Recent advances in retention and permeation of CO2 gas using MXene based membranes
Introduction
The escalating challenges posed by global climate change have intensified the focus on CO2 emissions and their substantial role in exacerbating global warming1,2,3. Carbon reduction strategies are pivotal, not only to the Intergovernmental Panel on Climate Change (IPCC) commitments by international governments during the Paris Agreement but also to offer a feasible pathway for corporations to meet their greenhouse gas (GHG) reduction targets4. The Paris Agreement, a landmark accord within the United Nations Framework Convention on Climate Change (UNFCCC), was adopted in 2015 to address and mitigate the impacts of climate change. A central aim of this agreement, as outlined in the IPCC Special Report on Global Warming of 1.5 °C5, is to limit the global average temperature increase to well below 2 °C above pre-industrial levels, while pursuing efforts to limit the warming to 1.5 °C. This more ambitious target of 1.5 °C is deemed crucial to significantly reduce the risks and impacts of climate change. Furthermore, governments of the signatory countries have committed to nationally determined contributions (NDCs) under the Paris Agreement, which are essentially pledges to reduce national emissions and adapt to the impacts of climate change. These commitments are varied and include measures such as cutting down carbon emissions, increasing the use of renewable energy, and investing in carbon reduction solutions6. The agreement also establishes a global stock-take every five years to assess collective progress towards achieving its long-term goals, as discussed in Climate Policy7. The commitment to limit warming to 1.5 °C carries profound implications. According to the IPCC report, this would require rapid and far-reaching transitions in land, energy, industry, buildings, transport, and cities, and global net human-caused emissions of carbon dioxide would need to fall by about 45% from 2010 levels by 2030, reaching ‘net zero’ around 20508. In addition, the urgency to meet the targets set by the Paris Agreement underscores the critical need for innovative technologies in carbon reduction. Given the ambitious goal of limiting global warming to 1.5 °C, as emphasized in the IPCC Special Report, technological advancements play a pivotal role in this endeavor. Advanced carbon capture and storage (CCS) technologies, renewable energy solutions, and energy-efficient infrastructures are essential to achieve the drastic emission reductions required.
Natural gas primarily consists of methane (CH4), with minor quantities of other hydrocarbons like ethane (C2H6), propane (C3H8), and butane (C4H10), along with various impurities such as CO2, H2S, N2, and O29. In addition, the CO2 concentration was reported to be >400 ppm in 2019 and <380 in 2005, showing clear evidence that imperative actions should be taken to avoid further escalation1,9,10. Different technologies are employed for gas separation, including membranes, adsorption, absorption, and cryogenic distillation11. Membrane separation has gained significant attention over the years because of its low energy requirements, affordable capital costs, and straightforward operation12,13. The development of polymeric materials has revolutionized membrane technology14,15,16, followed by advances in inorganic membranes17,18 and mixed matrix membranes (MMMs)19,20. Most reportedly, the polymer membrane, however, suffers from a trade-off between permeability and selectivity21,22,23. Several reports have investigated methods to eliminate those limitations24,25,26. Using an inorganic filler is one of the methods proven to be effective12.
In this regard, 2D Ti3C2Tx MXene-based membranes are applied due to their exceptional properties, which include a vast surface area, robust thermal and chemical stability, and pronounced hydrophilicity. MXenes, a new class of 2D layered materials discovered by Gogotsi and co-workers in 201127,28. MXenes are synthesized by etching the “A” layer from MAX phases (Fig. 1), a large family of ternary transition metal nitrides, carbides, and carbonitrides with a general formula of Mn+1AXn. In MAX phases, M refers to an early transition metal (Ti, V, Cr, Nb, etc.), A refers to an element of group 13 or 14 elements (Al, Si, Sn, In, etc.), X refers to nitrogen, carbon29, boron30, or oxygen31,32 and n = 1, 2, 3 or 433. This selective etching process for A species results in the formation of a terminal group (Tx) typically -OH, -F, and -O groups34. The synthesis method plays a crucial role in determining the surface chemistry of MXenes, which significantly influences their interaction with gas molecules. The surface properties, including the presence of functional groups, surface charges, and defects, are directly influenced by the synthesis conditions. In many studies, to reduce the toxicity of the etchant, HF, alternative etching methods using acid and fluoride mixtures have been explored, such as LiF, NaF, and CaF2 in HCl or H2SO4.
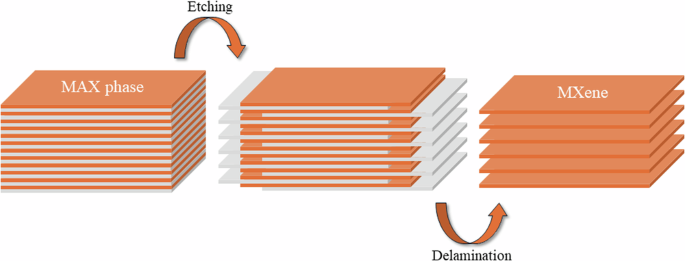
The MXene (Ti3C2) synthesis from the MAX (Ti3AlC2) phase.
Although there have been several reviews published on gas separation using MXenes, these reviews typically focus on aspects such as MXene fabrication methods, membrane preparation techniques, and the reported separation performance35,36,37,38. However, they often overlook critical aspects like retention and permeation, which are essential for understanding the efficiency and practicality of MXene-based membranes in the separation process. Therefore, this review aims to provide a comprehensive analysis by integrating the concepts of retention and permeation in the context of MXene-based membranes for gas separation. By doing so, it offers a more holistic view of the factors that influence membrane performance, beyond just fabrication and separation outcomes. Further, this review provides valuable insights into how MXenes can be effectively utilized to selectively retentate and permeate specific gas, such as CO2, and explores the underlying mechanisms responsible for selective separation. By examining the principles of MXene-based gas separation, the review offers a comprehensive perspective that can guide researchers in tailoring MXene materials for the efficient separation of single or multiple gas components. This framework will aid scientists in optimizing MXene properties to enhance performance for various gas separation applications, thus contributing to the development of more efficient and sustainable technologies.
