Targeted memory reactivation with sleep disruption does not weaken week-old memories

Introduction
The hours we spend asleep are critical for transforming memories made when we are awake. This transformation can lead to robust memories retrieved on subsequent days. One mechanism commonly thought to be responsible for sleep-based memory consolidation involves memory replay or reactivation naturally occurring during sleep, specifically the stages known as nonrapid eye movement sleep stage 2 and 3 (NREM N2 and N3)1. Replay may induce neurological changes that make memories more resilient to disruption and forgetting2. Replay may also transform memory content, for instance, by integrating several instances of an event into a generalized representation3.
A powerful method for probing memory replay during sleep is Targeted Memory Reactivation (TMR)1,4. Most TMR studies to date have focused on declarative memory, which is defined as memory assessed through recall or recognition of facts and events. The TMR method typically requires a pre-sleep learning period when specific stimuli are associated with new memories. These stimuli can subsequently be presented during N2 or N3 sleep. When memories for previously learned information are reactivated in this manner, retrieval performance is improved in tests performed after waking4,5,6,7. Several mechanisms may explain the improvements in recall performance, including strengthening cortical engrams2, rescaling of synapses to improve signal-to-noise ratio2, and retrieval-induced forgetting of irrelevant information8.
Sleep may be disturbed in various circumstances, such as due to environmental noise or respiratory events, which could disrupt naturally occurring memory reactivation9,10,11. The use of TMR with sleep disruption (TMR-SD), a variant of TMR where memory cues purposely cause sleep disruption, may be construed as an experimental model of these real-life scenarios. Studies with TMR-SD thus enable the systematic investigation of the potential costs of sleep disruption for memory function.
Two previous studies found that sleep disruption caused by TMR cues selectively weakened rather than strengthened memories12,13. The sleep disruption was unintentional in these two studies, as TMR is typically conducted with the aim of presenting sensory cues without disturbing sleep. On the basis of those results, we designed a study using TMR-SD to intentionally cause arousals, and we observed selective memory weakening14. Spatial memories were formed about 1 hour before a nap, and then TMR-SD cues were presented during the nap both to reactivate certain memories and to disrupt sleep. Whereas a typical TMR study produces an improvement in memory storage, with TMR-SD the reactivated memories were weakened14.
What mechanisms might explain the memory-weakening effects of TMR-SD? TMR may normally involve two steps, selection of a particular memory followed by modification of storage. In TMR-SD, the selection step presumably occurs as in any TMR study. However, the second step may be changed such that storage is weakened. One possibility is that TMR-SD prevents sleep-based consolidation from taking place. Thus, when a recent memory is reactivated with concurrent sleep disruption, consolidation may not move forward, so instead, the memory is rapidly forgotten14. Another possibility is that TMR-SD actively weakens memories, even well-established memories, as has been shown for amnestic drugs, protein-synthesis inhibitors, and electroconvulsive therapy15.
In this study, we sought to distinguish these two potential mechanisms by extending the time between learning and TMR-SD. We used the same procedures as in our previous experiment14, except we interposed a 1-week delay after learning. The first experimental session comprised a brief period of spatial learning. Memory reactivation occurred 7 days later, so that the average recall accuracy at the time of the nap was slightly lower than in the prior study. We predicted that if TMR-SD weakens memories by preventing consolidation, TMR-SD applied 7 days after learning would have little effect on memory storage. In contrast, if TMR-SD can weaken memories even after some consolidation over the course of a week, then these memories would be degraded by TMR-SD just like recently established memories in the prior study14.
Results
Each participant visited the lab twice, separated by a delay of 7.33 days on average (range: 6–10 days). On the first day of the study, participants learned 74 unique object-location pairs while hearing an object-congruent sound on each trial. Learning was followed by a 5-min break and then a test on the location for each of the 74 objects (Fig. 1). At the next session, participants were tested on object locations, took a nap with TMR-SD, and then were tested again on object locations.
