Real-World outcomes with sacituzumab govitecan among breast cancer patients with central nervous system metastases

Introduction
Central nervous system (CNS) metastases, including brain metastases (BM) and leptomeningeal disease (LMD), occur in one-quarter of patients with metastatic breast cancer (MBC), with varying incidence across different subtypes1,2. As the overall survival (OS) of patients with MBC is increasing, and the incidence of CNS metastases continues to rise across all tumor subtypes without any signs of plateauing, managing patients with CNS metastases from breast cancer is a major challenge1,3.
CNS metastases encompass distinct clinical scenarios with specific therapeutic implications and prognosis. The FDA has defined brain metastases based on their clinical status as (1) stable or (2) active. Stable brain metastases refer to patients who have previously received local therapy, such as radiation or resection, and their CNS disease is stable at the time of therapy initiation. Active brain metastases refer to patients with new untreated lesions or previously treated lesions that have not been subjected to CNS-directed therapy since documented progression4. There are no FDA-approved systemic therapies that improve progression-free survival (PFS) or OS in patients with active or stable HER2-negative BM. Evaluation of novel agents for these patients is urgently needed.
Antibody-drug conjugates (ADCs) are an emerging therapeutic class. Both trastuzumab emtansine and trastuzumab deruxtecan (TDXd) have demonstrated intracranial response improvement in randomized trials of patients with HER2-positive CNS metastases5,6,7. The Phase 3b/4 DESTINY-Breast study12 evaluated T-DXd in patients with HER2+ breast cancer, including those with active or stable BM (n = 263). These patients had an objective response rate in the CNS (ORR-CNS) and a 12-month CNS progression-free survival (CNS-PFS) rate of 79.2% (95%CI 70.2–88.3) and 57.8% (95%CI 48.2–66.1) for patients with stable BM and 62.3% (95%CI 50.1–74.5) and 60.1% (95%CI 49.2–69.4) for patients with active BM, respectively8.
Limited data are available for patients with CNS metastases from hormone receptor-positive and triple-negative breast cancer (TNBC) MBC, which represent 45% and 25% of patients with CNS metastases, respectively1. Sacituzumab govitecan (SG) is an ADC composed of an anti-TROP2 antibody linked to an active metabolite of irinotecan. SG has demonstrated significant PFS and OS benefit over standard chemotherapy in pretreated patients with TNBC and HR-positive/HER2-negative (HR + /HER2-) MBC6,7. However, these trials excluded patients with active CNS metastases or LMD. A Phase 0 study demonstrated that the SG payload achieved therapeutic levels in brain metastases and induced intracerebral responses with ORR-CNS of 50% in 13 patients with MBC of all subtype9; however, limited data on intracranial outcomes in MBC patients exist.
In this retrospective, observational, real-world data study, we report the activity of SG in patients with HR+ /HER2- and TNBC MBC using a prospectively maintained institutional database of patients with MBC at Dana-Farber Cancer Institute (DFCI) and electronic health records collection. The objective of our study was to evaluate the real-world activity of SG in patients with CNS metastases, considering whether the metastases were active or treated/stable, and LMD, using the RANO response assessment criteria for BM and LMD, with confirmation through central radiologic review10,11. Treatment response was retrospectively categorized as objective response, stable disease, and progressive disease in the CNS and exta-CNS. An independent radiologist confirmed CNS-ORR using RANO criteria10. Because clinical status was not uniformly available at each restaging, we used a modified version of the RANO criteria where clinical status was not included in the response assessment.
Thirty-three patients were included (Table 1), with 18 (54.5%) having treated/stable, 7 (21.2%) having active CNS metastases, and 8 (24.3%) having LMD. Ten (30.3%) and 23 (69.7%) were HR+ /HER2- and TNBC, respectively. The median age at SG initiation was 56.7 years. Only one patient had CNS metastasis with no extra-CNS involvement; while 18/33 (54.5%) patients had at least pulmonary and/or hepatic involvement, with a median of 3 (range, 1–6) metastatic sites at SG initiation. Patients were heavily pretreated, with a median of 3 (0–10) lines of therapy in the metastatic setting before SG initiation, and three patients received prior TDXd. In patients with stable brain metastases, the median time from prior CNS-directed radiotherapy to SG start was 2.3 months (range 0.5–44.0). Among patients with LMD, 7/8 had associated BM, and all patients had extra CNS metastases. Two had a positive cerebrospinal fluid (CSF), one had a negative CSF, and five did not have a lumbar puncture. The median follow-up for the entire cohort was 6.7 months (IC95% 3.1–10.0) (Fig. 1).