Gas transport in membranes
Gas transport properties emerge when gas molecules diffuse through the membrane via its cavities and dissolve at the membrane’s surface. Permeability results from the interplay of these two processes, as illustrated in Eq. 139:
In this equation, (P) represents permeability (related to gas partial pressure), (D) is the diffusion coefficient (a kinetic factor), and (S) is the sorption coefficient (a thermodynamic factor). The sorption coefficient reflects the physicochemical attributes of the materials and the condensability of the gas. Henry’s law establishes a direct relationship between the amount of gas dissolved in a membrane and its partial pressure, while Fick’s law describes the diffusion coefficient40, which indicates the energy needed for gas molecules to diffuse through the membrane. This coefficient is influenced by the size and shape of the gas molecules41. Ideal selectivity ((alpha)) is used to assess the gas transport characteristics of the membrane (Eq. 2). It is defined as the ratio of permeability for gases a and b. According to Eq. 1, it can also be represented in terms of diffusion selectivity (the ratio of diffusion coefficients) and adsorption selectivity (the ratio of sorption coefficients), as illustrated in Eq. 240.
Potential membranes for CO2 separation
Membrane-based gas and vapor separation technology offers a competitive alternative to traditional methods such as cryogenic distillation, absorption, and pressure swing adsorption, mainly due to its economic, safety, and environmental advantages. Initially developed for hydrogen recovery, membrane systems now serve a variety of industrial applications, including nitrogen and oxygen production, hydrogen recovery in refineries, and CO2 separation from natural gas. Their compact size makes them especially suitable for offshore operations, with potential growth in areas like flue gas CO2 recovery, vapor/gas separation, and methane/nitrogen separation42.
The experimental gas separation results are often based on pure (single) or binary gas measurements for new materials with low temperature and pressure conditions43. On the other hand, the industrial gas streams contain multi components with different conditions (Table 1). Hence, solely comparing experimental results based on permeation and selectivity between the industry and experimental/theoretical studies will result in far offset values. Although simplified conditions are necessary for the first step with new materials for screening, a more realistic conditions are still required in the lab work43.
The main challenges that hinder widespread industrial adoption include stream pretreatment, membrane durability, selectivity, and permeability. Research efforts are focused on enhancing membrane materials, such as polymeric, carbon-based, and hybrid materials, to withstand harsher conditions and improve overall performance. Inorganic membranes show promise for high-temperature or hydrocarbon vapor applications but require additional development9,42. Experimental and theoretical studies have highlighted that the ideal gas separation membrane should have the following characteristics to achieve high gas selectivity (>50%) and permeability: a separation selective layer at the nanometer scale (0.1–1.0 µm) and deposited on high permeable porous support to enhance the mechanical properties of the overall membrane, molecular-level gas permeation channels with a regular arrangement and high orientation, and strong mechanical properties along with excellent physicochemical stability as newly fabricated thin film membranes will often lose 25% of their permeability in a couple of days and another 25% over the first two weeks. Regardless of the permeability of the fabricated membranes, large scale membranes are essential for industrial usage43.
CO2 gas retention using MXene-based membranes
MXene membranes are distinguished by their unique two-dimensional structure, which facilitates selective gas permeation, making them a cutting-edge solution in the fight against climate change. Their development represents a significant stride in creating sustainable and environmentally friendly technologies for reducing greenhouse gas emissions. This section discusses the CO2 gas retention using unmodified and chemically modified MXene membranes, with a summarized tabulated experimental conditions and results serving as a comparative guide among the reported studies (Table 2).
Self-standing Ti3C2Tx membranes
A self-standing membrane of laminated 2D Ti3C2Tx with an ordered structure was reported by Wang et al. for selective H2 gas separation44. The typically prepared Ti3C2Tx was vacuum filtrated on aluminum oxide porous support, as shown in Fig. 2A, B. After drying in a vacuum oven (Fig. 2C), Ti3C2Tx can be peeled off easily (Fig. 2D–F), confirming its excellent flexibility. Further, the 2 µ thick membrane exhibited 50 MPa tensile strength and 3.8 GPa Young’s modulus, indicating good mechanical properties. The free-standing membrane’s gas performance illustrated a high permeation for the gas molecules with a diameter smaller than the interlayer spacing of Ti3C2Tx sheets such as He and H2, as shown in Fig. 2G. The dominance in permeation based on the kinetic diameter of gas molecules indicates that the separation process using Ti3C2Tx is based on a molecular sieving mechanism44. Nevertheless, CO2 trapping was higher than N2 while being smaller in size than the latter, since the former has a larger quadrupole moment. Further, it is worth noting that despite the preferential adsorption of CO2 on Ti3C2Tx than other gases, the adsorbed CO2 molecules play a rule in blocking the nano-spacing and increase the diffusion resistance for CO244. While such phenomena is absent, the membrane exhibited an outstanding H2 permeability of 1201 GPU and 166.6 H2/CO2 selectivity44. The high thermodynamic stability of Ti3C2Tx sheets is due to the covalent/metallic/ionic bonds45,46. Ti3C2Tx has been reported in high-temperature (320 °C) gas separation experiments47. Supported by aluminum oxide, the thin film composite membrane showed a stable performance over a long-term measurement at 320 °C (Fig. 2H). Nevertheless, the experiment did not implement oxidizing gases such as O2 and CO2, as pristine Ti3C2Tx can easily be oxidized at high temperatures48,49. The fabrication of a composite Ti3C2Tx based material has shown an improving results in enhancing the thermal stability for the material50. Recently, a highly thermal stable Ti3C2Tx/rGO was synthesized after reduction process on the surface of Zinc foil51. The composite membrane reported 809 GPU H2 permeation and 83 H2/CO2 selectivity at 120 °C.