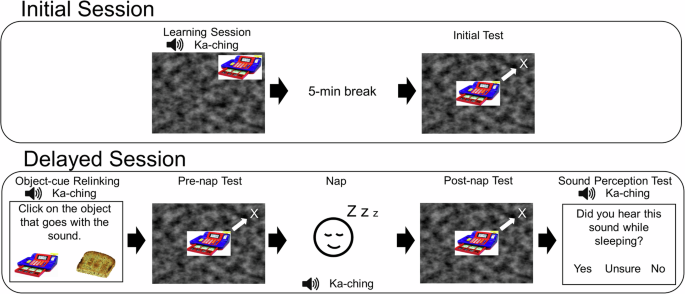
Participants learned a set of 74 object locations (objects from Bank of Standardized Stimuli33). Location recall was tested on the same day and again a week later. In each test, participants moved the object from the center to place it in the location they remembered from learning (as illustrated by the white arrow).
As shown in Fig. 2a, memory performance differed between the initial and delayed sessions. Comparing the initial, pre-nap, and post-nap memory tests revealed a significant test effect [F(2,51) = 25.8, p < 0.001]. Post-hoc pairwise t-test comparisons with Benjamini-Hochberg correction showed a significant decline from initial test in spatial recall error both on the pre-nap test and the post-nap test (corrected p’s < 0.001). Pre-nap and post-nap recall did not differ (p = 0.53). Furthermore, recall error on the pre-nap test was significantly less than that expected from uniform random placement [uniform random mean error ± SEM = 19.4 cm ± 1.1, t(1017) = 29.1, p < 0.001 (Fig. 2b)], indicating that participants retained knowledge of object locations after the delay. Furthermore, the mean pre-nap error was significantly worse than in our prior study14, in which the delay from learning to test was approximately 1 hour [12.29 cm ± 1.91 vs. 6.80 cm ± 2.46, respectively; t(39.99) = -8.03, p < 0.001].
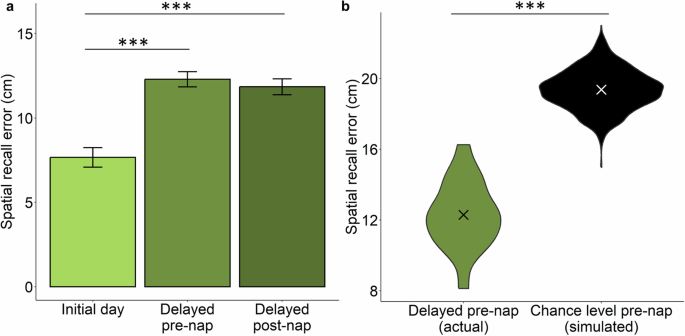
a Spatial recall error on the initial day immediately after learning object locations was smaller compared to after a week, prior to an afternoon nap with TMR-SD (Delayed pre-nap), and after the nap (Delayed post-nap). Error bars represent within-subject SEM. b Distribution of participants’ spatial recall error (group mean as X) at the one-week delay pre-nap (left). Evidence that recall was superior to chance levels was provided by data from simulated recall in 1000 participants who placed objects at uniformly distributed random locations (right). ***p < 0.001.
After the pre-nap test, objects were assigned to either the cued or uncued condition (37 objects in each condition) using an algorithm designed to match accuracy. To confirm that this matching procedure was successful, we compared recall accuracy on the pre-nap test for to-be-cued and not-to-be-cued objects. Recall error did not differ [t(17) = -0.05, p = 0.96]. Similarly, recall error between to-be-cued objects and not-to-be-cued objects did not differ on the initial test [t(17) = 0.41, p = 0.69].
We next evaluated whether presenting cues during sleep—cues intended to cause sleep disruption—affected memory fate. Prior studies of TMR with cue intensity set to avoid sleep disruption generally produced relatively less forgetting for items linked to those sounds. As shown in Fig. 3a, this effect was not reliably present, although there was a trend for a lower forgetting ratio for cued compared to uncued objects [t(17) = 1.74, p = 0.10]. The forgetting ratio was computed in centimeters as spatial error on the post-nap test divided by spatial error on the pre-nap test. A forgetting ratio (FR) > 1 indicated forgetting from pre- to post-nap; a FR < 1 indicated improvement from pre- to post-nap. The mean forgetting ratio was 0.94 ± 0.03 for cued objects and 0.99 ± 0.03 for uncued objects (SEM corrected for overall individual differences).