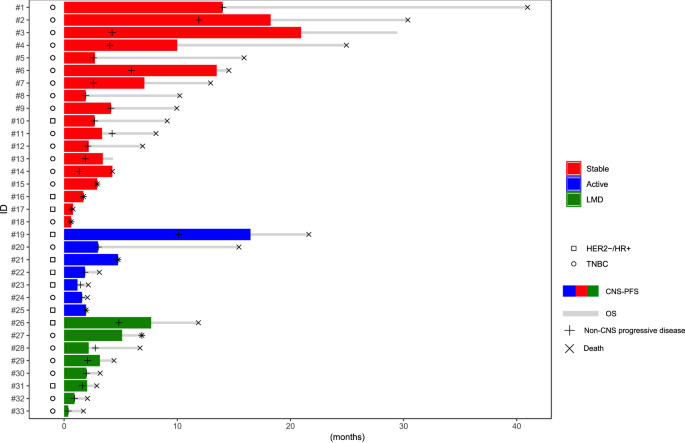
Swimmers plot illustrating Central Nervous system progression free survival (CNS-PFS, colored line) and overall survival (OS, gray line), occurrence of extra-CNS progression and death in metastatic breast cancer patients with CNS metastases treated with sacituzumab govitecan. LMD: leptomeningeal disease.
Thirty (90.9%) and 29 (87.9%) patients had evaluable responses in the CNS and extra-CNS, respectively, with a time interval between initiation of SG and first imaging restaging of 1.6 months (95%CI 1.4–2.3) for CNS responses and 2.1 months (95%CI: 1.8–2.6) for extra-CNS responses. The CNS-ORR was 12.5% for patients with treated/stable BM, 0% for patients with active BM; 11.1% for HR + /HER2-, and 14.3% for TNBC (Table 2, Fig. 2 and Supplementary Table 1). “Furthermore, no extra-CNS objective responses (extra-CNS-ORR) were observed in patients with either treated/stable or active BM. The CNS clinical benefit rate (CNS-CBR: response + stable disease ≥6 months) was 37.5% and 14.3% for patients with treated/stable and active BM, respectively. 7/8 patients with LMD were evaluable for response according to RANO-LM criteria11. For them, the CNS-ORR and CNS-CBR were 28.6% and 14.3%, respectively.
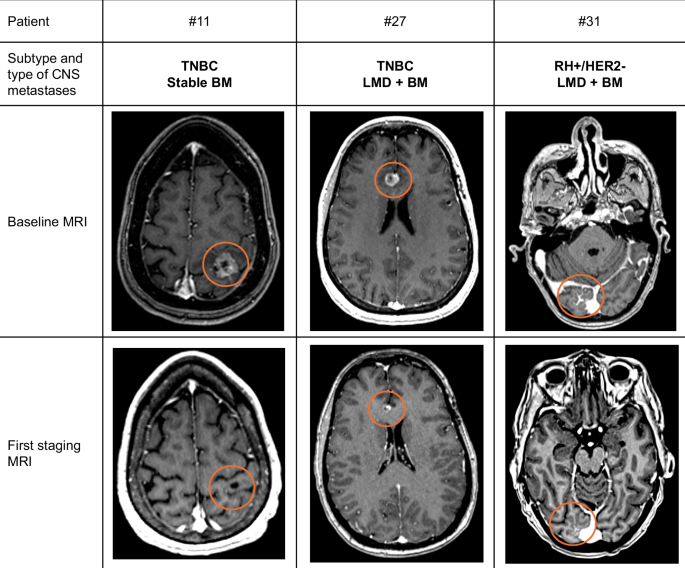
Baseline and Central Nervous system (CNS) response assessment by MRI Brain imaging of patients with metastatic breast cancer patients with CNS metastases treated with sacituzumab govitecan. LMD: leptomeningeal disease;BM: Brain Metastases. Patient #11 with stable BM received RT (SRS) less than two months before the initiation of OS. Patient #27 was irradiated for 9 months on another lesion (temporal) and patient #31 did not receive any irradiation before the SG.