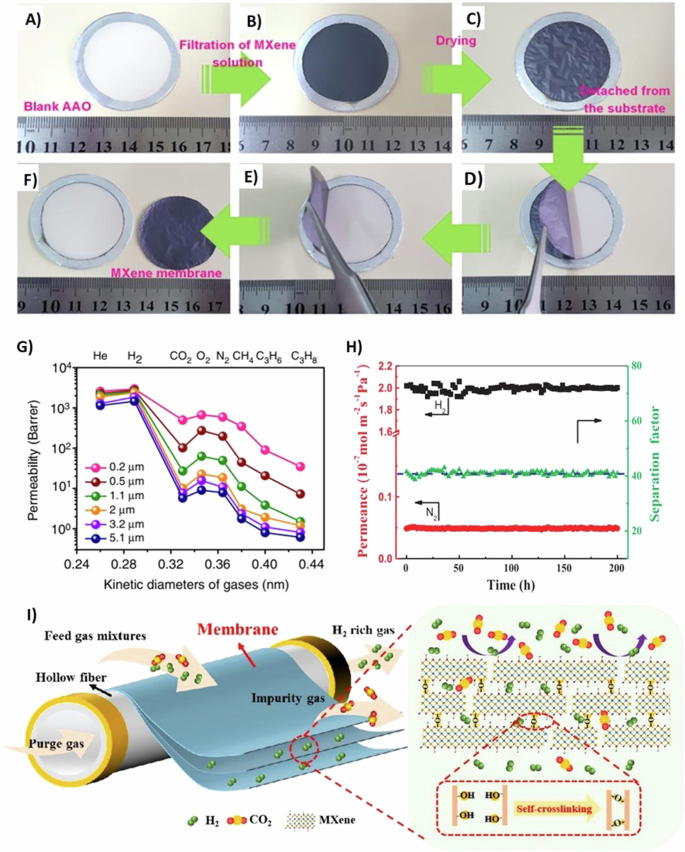
A Anodic alumina oxide supported porous membrane. B Filtrated wet MXene (Ti3C2Tx) on porous anodic aluminum oxide membrane. C Dry Ti3C2Tx on porous anodic aluminum oxide membrane. D, E Removal of flexible Ti3C2Tx. F Self-standing Ti3C2Tx peeled off from the porous anodic aluminum oxide support. Adapted under terms of the Creative Commons license from supporting file of ref. 44. Copyright © 2018, Springer Nature. G Gas permeability for different membrane thicknesses using self-standing Ti3C2Tx at 25 °C and 1 bar. Adapted under terms of the Creative Commons license from ref. 44. Copyright © 2018, Springer Nature. H Stability test for hydrogen (H2) gas separation from nitrogen (N2) gas using Ti3C2Tx at 320 °C. Reprinted from ref. 47. Copyright 2019 Elsevier. I Self-crosslinking of Ti3C2Tx MXene under thermal treatment supported on yttria-stabilized zirconia hollow fiber membrane. Reprinted with permission from ref. 53. Copyright 2021 Elsevier.
Using support is one of the methods used to enhance the mechanical properties of membranes and provide a reliable and facile approach for the scale-up fabrication of 2D material membranes. On one side, polymeric membranes suffer from the trade-off between selectivity and permeability. On the other hand, hollow fibers are featured by tolerance for temperature and pressure as well as a porous structure with low gas transport resistance52. In this regard, Xu et al. supported self-crosslinked MXene sheets on hollow fibers of yttria-stabilized zirconia (YSZ)53. Ti3C2Tx MXene was fabricated and deposited using vacuum filtration on YSZ and underwent a thermal treatment process at 140 °C for 10 h to self-crosslink Ti3C2Tx layers on YSZ support. The thermal treatment reduced the interlayer spacing of Ti3C2Tx nanosheets to 0.31 nm, falling between the kinetic diameter of H2 (0.29 nm) and CO2 (0.33 nm), making the membrane more efficient at sieving H2 molecules. Size reduction in nanochannels of Ti3C2Tx is attributed to the reaction during the thermal treatment between (-OH) functional groups forming Ti–O–Ti bonds and H2O molecules that evaporate, as illustrated in Fig. 2I53. The H2 separation performance using self-crosslinked Ti3C2Tx exhibited a selectivity of 30.3, which was five times that of the pristine Ti3C2Tx. More importantly, the effect of membrane thickness on gas separation is drastic. Decreasing the thickness of the Ti3C2Tx membranes resulted in an uneven distribution of the nanosheets, creating pin holes on the membrane surface and exhibiting high permeance and low selectivity53,54. In addition, as the thickness increased, pin-holes are covered up by the added layers, forming a dense structure of laminated nanosheets exhibiting low permeance and high selectivity53. A 20 nm thick Ti3C2Tx membrane was reported using vacuum filtration after spin-coating with dilute polyether block amide to cover surface defects54. Reaching such thickness was attributed to filtering a highly diluted Ti3C2Tx solution on small diameter (2 cm) membrane54.