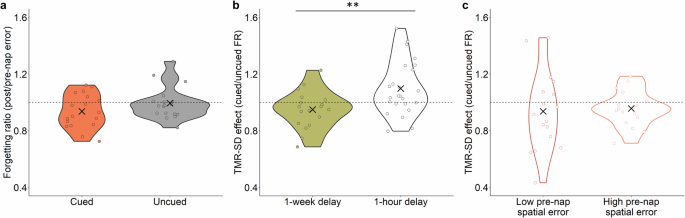
a Forgetting ratio (FR) of cued and uncued items (individual FR shown as circles and group mean as X). Dashed line denotes FR of 1, corresponding to no change in spatial recall error between pre- and post-nap tests. b TMR-SD effect (cued FR/uncued FR) in the present study (1-week delay) and in the study of Whitmore and Paller14 (1-hour delay). Dashed line denotes a TMR-SD effect of 1, corresponding to no difference in FR for cued and uncued items. c TMR-SD effect in objects with low and high pre-nap spatial error, as determined by a median split. **p < 0.01.
Given that highly similar procedures were used by Whitmore and Paller14 and in the present study, we compared the effect of TMR-SD in the two studies. The key difference in design was a post-learning delay of 1 hour versus 1 week, respectively. The TMR-SD effect was quantified as the forgetting ratio for the cued condition divided by that for the uncued condition. As shown in Fig. 3b, the TMR-SD effect differed between the two studies [t(39.84) = 2.99, p = 0.005]; the TMR-SD effect was greater in the prior study than in the present study.
Even though the two studies used the same cueing procedure, with sounds intentionally presented at a higher intensity than in typical TMR studies, we ran a further analysis to confirm that these divergent results cannot be attributed to less sleep disruption in the present study. The mean sleep fragmentation index (SFI) was defined as the number of awakenings or sleep-stage shifts per hour of sleep16. The SFI in the present study was significantly greater than that reported by Whitmore and Paller14 [t(35.94) = 3.52, p = 0.001, present study SFI = 31.6 ± 2.2, prior study SFI = 21.6 ± 1.9]. Additionally, we confirmed that the difference in the TMR-SD effect between the two studies was not due merely to weaker memories here, given the 1-week delay. We conducted a median split on pre-nap spatial error for cued and uncued objects per individual and then calculated the TMR-SD effect for objects with low versus high pre-nap error. As shown in Fig. 3c, there was no significant difference in the TMR-SD effect as a function of low versus high pre-nap error [V = 65, p = 0.611], indicating a similar lack of memory decline. Whereas pre-nap accuracy was lower overall in the present study than in the prior study, this was not the case for the low-error subset of trials. In fact, the level of accuracy for low-error trials in the present study was significantly higher than the overall pre-nap spatial error from Whitmore and Paller14 [t(37.51) = 2.64, p = 0.01], suggesting that the absence of a memory decline after TMR-SD here cannot be attributed to lower memory accuracy prior to sleep.
We also evaluated whether arousal after cue presentation modulated forgetting. We used the EEG time series of 10 seconds before and after cue presentation to determine whether a cue presentation disrupted sleep as intended (Fig. 4a). Arousal was determined offline by visual inspection of the EEG using the American Academy of Sleep Medicine (AASM) arousal scoring rule17. We found that 62% of the cues caused an arousal and 38% did not, which is similar to results from Whitmore and Paller14 (57.9% and 43.0% of cues caused and did not cause an arousal, respectively). As shown in Fig. 4b, the forgetting ratio for cues that caused an arousal, cues that did not cause an arousal, and uncued objects, was similar [(H(2) = 2.21, p = 0.34].
