Reversibly thermosecreting and convertible lubricative oil-in-water mixtures using multiple hydrogen bonding interactions
Introduction
Oil and water can only be mixed by the formation of small droplets of one liquid in the other. These liquid droplets are easy to undergo coalescence to form single large droplets and eventually lead to complete phase separation over a sufficiently long period due to the high total interfacial free energy of the binary liquid system1. This is why a third component (e.g., a surfactant and particle) capable of generating a thin, surface-active layer at the oil/water interface that can effectively separate neighboring droplets during the Brownian motion is usually necessary to stabilize an oil/water mixture2,3,4,5, excluding few specific occasions such as water-in-liquid crystal emulsions and liquid systems containing additional hydroxide ions providing charge repulsions or impurities acting as surfactants1,5,6. For a liquid mixture that is able to undergo on-demand, reversible complexation and dissociation, surfactants and/or particles with tuneable interfacial activity are further required7,8,9,10,11,12. The introduction of these additional responsive surface-active materials may not only raise potential biosafety and environment concerns as they can be toxic and pollutive but also significantly increase the expense for obtaining an oil/water mixture as the preparation of these materials can be relatively complicated, time-consuming, and high-cost.
Recently, Nannette et al. have demonstrated the feasibility of the spontaneous formation of long-term stable water droplets in polymeric oils (molecular weight above 5000 g mol−1) in the absence of any surfactants, particles, and solvents when the thin fluid film of oil molecules separating adjacent water droplets gave rise to adhesive interactions between the droplets13. Nevertheless, in comparison, the possibility of creating highly stable oil-in-water mixtures consisting merely of water and a relatively low-molecular-weight oil remains largely unknown. In addition, the previously reported water-in-polymeric oil mixtures still cannot achieve an effective control over their switchable formation and destabilization. It also should be noted that oil/water mixtures are ideal candidates for serving as liquid lubricants in light of their unique capability in integrating the lubricity of a lubricating base oil and the cooling efficacy of water11,12,14,15. However, to date, there have been no reports on a complex liquid lubricant solely constituted of oil and water.
In this work, we produced stimuli-responsive and lubricative oil-in-water mixtures using ultrapure, deionized water and a relatively low molecular (~926 g mol−1) lubricating base oil trimethylolpropane trioleate (TMPTO). The TMPTO molecules can form multiple, high-strength hydrogen bonding with water molecules at the contact interface, thus resulting in ultralow interfacial tensions of the entire complex liquid system and generation of uniformly dispersed oil droplets capable of maintaining a good colloidal stability over a prolonged period at temperatures below 40 °C even when the oil-to-water volume ratio (Vo:Vw) attained as high as 8:1 (Fig. 1a). At higher temperatures, disruption of the intermolecular hydrogen bonding would give rise to loss of the pseudo-surface activity of TMPTO molecules and complete secretion of the oil microdroplets from the continuous aqueous phase, thereby further endowing the oil/water mixtures thermal responsiveness. Such thermosecreting behavior was reversible and would trigger prominent changes in the tribological performance of the binary liquid system, which may lead to additive-free smart liquid lubricants especially given that an enhanced lubricity compared to pure TMPTO oil could be achieved at a Vo:Vw of 1:4 owing to an effective combination between the lubrication of TMPTO and the heat dissipation of water. The oil/water mixtures were also found to possess excellent biocompatibility and biodegradability that contribute to minimizing their potential health and environmental concerns. Our study may provide useful guidance for preparing very pure, low-cost, smart, and green liquid materials that are anticipated to find extensive industrial applications.

a Schematic illustration of the formation and thermosecreting mechanism of TMPTO microdroplets in water. b Photographic illustration of the colloidal stability of the TMPTO/water mixture (upper phase). Scale bar = 1 cm. c Fluorescent and d optical micrographs of the TMPTO/water mixture. The numbers refer to the Vo:Vw used for preparing the binary liquid mixtures. Scale bars = 50 μm. pH = 7.0. T = 20 °C.