Ion-intercalated Ti3C2Tx membranes
Numerous studies have explored methods to modify the spacing of 2D nanosheets, viewing this approach as a promising strategy to improve gas separation performance26,55,56,57. Among those studies, Ni2+ ions intercalated Ti3C2Tx sheets supported on alumina hollow fibers were fabricated for H2 gas separation58. The ion-intercalation resulted in a noticeable shift of the 002 peak for Ti3C2Tx MXene to a lower angle. The shift from 9.52° to 7.74° upon the ion-intercalation demonstrates the increase in interlayer spacing of Ti3C2Tx MXene sheets. The fabricated Ni2+/Ti3C2Tx membranes displayed 615 H2/CO2 selectivity, three times that of the pristine Ti3C2Tx membranes58. Moreover, the Ni2+/Ti3C2Tx membrane gas separation performance was above the 2017 upper bound for H2/CO2 separation, outperforming other reported membranes59,60,61,62,63,64,65. The unprecedented preferential H2 permeation is owed to the electrostatic repulsion of Ti3C2Tx nanosheets due to the Ni2+ ion-intercalation, making a well-organized structure and capturing CO2 molecules by the cations58. Similar work was done by intercalating Pd2+ ions on Ti3C2Tx MXene66. The benchmark Pd2+/Ti3C2Tx membrane separation performance exceeded previously reported membranes67,68,69,70 and the 2017 upper-bound H2/CO2 separation membranes. Two routes for gas diffusion were proposed to further elaborate on the high H2 separation. As shown in Fig. 3A, the Pd2+/Ti3C2Tx membrane can allow the gas molecules to diffuse in a straightforward pathway created from defects and through tortuous nanochannels created by wrinkles and interlayers of the stacked nanosheets. The permeation of H2 was calculated using a mathematical model at different temperatures and compared to the overall experimental values to determine the dominant permeation-diffusion pathway. Figure 3B displays high tortuous theoretical values at all temperature ranges, indicating that the H2 permeation-diffusion is more prevalent in the tortuous nanochannels66. One could attribute this result to the abundant tortuous nanochannels over the straightforward pathways. Moreover, the intercalation of Pd2+ ions has modified the surface of Ti3C2Tx MXene sheets, enhancing H2 adsorption, forming Pd–H bond and diffusing through Ti3C2Tx nanosheets66. The mechanism (Fig. 3C) is based on the breakaway of hydrogen molecules into atoms on the surface of the Pd atoms. After the occupation of H2 atoms on all Pd atoms, H2 atoms will diffuse to the Ti3C2Tx surface due to the concentration gradient. Finally, the H2 atoms will be desorbed to H2 molecules. H-spillover combined with molecular sieving is assumed to be the tortuous effect in the hydrogen separation process66. Confirmed by molecular dynamics, diffusion coefficient for CO2 gas decreases as metal ion charge increases due to a stronger electronegativity of oxygen in comparison to that of nitrogen and hydrogen71.
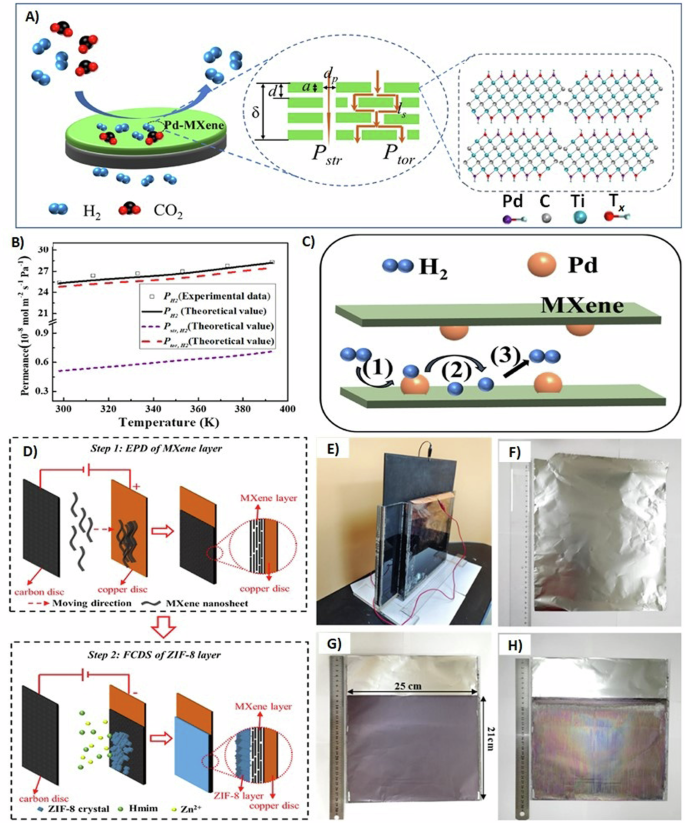
A Gas diffusion routes on palladium/MXene (Pd2+/Ti3C2Tx) membranes. B A comparison of hydrogen permeation at different temperatures between theoretical values of the proposed two routes and the experimental data. C Hydrogen spillover mechanism. Reprinted with permission from ref. 66. Copyright 2022 Elsevier. D Preparation method for MXene/zeolitic imidazolate framework-8 (Ti3C2Tx/ZIF-8) membranes. (Step 1) Electrophoretic deposition (EPD) of Ti3C2Tx sheets on a copper disc at a direct voltage of 20 V in 1 minute. (Step 2) Fast current-driven synthesis (FCDS) of ZIF-8 particles on Ti3C2Tx MXene layers at an applied current density of −0.5 mA/cm2 in 20 minutes. E The device used to prepare large-scale membranes of Ti3C2Tx/ZIF-8 on aluminum foil. F Aluminum foil substrate. G First layer of Ti3C2Tx MXene deposited on aluminum foil covering a 25 × 21 cm2 area. H Front side of dual-layered Ti3C2Tx/ZIF-8 membrane (ZIF-8 covering Ti3C2Tx MXene layer) on aluminum foil. Reprinted with permission from ref. 72. Copyright 2022 Elsevier.
Zeolitic imidazolate framework (ZIF) modified Ti3C2Tx membranes
Other strategies are described in the literature to enhance the gas separation performance of Ti3C2Tx membranes. For instance, Wang et al.72 controlled the thickness of a dual-layered ZIF-8 supported on Ti3C2Tx MXene membranes using electric field. First, carbon and copper discs were immersed in Ti3C2Tx solution, and a direct voltage was applied. As shown in Fig. 3D, Ti3C2Tx sheets were first deposited on a copper plate, forming 800 nm laminated Ti3C2Tx layer. And by taking advantage of the high electrical conductivity of MXene73, ZIF-8 crystals were grew on Ti3C2Tx sheet surface, forming 450 nm thick layer. Finally, the deposited dual-layered Ti3C2Tx/ZIF-8 membrane was peeled off the copper plate72. It was noticed that achieving a thinner MXene layer would weaken the membrane’s mechanical strength, making it difficult to be peeled off. The dual-layer membrane with optimized thickness enhanced H2/CO2 selectivity from 44.4 (for pristine Ti3C2Tx) to 77.4. Furthermore, a large-scale membrane (25 × 21 cm2) was easily fabricated on commercial aluminum foil, as shown in Fig. 3E–H. This fabrication method can be applied not only to create various multilayer membranes but also to produce large-scale commercial membranes for gas separation applications72.