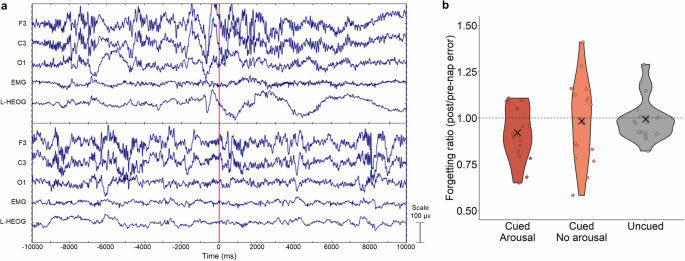
a Illustrative examples of a cue with arousal (top) and without arousal (bottom). The vertical red line indicates cue onset time. b Forgetting ratio (FR) for cued objects that caused arousals, those not causing arousals, and uncued objects did not differ significantly (individual FR as circles and group mean as X). Dashed line denotes FR of 1, corresponding to no change in spatial recall error between pre- and post-nap tests.
Next, we examined whether sleep measures were related to the forgetting ratio of cued objects or the effect of TMR-SD. The sleep measures we probed included total nap duration, wake, N2 and N3 durations, and SFI using Spearman rank correlation with Benjamini-Hochberg p-value corrections (Table 1). The forgetting ratio of cued items was not significantly correlated with total nap duration, N2 or N3 duration, or SFI [-0.30 ≤ ρ’s ≤ 0.53, 0.11 ≤ corrected p’s ≤ 0.73]. The TMR-SD effect was also not significantly correlated with any sleep measures [-0.05 ≤ ρ’s ≤ 0.21, corrected p’s = 0.85]. We also did not find that the forgetting ratio of cued items or TMR-SD effect significantly correlated with alpha (7.5-12.5 Hz) and beta (16-24 Hz) mean band power 5-seconds before the cue [-0.18 ≤ ρ’s ≤ 0.27, corrected p’s = 0.67] or after the cue [-0.18 ≤ ρ’s ≤ 0.17, 0.71 ≤ corrected p’s ≤ 0.97].
In typical TMR studies, participants may wake up and report hearing one or more cue sounds. Given the higher intensity of sound cues here, those experiences occurred more often. We asked participants about hearing each of the 74 sounds in the post-nap perception test. We considered whether these experiences with cued sounds were related to the cued-item forgetting ratio. Participants reported that they definitely heard 11.9% of sounds played during the nap, possibly heard 28.6%, and did not hear 59.5%. We combined the first two categories for our analysis and found no significant difference in the forgetting ratios [V = 52, p = 0.43]. Sounds not played during the nap were also included in the test, and participants reported that they definitely heard 8.9%, possibly heard 29.7%, and did not hear 61.4% of them.
Given that data from three individuals were excluded due to a technical failure that prevented stimulus presentation times to be recorded during sleep, we considered how the missing data may have changed the behavioral results. With those data included, the memory contrast for initial, pre-nap, and post-nap tests remained significant [F(2,60) = 23.81, p < 0.001]. The pairwise corrected post-hoc t-tests comparing initial day spatial error to pre-nap and post-nap error [corrected p’s < 0.001] remained significant. The comparison of the forgetting ratio between cued and uncued objects remained not significant [t(20) = 0.76, p = 0.46]. Also, comparing the effect of TMR-SD across the present study and that of Whitmore & Paller14 remained significant [t(42.95) = -2.29, p = 0.03].
Discussion
Previously we found that intentional sleep disruption caused by TMR cues presented during a nap degraded 1-hour-old memories14, whereas memory degradation was not observed in the present experiment for 7-day-old memories. The broader TMR literature has shown convincingly that memory cues, when delivered without sleep arousal, lead to memory improvement, particularly on the same sort of spatial recall test used in the present experiment4. Results from these experiments together favor the general interpretation that memory reactivation prompted by unobtrusive sounds is beneficial, but when those sounds cause a momentary disruption of sleep, memory consolidation is not advanced. For week-old memories, there is little change, but for memories quite recently formed, reactivation with sleep disruption reverses consolidation and degrades the corresponding memories. By extension, we postulate that these conclusions apply for memory generally, including both stimulus-induced and spontaneous reactivation during sleep.