Results
Formation and stability of the TMPTO/water mixture
The highly stable, responsive binary oil/water mixture could be obtained in a broad Vo:Vw range from 1:8 to 8:1 by directly adding ultrapure, deionized water at its pristine pH value (i.e., 7.0) to TMPTO (molecular weight ~926 g mol−1, purity ≥99%) under magnetic stirring at 1500 rpm for about 10 min (see details in “Methods”). The resultant white turbid emulsions of oil/water mixtures could be obtained in the upper phase of the binary liquid system (Fig. 1b) and can be stored at room temperature for at least 30 days without any noticeable phase separations, corresponding to an excellent colloidal stability8,15,16. Such high stability could be maintained in a relatively wide pH range from 6.0 to 12.0 as displayed in Supplementary Fig. 1. Fluorescent micrographs in Fig. 1c demonstrate the formation of oil-in-water mixtures as they only exhibited fluorescent signals in the continuous aqueous phase in the studied volume ratio range after stained with a hydrophilic dye sodium fluorescein12,14,15. This can be confirmed by the droplet test in Supplementary Fig. 2 where the mixtures were found to fracture rapidly upon contact with water but were able to retain integral after being added to dodecane11,12,14,15. Optical micrographs in Fig. 1d and dynamic light scattering data in Supplementary Fig. 3 show that the homogenously dispersed, spherical TMPTO droplets all possessed an average size of ~38 μm at Vo:Vw between 1:8 and 2:1. And both the amount and size of oil microdroplets prominently reduced upon further elevating the volume ratio, which was likely owing to a relatively lower emulsifying efficiency as indicated by the declined turbidity of the upper emulsion phase. After being stored at ambient temperature for 30 days, the oil microdroplets did not exhibit significant changes in both their morphology and size distribution, which further verifies a high stability of the binary oil/water mixture.
Reversibly thermosecreting behavior of the TMPTO/water mixture
Supplementary Fig. 4 shows that such oil-in-water mixtures could be produced up to an external temperature of 40 °C and the oil and water were no longer able to undergo effective complexation at higher temperatures even after magnetically stirred for a prolonged period (i.e., 120 min). It suggests that these stable liquid mixtures were essentially triggered by the interaction between TMPTO and water molecules and the intermolecular interaction was thermo-controlled. Figure 2a depicts the evolution of the size and morphology of the oil droplets upon initially stepwise heating up from 10 to 60 °C and then gradually cooling down to 20 °C. It was found that the TMPTO molecules generated spherical droplets with an average size of ~22 μm at 10 °C. The droplet size was found to increase to ~43 μm at 30 °C. Further raising the external temperature to 50 and 60 °C led to a nearly complete vanishment of the microdroplets, indicating that the oil molecules were thermosecreted from the continuous aqueous phase17. And the oil microdroplets could be regenerated after the temperature decreased to 40 and 20 °C, suggesting that this thermosecreting behavior was reversible17. Such thermo-controlled complexation and dissociation between TMPTO and water can be confirmed by the viscosity measurements in Supplementary Fig. 5. It was found that the oil-in-water mixture could yield a shear viscosity above 1.7 Pa·s at an applied shear rate of 1 s−1 and an overall shear-thinning behavior can be discovered in the measuring shear rate range at temperatures below 40 °C. Further elevating the testing temperature gave rise to very thin binary liquids with ultralow, near-zero viscosity throughout the applied shear rate range as a consequence of thermally induced dissociation between the oil and water compositions.

a Optical micrographs of the TMPTO/water mixture upon heating from 10 to 60 °C and then cooling back to 20 °C. b Variations in the macroscopic appearance and turbidity of the TMPTO/water mixture upon alternately changing temperatures between 20 and 50 °C. Scale bar = 50 μm. pH = 7.0. VO:VW = 1:1.
Figure 2b illustrates the variation in the macroscopic appearance and turbidity of the upper emulsion phase upon alternately changing the external temperature between 20 and 50 °C. It can be seen that the pristine milk-white oil-in-water mixture with a near-zero transmittance would become relatively transparent with its transmittance value increasing to ~30% after heating to 50 °C, corresponding to the thermosecretion of dispersed oil microdroplets. The liquid mixture would regain its original high turbidity and appearance after cooling back to 20 °C and such thermally induced macroscopic changes could be repeated several times without any prominent alterations in the turbidity values, which therefore demonstrates the reversibility and longevity of the thermosecreting behavior. Supplementary Figs. 6 and 7 further illustrate no pronounced variations in both the microstructure and size distribution of TMPTO droplets after four consecutive heating-cooling cycles, confirming a good reversibility and reproducibility.