The median CNS-PFS was similar across patients with stable brain BM (3.4 months; [95% CI: 2.2–10.0]), patients with active BM (1.9 months; [95% CI: 1.2–16.5]), and those with LMD (2.1 months; [95% CI: 0.4–7.7]) (Fig. 3). The median OS in the population was 6.9 mo (95%CI: 3.1–10.2). OS did not differ significantly between patients with stable BM (10.0 months; [95%CI: 4.3–15.9]) and those with active BM (3.1 months; [95%CI: 1.9–21.6]; p = 0.17) but was longer in patients with stable BM compared to those with LMD (3.8 months; [95% CI: 1.7–11.9]) (Fig. 3). A total of 32 patients discontinued SG due to progression, and one patient due to toxicity. Most patients (53.1%) experienced both CNS and extra-CNS disease progression. Out of the five patients who died while receiving SG, two suffered from neurological death (Table 3).
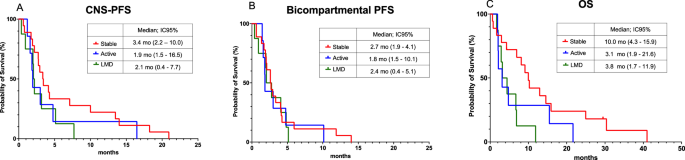
Kaplan Meier curve of (A) Central Nervous System progression free survival (CNS-PFS), (B) Bi compartmental PFS and (C) Overall Survival (OS).
In addition, subgroup analyses were performed based on tumor subtype and number of prior therapies (>3 and ≤3 prior lines). CNS-PFS (central nervous system progression-free survival) was similar in patients with HR + /HER2- and TNBC at 2.0 months ([95% CI: 1.2–7.7]) and 3.2 months ([95% CI: 2.2–5.1]), respectively. Similarly, CNS-PFS was similar for patients with >3 prior lines and ≤3 prior lines at 2.7 months ([95% CI: 1.2–4.8]) and 3.2 months ([95% CI: 2.0–7.1]), respectively. Results for bicompartmental PFS and OS by subgroup are shown in Supplementary Fig. 1.
In our cohort of 33 patients with MBC with CNS metastases, SG demonstrated modest CNS and extra-CNS activity with a CNS-ORR, median CNS-PFS, and OS of 4/30 (13.3%), 2.9 months (95%CI: 2.0–4.3), and 6.9 months (95%CI: 3.1–10.2), respectively. However, we did not observe any centrally-confirmed CNS responses in the seven patients with active BM. Furthermore, both patients with stable BM and CNS response after SG also received RT less than two months before the start of SG. Responding lesions were irradiated.
In our cohort, 3 patients showed exceptional responses to SG, with bicompartmental PFS exceeding 10 months. No novel prognostic factors were identified. All three patients underwent surgery and brain radiotherapy. Two were TNBC patients with stable BM treated early with SG (≤2 prior therapies). The third patient had HR + /HER2- MBC with low-velocity BM, characterized by a prolonged interval of 95 months between the diagnosis of BM and the initiation of SG.
This is the largest cohort of patients treated per current real-world practice, including CNS metastases data in MBC patients. Results align with a retrospective cohort of five patients with active CNS metastases, showing a median CNS-PFS of 2.7 months (95% CI 1.9–10.5) and a disease control rate of 42% (95% CI 13%–71%)12. We observed some efficacy of SG in pretreated patients with leptomeningeal involvement, with CNS-ORR, disease control rate (DCR), median CNS-PFS, and OS of 28.6%, 71.4%, 2.1 months (95%CI 0.4–7.7), and 3.8 months (95%CI 1.7–11.9), respectively. Although these results are still poor, they should be interpreted in the context of the known dismal outcome of patients with HER2-negative MBC, with an expected survival from the diagnosis of LMD of 3.7–6.0 months for HR + /HER2- and 2.0–3.0 months for TNBC13.