Comparisons across experiments can be challenging if experimental parameters differ, but in these two experiments we used the identical procedure aside from changing the time between learning and sleep. By comparing sleep fragmentation and arousal metrics, we confirmed that the levels of sleep disruption and arousal in this study were similar to or even higher than in our previous study where TMR-SD weakened memory. We thus infer that stimulation that would weaken newly formed memories did not weaken week-old memories. A reasonable explanation is that week-old memories were more resilient than newly acquired memories.
One factor that potentially confounded with the learning-sleep delay between experiments was the strength of these spatial memories at the moment of sleep onset. Newly formed memories would be expected to be stronger than week-old memories. Indeed, we documented such memory differences based on pre-nap testing. To address this possible explanation for the post-nap results, we compared the effect of TMR-SD for objects with low and high pre-nap spatial error, as determined by a median split from each participant. We found that the TMR-SD effect was similar for low and high pre-nap error groups. Further, the low pre-nap error was significantly lower than the overall pre-nap error with a 1-hour delay. These results rule out the idea that different TMR-SD effects with a 1-hour versus 1-week delay can be ascribed to weaker pre-nap memories in the present experiment. Furthermore, the fact that pre-nap accuracy did not modulate the effects of TMR-SD is likely due to memory transformation during the 1-week delay.
Our observation that memories become more resilient with time after encoding is consistent with findings from other studies where amnestic drugs18 and electroconvulsive therapy19 were used to produce memory deficits, as well as observations of temporally graded retrograde amnesia after brain damage20. Other approaches to experimentally weaken a specific memory have also found that younger memories are more vulnerable to disruption than older memories21,22,23. This increase in resilience over time has been considered one of the main hallmarks of systems consolidation20,24. We thus interpret our results as indicating that TMR-SD is effective for weakening memories only if consolidation has not yet transformed the memories sufficiently.
What other possibilities might account for the time-dependent effects of TMR-SD? One is that the link between the sound and the object location was lost over time, such that presenting the sound did not reactivate the object location. We consider this possibility unlikely given that participants practiced pairing the sounds with objects immediately before the nap session. Because our sounds were semantically related to the objects and the linkage was practiced immediately before sleep, it is unlikely the effect can be explained by the weakness of the cue-location link. A related possibility is that the memories were simply forgotten after 7 days, such that reactivation was not possible. However, we found that recall remained significantly above chance levels on the test just prior to sleep, indicating that participants retained information about the location of the objects that could be subject to reactivation.
Given that nocturnal sleep is different from afternoon sleep, further research is needed to determine whether the present findings generalize beyond afternoon naps. Whereas the TMR literature has an abundance of afternoon studies, similar memory effects have also been found in overnight TMR studies4. Another limitation is that we did not collect sleep data between the initial and delayed session, nor did we assess each individual’s circadian phase, which is known to impact cognitive performance25. Irregular sleep schedules are quite common in undergraduate students, and the detrimental effects of sleep restriction on cognition can linger for multiple days26. However, given the present within-subject study design, the influence of an individual’s sleep history and circadian phase on cognition would be expected to impact cued and uncued conditions in an equivalent way. Likewise, these factors are not likely to change results across the cued-arousal, cued-no-arousal, and uncued conditions. Another limitation is that we did not directly examine how TMR influences week-old memories when there is no sleep disruption. Our study did include analysis of forgetting for cued-no-arousal items, as a proxy for TMR with no arousal. However, further investigation is warranted because the presence of repeated arousal during sleep likely changes memory processing even for items cued with no apparent arousal14.
In summary, TMR-SD produces effects that vary with time since initial learning. TMR-SD did not impair week-old memories for object locations here, but did when the memories were newly acquired14. This pattern of results suggests that sleep disruption weakens memories specifically by interfering with consolidation that would otherwise transpire. This finding has relevance to understanding the mechanisms of sleep replay and the time-course of consolidation in general. Further, our results are pertinent to designing interventions to weaken unwanted memories, such as in post-traumatic stress disorder (PTSD). REM sleep disturbances are well regarded to exacerbate PTSD severity and symptomology27; however, findings concerning the contribution of NREM sleep to PTSD are mixed28. Our findings suggest that TMR-SD soon after an emotional or traumatic experience may weaken those memories and could serve as a potential treatment for PTSD, though only if a long delay is not interposed between trauma and TMR-SD. Finally, our findings may inform efforts to understand mechanisms of how memory is affected in populations where sleep disturbances are common, such as in shift workers, in those with sleep disorders causing repeated arousals like in sleep apnea29, and in aging30.