Mechanism of the high stability and reversible thermosecretion
Interestingly, as shown in Fig. 3a, the interfacial tension curve for the pure TMPTO/water interface at 20 °C was found to be quite similar to that of an oil/water interface containing adsorbed surfactants, where the interfacial tension was observed to decline from a relatively high initial value of ~35 mN m−1 to an ultralow equilibrium value of ~5 mN m−1 after 2400 s. It suggests that the interaction with water may make the oil molecules at the contact interface function as pseudo-surfactants to effectively stabilize the oil-in-water mixture by prominently reducing the total interfacial free energy of the binary liquid system. By comparison, the interfacial tension of both the pure paraffin oil/water and dodecane/water interfaces displayed negligible variations over the same measuring timescale as shown in Supplementary Fig. 8. High-resolution (600 MHz) carbon nuclear magnetic resonance (13C-NMR) spectra in Supplementary Fig. 9 show that the chemical shift corresponding to the carbonyl groups of TMPTO at 174.457 ppm exhibited evident changes after mixed with ultrapure water at 20 °C, indicating that the ultrahigh pseudo-interfacial activity of the TMPTO oil was likely to be a consequence of the strong hydrogen bonding interactions between its carbonyl moieties and the hydrogen atoms of water molecules at the contact interface18,19. Molecular dynamics (MD) simulations in Fig. 3b, c demonstrate that the pure oil molecules (20 in total) were exactly capable of spontaneously self-assembling into a droplet-like aggregate in pure water, in which their relatively hydrophilic carbonyl moieties were inclined to expose to surrounding water molecules and maintain strong hydrogen bonding interactions with the hydrogen atoms in water whereas their long, hydrophobic alkyl chains were embedded into the core of the droplet at 20 °C. The simulated number of as-formed intermolecular hydrogen bonds was ~54 in total (Fig. 3d), suggesting that all the TMPTO molecules and most of their carbonyl groups participated in the formation of hydrogen bonding.
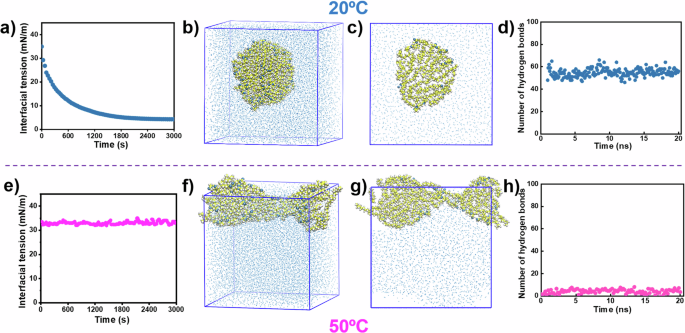
a, e Interfacial tension measurements of pure TMPTO/water interface (pH = 7.0) at 20 and 50 °C. Simulated b, f 3D and c, g 2D self-assembled microstructures of TMPTO molecules in water at 20 and 50 °C. The yellow, blue, and white symbols refer to the C, O, and H atoms, respectively. d, h Simulated numbers of the intermolecular hydrogen bonds generated at 20 and 50 °C.
In contrast, as illustrated in Fig. 3e, the interfacial tension of pure TMPTO/water interface became fully independent of time as those of the paraffin oil/water and dodecane/water interfaces as shown in Supplementary Fig. 8 at 50 °C, indicative of loss of the high pseudo-interfacial activity of the oil molecules and disruption of their intermolecular hydrogen bonding with the surrounding water molecules. 13C-NMR spectra in Supplementary Fig. 9 confirm the dissociation of the multiple, high-strength hydrogen bonding between the oil and water molecules at 50 °C as the chemical shift of carbonyl groups in the presence of an equal volume amount of water nearly completely recovered to its pristine value18,19. MD simulations in Fig. 3f–h also show that the oil molecules can no longer be dispersed and self-assemble into a droplet in water and the number of intermolecular hydrogen bonds significantly decreased to less than 8 in total at 50 °C. Our results suggest that the unusual high stability of the oil-in-water mixture and its reversibly thermosecreting behavior were essentially triggered by the invertible multiple hydrogen bonding interactions between the oil and water molecules at the contact interface as schematically illustrated in Fig. 1a.