CO2 gas permeation using MXene-based mixed matrix membranes
Mixed matrix membranes (MMMs) have attracted significant interest because traditional polymeric membranes face a compromise between gas selectivity and permeability, often referred to as the Robeson upper-bound limit74. In contrast, inorganic membranes struggle with issues such as inconsistent production quality and high manufacturing costs75. MMMs are composed of a polymer matrix combined with filler particles, each exhibiting distinct flux and selectivity properties76. These fillers are integrated into the continuous phase of the polymer network. A typical representation of a mixed matrix membrane is illustrated in Fig. 4. It is essential for the polymer matrix to form strong connections with the filler components, enhancing gas selectivity while also providing robust mechanical and thermal properties77. Commonly used polymers in gas separation include Pebax78,79,80, polysulfone (PSF)81,82,83, Matrimid84,85,86, and 6FDA-based polyimides87,88,89. In addition, many fillers concerning zeolites90,91,92, MOFs93,94, and carbon materials95,96, have been extensively studied. Notably, incorporating small quantities of molecular sieves has been shown to significantly boost the gas selectivity of the membranes. This enhancement is attributed to the superior separation efficiency of molecular sieves compared to the pure polymer, enabling MMMs to exceed the Robeson upper-bound limit97. MMMs are also being developed to improve the mechanical and electrical properties of polymers98,99,100. Particularly, MXene additives are distinguished by their unique two-dimensional structure, which facilitates selective gas permeation, making them a cutting-edge solution in CO2 gas separation. This section discusses the CO2 gas permeation using different MXene-based mixed matrix membranes.
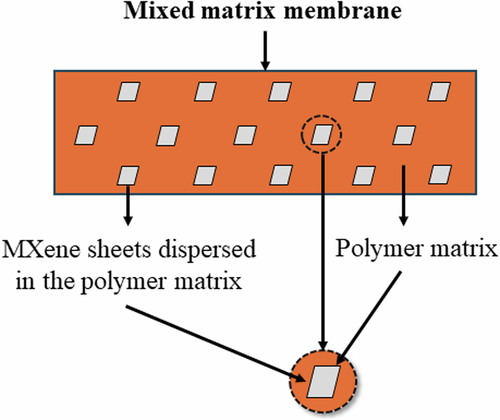
Schematic illustration of a typical mixed matrix membrane.
Pebax/MXene mixed matrix membranes
A robust commercial membrane based on Ti3C2Tx and Pebax-1657 was reported101. Ti3C2Tx and Pebax were mixed in a water/ethanol solution. The mixture was then cast on polyvinylidene fluoride (PVDF) support after being coated with a gutter to prevent the polymer solution from penetrating the porous support. The gas separation test resulted in high permeation and selectivity of CO2 gas, owing to strong interactions between CO2 gas and polyethylene oxide (PEO) group. In particular, 1986.5 GPU and 41.8 and 6.1 were the permeation of CO2 and CO2/N2 and CO2/H2 selectivity values, respectively101. The outstanding performance of the membrane is contributed by the effect of solubility and diffusivity, as shown in Fig. 5A. The CO2 molecules are more favorable to be adsorbed on the -OH functional group of Ti3C2Tx sheets. The interlayer spacing between Ti3C2Tx sheets provides molecular sieving, enhancing permeability, solubility selectivity, and diffusivity selectivity. In addition, Ti3C2Tx sheets played a critical role as a filler in Pebax chains, decreasing the crystallinity of the hard segments, increasing the phase separation, and enhancing the permeability. As such, the introduction of nanofillers into block copolymers can change the phase separation, which strongly depends on the nanomaterial’s size and shape and its interaction with the polymer chains102,103. Lastly, the random orientation of Ti3C2Tx nanosheets in the matrix can affect the transport pathway, increasing the diffusivity selectivity. While the lateral size of the nanosheets has an inversely proportional relationship with the selectivity and diffusivity of CO2 gas, small sheets were used (0.5 µm)101. It is worth noting that the selectivity of thin film composite membranes compared to that of the self-standing MMM with the exact composition of the filler was almost halved101. This reduction in selectivity could be ascribed to the uneven free volume formed by the fast fabrication within the thin film104. Moreover, the permeation and selectivity of single gas using Ti3C2Tx/Pebax membranes were lower than that of mixed gas, which is attributed to the nonideal behavior between mixed gases infiltrating the membranes101.