Methods
Participants
To achieve a comparable sample size to that of Whitmore & Paller14, we aimed to include 20 participants from the local community of Northwestern University. We enrolled 37 right-handed participants between the ages of 18-33 years old (mean = 23.1, SD = 4.6, 29 females). Data from 18 were included in primary analyses. Factors that warranted exclusion for the other 19 were: not returning for the second session (6), not reaching stable N2 or N3 sleep (8), technical failure that prevented stimulus times to be recorded during sleep (3), and other technical errors (2). We asked participants to sleep 1 hour less than normal the prior night and not to ingest nicotine or caffeine on the day of the nap. Participants gave written informed consent and received monetary compensation. All procedures were approved by the Northwestern University Institutional Review Board.
Procedures
Initial Session
Learning
The study began with an object-location task. Participants learned the unique locations of 74 common objects presented on a Perlin noise background. The learning session consisted of two parts. First, participants were shown each object’s correct location while the object-congruent sound associated with the object was played. Then they were immediately tasked with moving the object from the middle of the screen to its correct location. Participants were then shown the correct location with visual feedback (“correct” if the object was placed within 3 cm from its correct location or “incorrect” if the object was placed any further away). The object’s sound was played whenever the participant made a correct response.
In the second part, participants learned each location without being shown the correct location at the beginning of each trial. Each object appeared in the middle of the screen and participants were asked to move it to its correct location. The criterion was again 3 cm. As before, the object’s unique sound was played with every correct response. Objects were presented in a random order. Once a correct response was achieved for an object, it was not shown in the learning session again. Other objects were repeatedly presented until a correct response was recorded. The learning session ended when all objects were placed in their correct location.
Initial memory test
Participants took a 5-minute break after the learning session. After the break, participants began the initial memory test. The same objects were presented in a random order against the same Perlin noise background. Each object appeared once and no feedback or sounds were used. After the test, participants were explicitly informed that they would be tested in the same way during the next session a week later.
Delayed Session
Bioelectrical recording
At the beginning of the session, electrodes were applied for electroencephalography (EEG), electrooculography (EOG), and electromyography (EMG). There were 26 scalp EEG channels at standard locations referenced to right mastoid, horizontal and vertical EOG (HEOG, VEOG, respectively), and EMG from the chin. EEG, EOG, and EMG were continuously recorded throughout the study using a Synamps2 system (Neuroscan Inc, North Carolina, USA). Data were recorded at 1000 Hz with a high-pass filter at 0.1 Hz and a low-pass filter at 100 Hz.
Object-cue relinking
Each object-associated sound was played over a set of speakers. On each trial, participants viewed two objects on the screen and were asked to select the one associated with the sound. If the wrong object was chosen, the trial was repeated. The procedure continued until a correct response was obtained for every cue sound.
Pre-nap memory test
The pre-nap memory test was identical to the initial memory test. After the test, objects were divided into two equal sets: 37 objects to be cued during sleep and 37 objects to not be cued during sleep. Objects were ranked by accuracy and sequentially assigned to one of the two groups to assure that the two sets had approximately equal accuracy. We verified that recall accuracy did not differ between the two sets using a paired t-test on mean error.
Sleep period
Participants slept on a futon in the same sound-attenuating chamber where they completed behavioral tasks. Once a participant reached stable N2 sleep (as determined by on-line sleep staging by the experimenter), their initial arousal threshold was determined by presenting a probe sound (a bike bell). The probe sound was unrelated to any object presented in the memory task. If the probe sound did not elicit an arousal, as defined by AASM17, intensity was increased and the sound was played again until the probe sound elicited an arousal. The intensity that elicited an arousal was then used when TMR-SD cueing began.