Tribological performance and convertible lubrication of the TMPTO/water mixture
Figure 4a, b shows that the TMPTO oil could yield a very low coefficient of friction (CoF) and wear volume of ~0.05 and ~0.23 × 106 μm3, respectively, for two contact steel surfaces over the measuring period (i.e., 30 min), featuring an excellent lubricity. However, interestingly, although water was uncovered to possess a relatively poor lubricity as indicated by its high CoF and resultant wear volume of ~0.25 and ~2.9 × 106 μm3, respectively, the oil-in-water mixtures all exhibited a comparable friction and wear resistance to that of pure oil even at very low VO:VW values such as 1:8 and 1:4. And an exceedingly comparable tribological property could be achieved at the oil-to-water volume ratio of 1:4 as suggested by the nearly halved resultant wear volume of ~0.12 × 106 μm3. Moreover, as displayed in Supplementary Fig. 10, water could provide a relatively high thermal conductivity of ~0.64 W m−1 K−1, indicative of a favorable cooling capacity. Whereas the oil was discovered to possess an unsatisfactory heat dissipation efficacy due to its relatively low thermal conductivity of only ~0.18 W m−1 K−1. The cooling performance of the binary liquid mixture generally increased as a function of the water content and a more than two-fold larger thermal conductivity (~0.42 W m−1 K−1) relative to that of the oil was obtained at a VO:VW of 1:4. Our results thus indicate a simple and low-cost strategy for creating functional liquid lubricants using the VO:VW = 1:4 mixture that can not only furnish an enhanced lubrication compared to the well-established industrial lubricating oil TMPTO but also an excellent cooling capability that is crucial for metalworking and heavy equipment operation20,21,22. The relatively high cooling efficacy can also make the liquid mixture capable of overcoming the issue of friction-generated heat-induced phase separations as demonstrated by the OM observations in Supplementary Fig. 11, in which the VO:VW = 1:4 mixture was able to retain its pristine internal droplet size and morphology after the friction tests. A highest lubricity of the binary liquid mixtures acquired at the oil-to-water volume ratio of 1:4 was likely due to the balance between the lubrication of TMPTO and the heat dissipation of water attaining an optimum state at this ratio.

a CoF and b wear volume of the steel/steel tribopair after lubricated with various liquid materials under an applied normal load and sliding velocity of 15 N and 80 mm s−1, respectively, at 20 °C. c CoF of different studying liquid materials under an external normal load of 15 N and sliding velocities between 1 and 120 mm s−1 at 20 °C. d Schematic illustrations of the boundary and mixed lubrication regimes of the VO:VW = 1:4 oil/water mixture. e CoF of the VO:VW = 1:4 oil-in-water mixture at different external temperatures. Normal load = 15 N. Sliding velocity = 80 mm s−1. f CoF of the VO:VW = 1:4 liquid mixture upon alternately changing the testing temperature between 20 and 50 °C at 15 N and 80 mm s−1. pH = 7.0. All the statistical data in b, c, e, f are expressed as mean ± standard deviation (n ≥ 3).
The lubricity of different studied materials can be evidenced by the scanning on the surface topography of the abrasion on a steel surface (Supplementary Figs. 12 and 13), in which the total area of resultant wear tracks was overall in line with the results of the measurements on the wear volumes and a minimum abrasion was acquired by using the liquid mixture with an oil/water volume ratio of 1:4. Supplementary Fig. 14 further shows that the VO:VW = 1:4 oil-in-water mixture was able to yield a CoF of ~0.06 even when the external applied normal load attained as high as 800 N, corresponding to a favorable load-carrying capacity that is also very important for metalworking and heavy machinery14,20,23. Such extreme-pressure lubricity can be confirmed by the maximum weld point (PD) and non-seizure load (PB) of the liquid mixture at ~1560 and ~830 N, respectively, which thus makes the compound liquid lubricant ideal for maintaining the normal operation of a heavy equipment and machining of difficult-to-cut metallic materials12,20,24. The high PB value also suggests that the liquid mixture could generate a sturdy, physically adsorbed film on a steel surface and consequently provide effective lubrication25. Supplementary Fig. 15 illustrates that the binary liquid mixture was able to retain a low and stable CoF of ~0.03 after being continuously used for 12 h without measurable increments and fluctuations throughout the whole friction test, indicative of an effective long-lasting lubrication that is essential for putting into practical applications.