Our results are comparable with the prospective data from the ASCENT trial, which included 32 patients with stable/treated BM TNBC randomized to SG. In this subgroup analysis, the median extra-CNS -PFS and OS were 2.8 months (95%CI: 1.5–3.9) and 7.0 months (95%CI: 4.7–14.7), and the extra-CNS -ORR was 3%14. Conversely, the performance of SG in our cohort was inferior to what reported from the overall population (CNS and extra-CNS) of the TROPICS-02 study in HR + /HER2- patients: median extra-CNS-PFS and OS of 5.5 months (95%CI, 4.2–7.0) and 14.4 months (95%CI 13.0–15.7) with a extra-CNS -ORR of 21%15. Finally, the intracerebral response rates in our cohort were lower than those observed in the phase 0 study by Balinda et al, which reported a response rate of 50% (37% stable disease, 25% partial response, 25% complete response)9. This study included patients with all subtypes of MBC eligible for BM resection, unlike our cohort of heavily pretreated TNBC and HR + /HER2- patients. The ongoing Phase II Southwest Oncology Group (SWOG) trial is expected to provide prospective data on the intracerebral efficacy of SG in patients with TNBC (NCT04647916).
Our study has several limitations related to the small sample size and the retrospective nature. However, our results are comparable with data from prospective studies, and an independent review of responses reduces the risk of information bias. We did not perform a multivariate analysis due to the small sample size, but CNS involvement is a recognized poor prognostic factor. Finally, our study does not include comprehensive safety data or symptoms, particularly neurological ones, as there was incomplete data on retrospective chart abstraction.
In conclusion, we report modest activity of SG in pretreated patients with active and stable/treated CNS metastases and possible activity in patients with LMD. Additionally, the efficacy of CNS and systemic metastatic involvement is comparable.
Methods
Study objective(s), design, data sources and variables
The objective of our study was to evaluate the activity of SG in patients with CNS metastases, considering whether the metastases were active or treated/stable, and LMD, using the RANO response assessment criteria for brain metastases and LMD.
We conducted a retrospective, observational, real-world, single-center study using the prospectively maintained institutional EMBRACE database of patients with MBC (DF/HCC IRB #09-204) supplemented by a manual review of the EHRs. Patients provided written informed consent to DF/HCC IRB #09-204 PI Nancy Lin. The study was performed in accordance with the principles of the Declaration of Helsinki. We followed and used the checklist of ESMO-GROW recommendations for real-world evidence studies in Oncology16.
The selection criteria included patients with CNS metastases who had received at least one dose of SG between 2018 and 2022 as part of their routine clinical care. Patient enrolled in clinical trials of SG were excluded. Demographic, clinical, pathological, and treatment data were extracted from the prospectively maintained institutional database. The types of CNS metastases, responses, PFS, and information on neurological deaths were extracted from the EHRs.
The subtypes were chosen according to the immunohistochemistry results of the last biopsy before the initiation of SG. Patients with stable/treated BM were defined as having previously received CNS-specific treatment, and their CNS disease was stable at the evaluation before the initiation of SG. Patients with active BM were defined as those exhibiting new or progressive CNS metastases that had not been treated with CNS-directed therapy since documented progression. LMD was defined as patients having metastases in the leptomeningeal space with or without BM4. Response assessment was divided into response rate, stable disease rate, and progressive disease rate in the CNS and extra-CNS according to RECIST1.117. An independent neuro-radiologist confirmed CNS-ORR using RANO criteria.
Statistical analysis methods
Quantitative variables were described using median and range (min; max). Qualitative variables were described using frequency, percentage. Survival endpoints and follow-up were described using the reverse Kaplan-Meier method and reported with a 95% confidence interval. Comparison used Log-rank (Mantel-Cox) test. PFS (CNS, extra-CNS, and bicompartmental) were defined as the time from initiation of SG to disease progression (CNS, extra-CNS or either) or death from any cause. Overall survival is defined as the time from initiation of SG to death from any cause. The software GraphPad Prism V10 and R Studio were used to create the figures and perform the statistical analyses.

Responses