After the arousal prompted by the probe sound, participants were left undisturbed until they returned to stable N2 sleep. Once stable N2 sleep was detected, presentation of sound cues began. Cues were presented in a random order at a mean sound intensity of 50 dB (measured using Decibel X on an iPhone 13 R placed at the location of the participant’s head). Sound intensity was continuously adjusted to provoke brief arousal but avoid a prolonged awakening. Cues were presented with at least a 10-s interstimulus interval. After an arousal, cues were paused until stable N2 or N3 was reached. Once all cues were presented, participants slept for 5 min and then were awoken by the experimenter. Immediately after being awoken, participants were told that sounds were played during sleep and were verbally asked if they remember hearing any sounds.
Post-nap test and sound-perception test
After awakening, the participant stood in the brightly lit chamber for a few minutes, and then sat down for the post-nap test, which was identical to the pre-nap test except for the use of a different random order. Memory performance was compared for initial test, pre-nap test, and post-nap test using a one-way analysis of variance test. After the post-nap test, each object-associated sound was played, and participants indicated whether they remembered hearing the sound during the nap. Three options were given: Yes, Unsure, or No. This sound-perception test was only conducted in n = 16 participants due to technical error.
Memory-performance measures
Forgetting ratio (FR) was defined as post-nap error (in cm) divided by pre-nap error (in cm), as in the study by Whitmore and Paller14 concerning 1-hour-delay TMR-SD. Prior to statistical analyses, we verified that the data met homogeneity requirements for parametric tests using the Levene test. A two-way repeated-measures t-test compared the forgetting ratio of cued objects and uncued objects. All statistical analyses were performed in R Studio (version 2022.02.1, with base R version 4.1.3). The effect of TMR-SD was defined by the forgetting ratio of cued objects minus the forgetting ratio of uncued objects. A two-way repeated-measure t-test compared the TMR-SD effect of the present 1-week-delay TMR-SD study and the 1-hour-delay TMR-SD study14. A Wilcoxon rank sum test compared the TMR-SD effect of low and high pre-nap error, as determined by a median split. Subsequently, an unpaired t-test compared the pre-nap error of the low pre-nap error and the 1-hour-delay pre-nap error.
To test whether participants still retained information after the 7-day delay, we simulated 1000 participants placing objects at uniformly distributed random locations. We then calculated the placement error for these simulated participants. We used a two-tailed unpaired t-test to compare observed error values at pre-nap test to the simulated participants placing items randomly.
Bioelectrical analysis
EEG data processing
EEG data were analyzed in EEGLAB (version 2022.1). Prior to analysis, noisy channels were spherically interpolated using neighboring channels. EEG recordings were re-referenced to the left mastoid prior to analysis in 6 participants due to noisy right mastoid signal. ICA was used to remove sweat artifact in one participant.
Sleep staging
Sleep was first staged automatically using YASA (version 0.6.3), a validated automated sleep staging algorithm31. Data from Cz, HEOG, and chin EMG were input to YASA. Subsequently, an experienced sleep scorer (E.Y.) verified and corrected the sleep stages produced by YASA. Time spent in wake, N2, and N3 were correlated with the TMR-SD effect and with FR of cued objects. Sleep Fragmentation Index (SFI) quantifies the extent of sleep disruption and is defined as the number of awakenings or sleep stage shifts per hour16. We compared SFI in the current study and 1-hour delay TMR study14 to assess the similarity of sleep disruption in the two studies.
Arousal analysis
Off-line arousal analysis was performed to determine whether cues played during the nap provoked an arousal. Using the EEG 10 s before and after cue presentation, each cue was scored as arousal-provoking or not according to AASM arousal scoring rule17 (as determined by author E.Y.). Kruskal-Wallis one-way analysis of variance test was used to compare forgetting ratio for objects that caused arousals, did not cause arousals, and uncued objects.
EEG band power analysis
We calculated band power 5 s before and after cue presentation during sleep using a multi-taper method32 with 5 tapers. To determine whether power in alpha (7.5–12.5 Hz) or beta (16–24 Hz) bands were associated with memory fate, we correlated these measures with the forgetting ratio for cued items as well as the TMR-SD effect.
Responses