Figure 4c shows that the CoF of both pure water and oil was relatively independent of the applied sliding velocity, featuring a typical boundary lubrication regime where the two sliding steel surfaces remained in direct contact with each other and consequently led to squeeze-out of the liquid materials from the gap. By contrast, the VO:VW = 1:4 oil-in-water mixture displayed the same behavior at sliding velocities below 6 mm s−1. At higher velocities, its CoF generally declined as a function of the sliding speed, suggestive of transition of the physical lubrication mechanism to mixed lubrication, in which the liquid mixture continuously flowed into the contact zone and served as lubricants, thereby resulting in an enhanced wear inhibition compared to pure TMPTO oil at a relatively high studying sliding velocity (e.g., 80 mm s−1). Supplementary Fig. 16 shows that the TMPTO oil could yield an electrical contact resistance (ECR) as high as ~0.6 Ω on a steel surface, indicative of its capacity in generating a high-strength lubrication film via physical adsorption11,12,14. In contrast, a near-zero ECR value would be obtained when using the ultrapure water, corresponding to its poor adsorption ability on steel surfaces. After mixing the two kinds of liquids together at an oil-to-water volume ratio of 1:4, a slightly enhanced ECR to ~0.8 Ω would be attained likely as a result of transition in the physical lubrication mechanism from boundary to mixed lubrication. It further verifies that the liquid mixture could generate a sturdy physically adsorbed film and consequently provide effective lubrication. Figure 4d shows that the binary liquid mixture could retain its inherent low CoF of ~0.05 up to an external temperature of 40 °C. The CoF dramatically elevated to ~0.18 at higher temperatures due to the thermosecretion of internal oil microdroplets and conversion of the lubrication mechanism into boundary lubrication as schematically illustrated in Fig. 4c. Such thermally induced prominent transition in the friction of the liquid mixture could be cycled for at least five times without noticeable changes in the CoF value (Fig. 4e), demonstrative of a good reversibility and longevity. The results hence suggest that the VO:VW = 1:4 liquid mixture may also be employed as a smart liquid lubricant to acquire an effective control over the tribological interactions between two contact machinery components and therefore achieve a precise control of the operating accuracy and efficiency of an entire machine or device17,26,27,28,29.
Biocompatibility and biodegradability
Supplementary Figs. 17 and 18 show that either the pure oil or oil-in-water mixtures would impose no measurable cytotoxic effects on both the human bronchial epithelial cells (BEAS-2B) and human skin fibroblast cells (HSF) even at a dosage as high as 500 μg mL−1, indicative of an excellent in vitro biocompatibility. Fluorescent micrographs in Supplementary Fig. 19 also demonstrate a negligible impact of the binary liquid mixtures on the morphology and growth of the two kinds of cells at the highest studied dosage. Figure 5a, b further illustrates a very good in vivo biocompatibility of the oil/water mixtures as all the mouse tissues including the heart, liver, spleen, lung, and kidney exhibited little or no defects after intraperitoneal injection with the mixture at a relatively high dosage of 10 mg kg−1 bodyweight. The long-term in vivo biocompatibility of the liquid mixture can be verified by the blood routine tests and in serum biochemical assays (Supplementary Fig. 20) on the hematology parameters in the whole blood of mice including the white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), blood platelets (BPLT), alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN) and creatinine (CREA), in which all the biochemical indexes showed no profound differences after intraperitoneal injection with the liquid mixture for 14 days. The excellent in vitro and in vivo biocompatibility hence ensures a favorable biosafety of the liquid mixture that can effectively rule out any potential health risks caused by either direct skin exposure or accidental oral intake during practical applications23,26.

H&E staining tissue sections from the mice after intraperitoneal injection with a saline and b the TMPTO/water mixture at a dosage of 10 mg kg−1 bodyweight for 14 days. Scale bar = 100 μm. c Cumulative CO2 production and d degree of biodegradation of the TMPTO/water mixture in comparison to the reference material. pH = 7.0. VO:VW = 1:1.