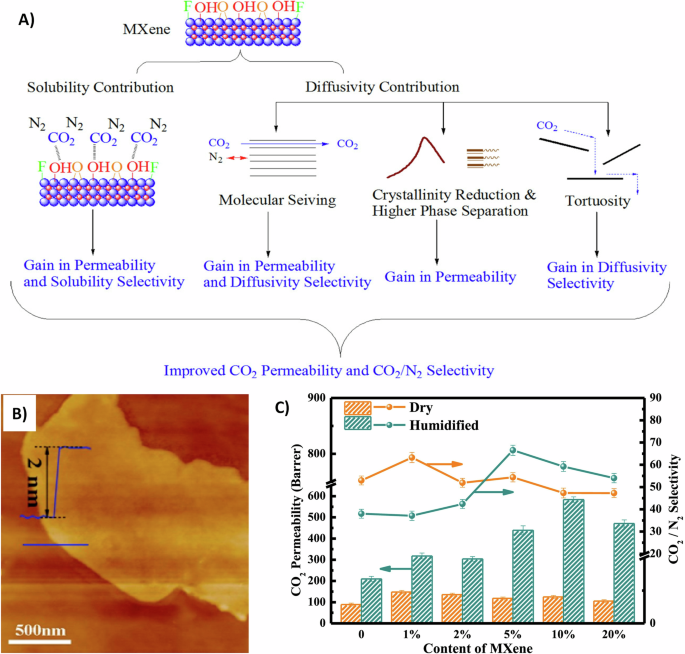
A Carbon dioxide (CO2) gas transport mechanism using Pebax/MXene (Ti3C2Tx) mixed matrix membrane (In brown the crystallinity reduction legend mimics the X-ray diffraction results for peak broadening caused by Ti3C2Tx and the high phase separation legend depicts the tuned polyamide (PA) and polyethylene oxide (PEO) segments in the Pebax matrix). Adapted with permission from ref. 101. Copyright (2020) American Chemical Society. B Atomic force microscopy image of Ti3C2 sheets. C Gas separation performance of Pebax/Ti3C2Tx mixed matrix membrane at wet and dry conditions. Reprinted with permission from ref. 105. Copyright 2021 Elsevier.
Wet MXene-based mixed matrix membranes
The moisture content of the filler can influence the gas separation efficiency of MMMs. In this regard, Pebax/Ti3C2Tx MMMs were prepared to exploit the unique properties of the humidified 2D filler on CO2 separation105. The single layer of Ti3C2Tx sheets displayed a 2 nm thickness and showed the adsorption of water molecules on the surface of Ti3C2 sheets, as shown in Fig. 5B. The gas performance for both the dry and humidified filler in the MMM for different contents of Ti3C2Tx MXene sheets is shown in Fig. 5C. The membrane in wet conditions seems to display a higher permeability at all given contents of Ti3C2Tx sheets compared to that in the dry state. On the other hand, the selectivity was only higher in the humidified state at high loading contents of Ti3C2Tx sheets. Such results indicated that the bound water molecules between the interlayer channels have a significant impact on the CO2 transport properties. Such behavior could be attributed to the fact that the trapped water molecules in the filler facilitate the adsorption of CO2 molecules, thereby increasing the solubility selectivity.
Unexfoliated MXene-based mixed matrix membranes
Following up with Pebax polymer, a mixture of Ti3AlC2 MAX and Ti3C2Tx MXene was incorporated into Pebax in a MMM106. The XRD pattern for the prepared Ti3C2Tx displayed a duo 002 peak at 9.8° and 6.5° along with a less intense aluminum peak at 39°, revealing the presence of a mixture of both Ti3AlC2 and Ti3C2Tx phases and the precursor was not completely transformed. Surprisingly, the fabricated membranes achieved much higher performance over the pristine Pebax membranes. At a low content of 0.15 wt% of the filler, the CO2 permeation exhibited 81% enhancement and more than 70% higher CO2/N2 selectivity106. This behavior could be attributed to the presence of polar groups in Ti3C2Tx sheets that increase the affinity of the membrane towards CO2 gas over N2 gas. In addition, the effect of temperature on the separation performance was investigated. The gas performance resulted in a graduate increase in CO2 permeation owned to enhanced gas diffusion at high temperatures and the swilling of the Pebax chains. On the other hand, the CO2/N2 selectivity decreased as the temperature increased. Based on the Arrhenius equation, the activation energy of CO2 gas is higher than that of N2 gas, indicating that the latter is more sensitive to temperature, resulting in increasing the permeation of N2 gas over CO2 gas.
The nanochannels of 2D Ti3C2Tx sheets incorporated into polymeric membranes led to a tedious gas pathway, thereby improving the selectivity; however, resulted in decreasing CO2 gas permeability101. Using pre-structured Ti3C2Tx MXene sheets ensures effective uniform channels107. This material is based on multilayered Ti3C2Tx MXene sheets in Pebax MMMs, which displayed promising results owed to the varied width of the nanochannels resulted from aluminum elements removal, causing molecular sieving effect. In addition, the hydrogen bonding between CO2 molecules and Pebax chains improves the selectivity permeability107.
Hybrid additives filled mixed matrix membranes
This section attempts to explore the synergistic effect resulted from combining 2D MXene nanosheets with 1D nanorods or 3D nanoparticles in MMMs. Nunes et al. incorporated hybrid fillers with different loadings into Pebax-based MMMs, Pebax/Ti3C2Tx/SiO2 and Pebax/Ti3C2Tx/halloysite nanotubes (HNTs) MMMs108. Compared to the pristine Pebax membranes, the dual filler-filled membranes displayed an outstanding gas separation performance. The maximum performance was observed for Ti3C2Tx/SiO2 filled MMMs, with 104% and 49% enhancements in CO2 permeation and CO2/N2 selectivity, respectively, compared to the pure Pebax membranes. The pristine Pebax membrane CO2 permeability was 1.06 GPU and the selectivity was 41. More importantly, the enhancement for a single filler (Ti3C2Tx) was below 1% for both permeation and selectivity108. Such performance illustrates the existence of the synergistic effect in hybrid fillers, which was more dominant for Ti3C2Tx/SiO2 than Ti3C2Tx/HNTs, due to a much better dispersity of SiO2 than HNTs.
