equal access to health care devoid of diagnostic bias. Indeed, it has been suggested that reduced diagnoses of PD among Black individuals may be due to practitioner bias64. Thus, race discrepancies in PD prevalence may reflect actual differences in the frequency of PD cases or a bias in diagnosis due to systemic factors, or both. We recognise results may be mediated by demographic, environmental, social and/or systemic factors that may leave certain groups more vulnerable to PD risk. This reinforces the notion that the limited studies on race/ethnicity in PD risk research is a major pitfall of the field. Considering genetic vulnerability, LRRK2 p.G2385R mutation has been found to be prevalent in Asian populations, whereas there is a marked absence of the LRRK2 p.G2019S55,65,66,67. However, the LRRK2 p.G2019S variant has been documented in European and North African populations21,67,68. Interestingly, our review identified a study demonstrating that among Ashkenazi Jews, the LRRK2 p.G2019S mutation seems to be more prevalent in females than males, suggesting a potential interaction between this mutation and gender58. While these findings highlight potentially important differences (or not) in PD risk factors by ethno-racial group, we note that these are a gross underrepresentation in the context of known risk factors in PD.
Some scientists may be hesitant to consider factors related to race/ethnicity in their investigations, as incorporation would necessitate adapting methodology, research design and potential recruitment strategies. Indeed, one might argue that past efforts to control errant variables and factors studied have been at the cost of generalizability and “real life” representation. Traditionally, scientific research has arguably suffered from a lack of inclusivity in across many domains, including sex and gender, for example, of all the Canadian Institute of Health Research-funded grants from 2009 to 2020, fewer than 3% considered sex and/or gender69.
Given the well-defined sex differences in many areas of health research, including PD, when males are 1.5 times more likely to have the disease70,71,72,73, and females tend to have a later onset of PD symptoms compared to males74,75, the lack of sex and gender inclusion is a major downfall. Clinical phenotypes also differ between males and females with respect to both motor and non-motor symptoms76, as females are more likely to develop a tremor38, and dyskinesia, while males are more likely to experience rigidity and increased sleep during day77. The mechanisms underlying these sex differences are not well understood76,78, and recent work suggests that sex and gender differences may not be as consistent as previously thought, where Asian populations show decreasing sex differences in PD prevalence over time79; however, it is clear that inclusion of these variables in population research will improve risk prediction, prevention and treatment outcomes80. Similar to the added value of including sex as a biological variable, consideration of other underrepresented populations in research—notably from diverse race and/or ethnic backgrounds along with different socioeconomic groups, is key to improved research generalizability and success in clarifying disease models and targeting interventions. Notably, results may not always highlight direct effects, but rather race/ethnicity may impact health disparities in interaction with other factors. It is well understood that gender-specific management of PD would greatly improve quality of life of those living with PD80, indeed African Americans, Hispanics and Asians with PD have poorer quality of life compared with White individuals81—further demonstrating the need to consider race/ethnicity and identify interactions to improve management, diagnosis, prevention and treatment similar to consideration of gender.
Call to action
It is critical to consider and report inclusion of race/ethnic populations in the results of studies, in addition to describing how classification was completed and the methodological reasoning for inclusion of the diverse groups in studies12. Indeed, several regulatory bodies and organizations have implemented excellent guidelines with respect to reporting and best practices for ethno-racial considerations in health research5,10,12,82,83. We recognise that inclusion of these variables together with sex and gender for example can become cumbersome in data analysis, particularly when trying to tease apart the underlying reasons for disparities such as social determinants of health. However, one might simply include these variables to test if differences exist and in future studies try to delineate underlying causes that can point to potential remediation. It is important to note that critical to increasing diverse population recruitment and research outputs is the need for reparations and trust between the scientific community and populations studied (for example maltreatment of Indigenous populations by the health system in Canada)84. Finally, we note that these recommendations are not restricted to clinical studies but should also be considered, where appropriate, in preclinical work, notably with the use of cell lines85.
It is also noted that there is geographical disparity in those studies which included race or ethnicity as an integral factor in their analysis, which may be due to differences in population heterogeneity (i.e. Asian population studies) and may also be due to restrictions in data collection with respect to ethno-racial variables. For example, several countries in Europe including France, Germany and Sweden restrict the collection of this information86,87,88. While the goals of these restrictions were likely rooted in anti-racism, the failure to include data which may highlight health disparities by ethnicity or race may in turn, impair our ability to improve health inequities86. Moreover, there is evidence that studies that directly investigate health disparities are underfunded by national health organizations such as the NIH89,90,91. As such, we would suggest that the inclusion of race and ethnicity variables is not only at the level of the individual study, but increased support is also needed at higher levels, such as large cohort design and policy making.
Our current work has identified that there is a lack of PD risk related literature considering race/ethnicity as a factor. Recognizing the societal implications of ethno-racial identity, it is important to (re)iterate that identified differences in PD risk across ethno-racial identification may not reflect a biological or genetic mechanism per se, but rather that social determinants of health could well be at play and disproportionately effect specific populations10. It is our hope that this perspective serves to advocate for and direct attention to the importance of including race/ethnicity and other intersectional variables within research to strengthen this field92,93. Thus, further research and inclusion of diverse populations in PD research is necessary to improve the generalizability of risk calculations and identify at-risk populations for targeted interventions.
Methods
A literature was conducted on July 3, 2024 in PubMED, MeSH (Medical Subject Headings) terms and key terms related to Parkinson’s disease, risk factors, incidence, prevalence, epidemiology, and diagnosis were included. Filters applied in the initial search string included literature published between 2000 and 2024, journal articles, texts published in English with human participants, while reviews were excluded. A research librarian at Carleton University assisted in development of the search strategy. All records were uploaded to Covidence for two-stage screening with two-independent reviewers, conflicts were resolved by consensus or with a third independent reviewer when needed. First, titles and abstracts were reviewed and assessed based on inclusion criteria listed in Table 2. Next, full texts were screened to either further exclude studies (wrong study type or outcome) or categorize inclusion of race/ethnicity. Inclusion of race/ethnicity was defined according to the text of each article, whereby the authors had to refer to a group specifically as a distinct ethnic group, race category, or from a specific ancestry. Categorization was conducted using a hierarchical process in descending order as shown in Table 3. Articles classified with race/ethnicity inclusion as an integral part of analysis were reviewed and data related to race/ethnicity was recorded in MS Excel, by two independent reviewers and consensus to resolve any conflicts.



Responses