The liquid mixture was also found to possess a very good biodegradability. One can see that the oil/water mixture could yield a relatively high amount of CO2 of ~90 g after metabolism by the microorganisms for 45 days (Fig. 5c), indicative of an active biological decomposition. The CO2 production rate and final output were both higher than those of the reference material (i.e., microcrystalline cellulose), and a relatively high degree of biodegradation of ~91% was acquired at the end of decomposition (Fig. 5d), which was also exceedingly comparable to that of the reference material. Our results hence suggest a good eco-friendliness of the liquid mixture that can further minimize its potential pollutive effects or burdens to the surrounding environment after discarded or discharged. It is worthwhile to note that the highly stable, reversibly thermosecreting, and convertibly lubricative oil/water mixture was also available for large-scale production taking advantage of its facile preparation, simple composition, and relatively low cost. Please refer to Supplementary Fig. 21 for a photographic illustration of our prepared 10 L, 9.6 kg TMPTO/water mixture, which was still able to retain its inherently reversibly thermosecreting property that allows it to spontaneously undergo destabilization and complete phase separation upon heating to 60 °C and recover to its pristine homogenous, highly stable state after cooling back to 20 °C (Supplementary Fig. 22). This further ensures a good prospect in practical industrial applications.
Discussion
So far, smart oil/water mixtures with high colloidal stability usually rely on the use of responsive surfactants and/or particles whose interfacial structures and properties can be effectively tuned by external stimuli7,8,9,10,11,12. The preparation of these surfactants and particles can be relatively difficult, high-cost, and time-consuming. They may also suffer the issues of relatively high toxicity upon skin exposure or accidental oral intake and high environmental burdens after being discharged or discarded. A recent study by Nannette et al. has demonstrated the feasibility of creating stable water-in-oil mixtures merely using deionized water and polymeric oils whose molecular weights are above 5000 g mol−113, but to develop their complements, i.e., highly stable oil-in-water mixtures produced solely with water and relatively low-molecular-weight oils remains challenging. Both the stimuli responsiveness and eco-friendliness of previously reported pure water/polymeric oil mixtures have not been verified. It is also worthwhile to note that oil/water mixtures can provide a unique platform for combining the friction and wear resistance of lubricating base oils and cooling efficacy of water that are of great importance for formulating high-performance liquid lubricants, especially under high load-bearing conditions11,14,15. However, a liquid lubricant consisting entirely of water and base oils has hardly been reported.
In this work, by exploiting a traditional commercially available, lowly molecular lubricating base oil TMPTO as both the dispersed phase and a pseudo-surfactant, long-term stable and thermal-sensitive oil-in-water mixtures fully absent of responsive surfactants and particles can be created in a broad VO:VW and pH value range building on multiple, high-strength hydrogen bonding interactions between the oil and water molecules at the contact interface. Owing to the formation of a strong physically adsorbed film on a steel surface, the resultant oil/water mixtures can function as additive-free liquid lubricants with extreme-pressure properties. As a consequence of the transition in the lubrication mechanism to mixed lubrication and the presence of a relatively high amount of water capable of providing efficient thermal conductivity, the binary liquid mixtures showed an optimum lubrication efficiency that was even higher than pure TMPTO when the poorly lubricative water component reached a volume fraction of 80%. Due to the inherent thermal responsiveness of the intermolecular hydrogen bonding, the liquid mixtures were reversible and convertibly lubricative, which can further be applied to develop not only smart liquid lubricants for controlling the tribological performance of various machinery components but also potential sustainable systems for achieving catalysts recovery, oil harvesting, drug release and products separation in different industrial applications8,12,16,30,31,32,33,34. In combination with the excellent biocompatibility and biodegradability that can largely eliminate the potential health and environmental concerns of the liquid mixtures, our study may provide insights into the design and preparation of very pure, low-cost, versatile, smart, and green liquid materials.
Methods
Materials
TMPTO (viscosity ~40 mPa.s) was purchased from Guangzhou Fufei Chemical Technology Co. Ltd, China. Hydrochloric acid (HCl, 36–38%) and sodium hydroxide (NaOH) were obtained from Sinopharm Chemical Reagent Co. Ltd, China. All the chemicals have a purity above 99% and were used as received.