Most recently, 2D Ti3C2Tx MXene and 1D carboxymethyl cellulose (CMC) were combined as fillers in Pebax-based MMMs for CO2 separation from flue gas109. The dual filler membranes possessed supreme tensile strength of 0.94 MPa and an elongation at break of 1351.11%, which are much higher than those of single additive-filled MMMs and pure Pebax membranes (Fig. 6A), indicating strong interfacial interactions between the fillers and the polymer matrix109. As expected, the gas separation testing displayed similar results, Pebax/Ti3C2Tx/CMC membranes showed 521 GPU of CO2 permeation and 40.1 CO2/N2 selectivity, as shown in Fig. 6B and compared with other studies110,111,112,113,114,115,116,117,118. The enhancement in the gas separation performance of Ti3C2Tx is mainly based on the abundant polar functional groups that increase the sorption selectivity for CO2 molecules and the nanochannels that enhance the diffusion of CO2 molecules. A novel two 2D nanofillers (g-C3N4 and MXene) were incorporated into Pebax MMM119 as a method to mend nonselective defects in g-C3N4. MXene was subjected to an in-situ hydrothermal oxidation method in the presence of g-C3N4 in order to obtain oxygen terminated MXene. Hence, inducing a hydrogen bond between -O terminal group in MXene and -NH2/-NH group in g-C3N4 creates defect-free nanofiller. The gas separation performance of g-C3N4-MX-O (1:1)/Pebax MMM illustrated an outstanding stability after 100 h test with 941 GPU CO2 permeation and 47 CO2/CH4 selectivity while possessing 0.6 MPa tensile strength119.
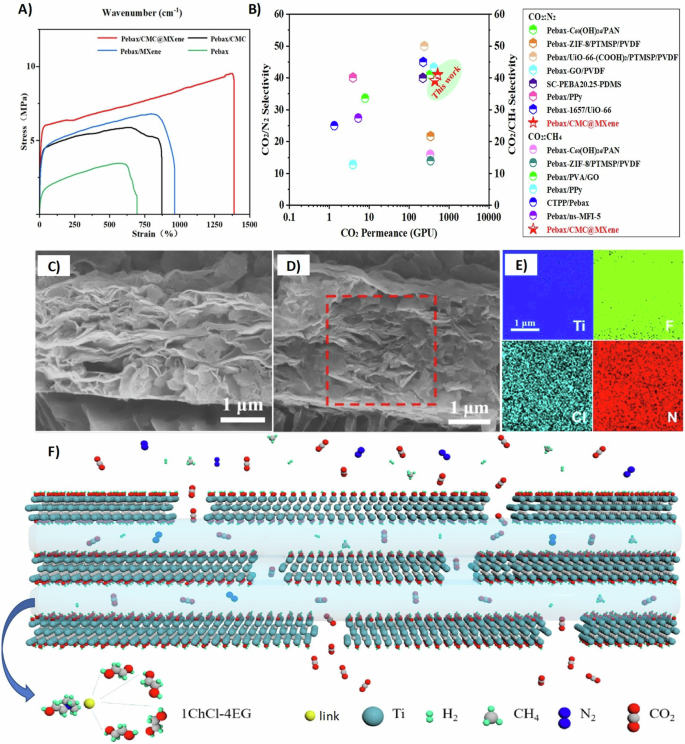
A Tensile test for Pebax-based mixed matrix membrane. B Gas separation performance of Pebax/MXene (Ti3C2Tx)/carboxymethyl cellulose (CMC) mixed matrix membrane compared with other studies. Adapted with permission from ref. 109. Copyright © 2022, American Chemical Society. C Cross-sectional scanning electron microscope image of Ti3C2 sheets. D Cross-sectional scanning electron microscope image of Ti3C2Tx/choline chloride (ChCl)-ethylene glycol (EG) mixed matrix membrane. E Element mapping from image D. F Solution-diffusion mechanism of Ti3C2Tx/ChCl-EG membranes. Reprinted with permission from ref. 122. Copyright 2021 Elsevier.
Other MXene-based mixed matrix membranes
Other matrices have also been used in MXene-based membranes preparation due to their simple synthesis from cheap and natural ingredients such as deep eutectic solvents (DESs). A facile mixing between salts (hydrogen bond acceptors, HBAs) and hydrogen bond donors (HBDs) such as ethylene glycol and phenol, can produce DESs. Their potential resides in using different combinations of HBDs and HBAs while varying their stoichiometry, making them favorable in many applications120,121. For instance, Peng et al.122 prepared a novel ionic liquid membrane by impregnating Ti3C2Tx MXene with DESs. First, the Ti3C2Tx additive was vacuum filtered on a polycarbonate substrate forming a horizontal stacking of laminated MXene sheets (Fig. 6C). The prepared membranes were then soaked in chlorine chloride-ethylene glycol (ChCl-EG) and then spin-coated to remove the excess of ChCl-EG, forming Ti3C2Tx/ChCl-EG membranes122. Figure 6D and E display a denser structure of these Ti3C2Tx/ChCl-EG membranes, confirming the successful impregnation of ChCl-EG through the nanochannels of Ti3C2Tx sheets. Element mapping further confirmed a well homogeneous distribution of the ionic solvent intercalated throughout the horizontal 2D MXene sheets. The rich terminal groups of Ti3C2Tx sheets can create a hydrogen bonding network with the DESs providing additional stability and durability for these membranes. Fabricating CO2-philic solvent imbedded in neoteric 2D Ti3C2Tx membranes resulted in high selectivity for CO2 gas over other gases (N2, CH4, and H2). The proposed gas separation mechanism of these membranes follows the solution-diffusion mechanism (Fig. 6F). The gas molecules can easily dissolve in the Ti3C2Tx/ChCl-EG membranes and diffuse due to the concentration gradient between the feed and permeate122. It is noteworthy mentioning that the prepared Ti3C2Tx/ChCl-EG membranes reported both permeation and selectivity above Robeson upper bound 2008123. Moreover, poly (ethylene glycol) (PEG) is extensively used in separation applications124,125. For example, Ti3C2Tx MXene/PEG MMMs were used for CO2 gas separation from N2 and CH4 gases126. Unlike the previous study122, Ti3C2Tx MXene sheets were first mixed with PEG chains and then filtered on polytetrafluoroethylene porous support membrane, as shown in Fig. 7A126. These fabricated membranes improved performance by exhibiting 32.18 CO2/N2 selectivity, 27.87 CO2/CH4 selectivity, and >1900 GPU CO2 permeation in mixed gas separation experiments (Fig. 7B and C)126. These interesting results are contributed to the presence of PEG polar ether oxygen functional groups that facilitate CO2 solubility127,128. Similarly, Jin et al.54 mixed borate and polyethyleneimine (PEI) with Ti3C2Tx MXene sheets, then the mixture was vacuum filtered on an anodic aluminum oxide substrate, forming membranes with a thickness of 20 nm (Fig. 7D). The interlocked borate and PEI in Ti3C2Tx membranes increased the selective adsorption of CO2 gas over H2 and CH4 gases and released the trapped CO2 molecules, facilitating its permeation and transforming the diffusion-controlled gas transport mechanism into solution-controlled one. The gas separation behavior under the influence of different feed pressures is rarely reported in the literature107. Figure 7E illustrates a steep decline in CO2 permeation as the feed pressure increases54. Such behavior can be attributed to the reversible reaction between the acidic gas molecules (CO2) and basic groups (amino groups in PEI)129. A similar CO2 permeation decay by increasing the feed pressure was reported in the literature129,130,131. Table 3 summarizes the CO2 permeation performance of MXene-based MMMs.