1H-NMR (600 MHz, DMSO-d6, δ, ppm) for TMPTO: 5.40–5.26 (m, 6H), 3.96 (s, 1H), 3.91–3.86 (m, 3H), 3.31 (d, J = 5.2 Hz, 2H), 2.27 (t, J = 7.3 Hz, 4H), 2.19 (t, J = 7.4 Hz, 2H), 2.09–1.85 (m, 11H), 1.50 (m, 7H), 1.42–0.95 (m, 62H), 0.86 (m, 9H), 0.83–0.77 (m, 3H).
13C-NMR (600 MHz, DMSO-d6, δ, ppm) for TMPTO: 174.46, 129.66, 129.63, 63.61, 33.64, 31.28, 31.27, 29.08, 29.05, 29.04, 28.83, 28.82, 28.72, 28.68, 28.67, 28.58, 28.52, 24.47, 24.45, 13.94, 13.93, 7.25.
Preparation and stability of the TMPTO/water mixture
The binary liquid mixtures were prepared by directly mixing the TMTPO oil and ultrapure water (ρ = 18.25 mΩ·cm) at volume ratios (VO:VW) between 1:8 and 8:1 in glass serum vials under magnetic stirring for about 10 min at temperatures below 40 °C. Aqueous HCl and NaOH solutions (1 mol L−1) were used to adjust the pH value of the continuous aqueous phase. The liquid mixtures were stored at 20 ± 0.5 °C for studying their colloidal stability. Destabilization of the liquid mixtures could be attained by incubating them at temperatures above 40 °C in a water bath for about 1 h. All the containers and magnetic stirrers were pre-cleaned with detergent and rinsed thoroughly using ethanol and deionized water to avoid contaminations on the day of experiment.
Characterization
Optical microscopy (OM, Axioskop, Zeiss, Germany) and FM (Axio Scope A1, Zeiss, Germany) were used to characterize the structure and morphology of oil microdroplets formed in the upper emulsion phase. A hydrophilic dye sodium fluorescein (Aladdin Biochemical Technology Co. Ltd., China) was used to stain the continuous aqueous phase for FM observations. Laser particle size analyzer (LS-POP, OMEC, China) was utilized to determine the size distribution of dispersed oil microdroplets in the upper emulsion phase. UV-vis spectrometer (TU-1810DSPC, Persee, China) was employed to measure the transmittance of the upper phase of the liquid mixtures. Nuclear magnetic resonance (NMR) spectrometer (600 MHz, Bruker, AVNEO 600, USA) was employed to acquire the 1H-NMR and 13C-NMR spectra of TMPTO and TMPTO/water mixtures at desired temperatures. The 1H and 13C chemical shifts were referenced to deuterated dimethyl sulfoxide (DMSO-d6) at 2.50 and 39.53 ppm, respectively, as internal standards. Multifunctional tensiometer (K100, Krȕss, Germany) was used to determine the interfacial tension of pure TMPTO/water interface using pendant drop configurations, in which the variation in interfacial tension over time was recorded after the oil phase was slowly injected into the aqueous phase. An MCR-302e rheometer (Anton Paar, Austria) with a plate-plate system and a sample gap of 1.0 mm was used to conduct the viscosity measurements of the liquid mixtures at different temperatures in a shear rate range between 1 and 1000 s−1.
Tribological measurements
UMT friction and wear tester (UMT-TRIBOLAB, Bruker, Germany) with reciprocating ball-on-disc configurations using commercial steel balls (d = 10 mm) and substrates (d = 24 mm) as counterparts were employed to study the tribological behavior of ultrapure water, TMPTO oil and the oil/water mixtures, respectively. Both the steel ball and disc had been cleaned ultrasonically using petroleum ether and methanol before each measurement. The applied normal load was between 15 and 800 N, the sliding velocity was between 1 and 100 mm s−1 and the reciprocating distance was set to be 2 mm. All the measurements were conducted by rigorously controlling the testing temperatures (i.e., 20, 30, 40, 50, 60, and 70 °C) at constant with an accuracy of ±1 °C. The convertibly tribological performance of the oil/water mixture was studied by alternately changing the testing temperature between 20 and 50 °C at a constant applied normal load and sliding velocity of 15 N and 80 mm s−1, respectively.
Multifunctional friction and wear tester (RTEC, MFT-5000, Germany) and field-emission scanning electron microscopy (FE-SEM, JSM-760F, Zeiss, Germany) were utilized to measure the wear volume and surface morphology of the steel substrates lubricated with different materials. The extreme-pressure anti-wear property was examined using the four-ball friction tester (MS-10A, TENKEY, China). All the friction and wear measurements were repeated at least three times to ensure good reproducibility.
Biocompatibility assays
The in vitro biocompatibility was assessed using cell counting kit-8 (CCK-8) assay for human skin fibroblast (HSF) and human bronchial epithelial (BEAS-2B) cells. Both the two kinds of cells were seeded in a 96-well plate (8 × 103 cells well−1) and cultured with or without the testing samples (including TMPTO and the TMPTO/water mixtures) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37 °C under 5% CO2 for 24 h. Afterwards, 10-fold diluted CCK-8 (0.1 mg well−1) was added to the cells and incubated for another 2 h. Finally, the cell viability was measured based on the optical density at 450 nm using a Victor X5 microplate reader (PerkinElmer, Akron, OH). All the cell viability data were expressed as mean ± standard deviation (n ≥ 10).
The live/dead cell imaging was conducted with an inverted fluorescent microscopy (FM, Axio Scope A1, Zeiss, Germany). Specifically, aqueous solutions of Calcein-AM/propidium iodide (2:1) were diluted with the culture medium at a ratio of 1:1000 and then added to the cell incubator at a dosage of 1 mL well−1 according to the protocol from the manufacturer. After incubated under dark conditions for about 15 min, the morphology and growth of the cells were detected using the FM. A 494 nm argon laser was used to excite the dyes.
The in vivo biocompatibility was evaluated by intraperitoneal injection of the TMPTO/water mixture (VO:VW = 1:1) into the ICR mice (male, 8 weeks old) at a dosage of 10 mg kg−1 bodyweight. After 14 days, blood was collected from the medial orbital venous plexus for biochemical assessments. The mice were then sacrificed and their major organs including the heart, liver, spleen, lung, and kidney were collected and tested with H&E staining to examine the potential toxic effects of the liquid mixture with the mice intraperitoneally injected with saline as controls. All the animal experiments were performed according to the National Research Council’s Guide for the Care and Use of Laboratory Animals and licensed by the Animal Experimentation Ethics Committee of Research Selection Biotechnology (Hangzhou) Co., LTD (approval number: YXSW2404259926).
Biodegradability tests
Biodegradability tests were performed according to the GB/T 19277.1-2011. Microcrystalline cellulose and the TMPTO/water mixture (VO:VW = 1:1) were employed as the reference and experimental materials, respectively. The testing system was fabricated by mixing the culture soils containing putrefactive heat-fermented organic fertilizer with the samples at a weight ratio of 6:1, and the data were recorded for continuous 45 days. By using the binary liquid mixture and microcrystalline cellulose as organic carbon sources, the testing mixtures were aerated with CO2-free air at a controlled rate and the degree of biodegradation was determined by measuring the CO2 production during the decomposition process.
Molecular dynamics simulations
Classic MD simulations were carried out to study the self-assembly behavior of TMPTO molecules in water. The cubic simulation box was constructed using the PACKMOL software35. It was of 10 × 10 × 10 nm in size and contained 20 TMPTO and 11226 water molecules in total. The GAFF molecular force field consisting of both the bonded and non-bonded interactions was utilized to describe the TMPTO molecules36. The non-bonded interactions can be further divided into the van der Waals and electrostatic interactions, respectively.
Specifically, energy minimization was initially conducted to relax the simulation box. Then, a canonical (NVT) ensemble where the temperatures were set to 293 and 323 K, respectively, with a 2.0 fs timestep was applied to optimize the simulation box. The simulating temperatures were maintained using the Nose-Hoover thermostat. The NVT optimization time was set to 10.0 ns, which was long enough to obtain a stable configuration. At last, the simulation box was further optimized using an isothermal-isobaric (NPT) ensemble for 20.0 ns with a timestep kept at 2.0 fs. During the simulation process, the motion of atoms was controlled with classical Newton motion where the velocity-Verlet algorithm was used to integrate the classical Newton equation. GROMACS 2020.7 package was used to conduct all the simulations37.

Responses