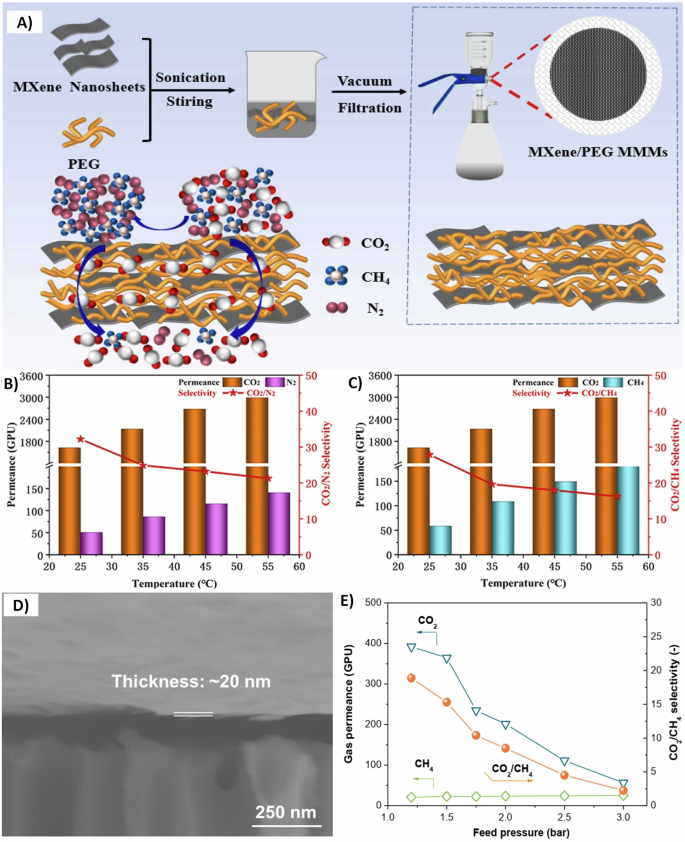
A A schematic diagram for MXene/poly(ethylene glycol) (PEG) mixed matrix membrane preparation. Mixed gas separation performance at different temperatures for B carbon dioxide (CO2)/nitrogen (N2) and C CO2/methane (CH4). Reprinted with permission from ref. 126. Copyright 2022 Elsevier. D Scanning electron microscope micrograph of MXene (Ti3C2Tx)/borate/polyethylenimine (PEI) mixed matrix membrane on an anodic aluminum oxide substrate. E CO2 separation performance at various feed pressures for Ti3C2Tx/borate/PEI mixed matrix membrane. Adopted with permission from the corresponding author (supporting file) ref. 54.
Conclusion and future directions
2D MXenes are gaining researchers’ interest in CO2 separation due to their remarkable tunable characteristics, attributed to their surface terminal groups and distinctive two-dimensional structure, which enhance selective gas separation. The concept of retention and permeation involves separating the desired products from unwanted impurities. Thus, the summarized research work describes how MXenes can be tailored to selectively retain or permeate CO2 gas, showcasing the potential of the MXene family for gas separation applications.
Studies have shown that self-standing MXene membranes result in the retention of CO2 gas while allowing the permeation of small-diameter gas molecules, owing to the narrow interlayer spacing of the sheets. In addition, the preferential adsorption of CO2 on the Ti3C2Tx surface is attributed to the terminal -OH groups, which increase the diffusion resistance for CO2 through the membrane. Several methodologies, such as ion-intercalation and surface modification of MXene, have been employed to enhance these properties. By introducing an additional tortuous effect to the molecular sieving mechanism of pristine Ti3C2Tx MXene, the diffusion of CO2 gas through the membrane is further hindered. On the other hand, MXenes are also used as nanofillers in mixed matrix membranes to further enhance gas separation performance by improving selectivity and permeability. According to the literature, highly soluble CO2 matrices have been used to fully intercalate MXene nanosheets. The -OH surface groups on the nanofiller facilitate CO2 adsorption, while the intercalated matrix increases selective solubility, resulting in improved CO2 permeation. Therefore, understanding the solubility behavior of each material is critical before fabricating a mixed matrix membrane.
MXenes have already shown promise for large-scale developments132. Nevertheless, the use of MXenes in gas separation processes is still in its developmental stage. Further studies and research are required for MXenes to achieve commercialization in the gas separation market. Existing studies have primarily focused on a single phase of Ti3C2Tx, despite the recent development of several subfamilies and structures of MXenes, such as 2D transition metal borides (MBenes)30, oxy-MXenes31,32, MXene quantum dots (QDs)133, and porous MXenes134, all of which are worth investigating. Furthermore, the literature lacks studies on industrial gas compositions and conditions, making current performance results less applicable to real-world scenarios.


Responses